Abstract
Systemic lupus erythematosus (SLE) is an autoimmune disease that may be associated with other autoimmune disorders. To date, limited data are available on thyroid dysfunction and SLE in Saudi Arabia. In this retrospective study, we reviewed the cases of 151 patients with SLE. The prevalence of thyroid dysfunction was 17.2%: 4% in the hyperthyroid group and 11.9% in the hypothyroid group. Euthyroid sick syndrome was found in 1.3% of patients. Moreover, 57% of patients with hypothyroidism were positive for antibodies to thyroglobulin and thyroid peroxidase. No correlation was found between the presence of thyroid dysfunction and higher SLE disease activity, according to the Safety of Estrogens in Lupus National Assessment–Systemic Lupus Erythematosus Disease Activity Index (SELENA-SLEDAI) score.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Systemic lupus erythematosus (SLE) is an autoimmune chronic inflammatory disease that may affect any organ or system in the body [1, 2]. Thyroid dysfunction, primarily autoimmune disorders, have been frequently described in patients with rheumatologic autoimmune diseases, such as SLE, Sjögren’s syndrome or rheumatoid arthritis. The prevalence of thyroid dysfunction in SLE varies between 3.8 and 22.2% among different populations and ethnicities. The most common reported thyroid dysfunction in patients with SLE is clinically overt and subclinical hypothyroidism. Several studies evaluating the correlation between thyroid dysfunction and SLE have shown varying and conflicting results [3,4,5]. Some studies evaluating the prevalence of anti-thyroglobulin antibodies (Tg) and anti-thyroid peroxidase antibodies (TPO) have shown high thyroid dysfunction in patients with SLE [6, 7].
The aims of this study were to determine the prevalence and characteristics of thyroid dysfunction among Saudi patients with SLE at King Fahad Medical City.
2 Materials and Methods
This was a retrospective study in which we reviewed a total 151 patients older than 18 years of age who had a confirmed diagnosis of SLE according to the Systemic Lupus International Collaborating Clinics classification criteria [11] and were treated at our rheumatology clinics.
Data on clinical manifestations and laboratory findings at the time of presentation were obtained from the medical records of the outpatient clinics of King Fahad Medical City, Riyadh, Saudi Arabia, between January 2014 and December 2019.
Data obtained from patients’ files included demographic data, sex, age at diagnosis of SLE, history of thyroid diseases and treatment for thyroid disease. Laboratory evaluations included complete blood count, thyroid function test (TFT), anti-nuclear antibody (ANA) detection by indirect immunofluorescence, anti-double stranded deoxyribonucleic acid (anti-dsDNA) antibody detection with a standardized enzyme-linked immunosorbent assay, serum complement (C3 and C4), TPO and Tg.
The Safety of Estrogens in Lupus National Assessment–Systemic Lupus Erythematosus Disease Activity Index (SELENA-SLEDAI) was used to determine disease activity in patients with SLE. Thyroid dysfunction was classified into three categories according to TFT: sick euthyroid, hyperthyroidism and hypothyroidism. Hyperthyroidism status was defined by low TSH levels and high values of free thyroxine (T4), free triiodothyronine (T3) or both; or by treatment with carbimazole or propylthiouracil. Hypothyroidism was defined by an elevated TSH concentration and low T4 and/or low T3; or by treatment with thyroxine replacement therapy. Thyroid autoantibodies, TPO and Tg were also assessed in patients with abnormal TFT.
Ethical approval for this study was obtained from the ethics committee at King Fahad Medical City.
2.1 Statistical Analysis
All data were entered and analyzed in SPSS version 22 software (SPSS Inc., Chicago, IL, USA). A two-tailed P-value of 0.05 was considered significant. Demographic and clinical characteristics of the patients are reported as medians (with 25th and 75th percentiles) for continuous variables and counts (with percentages) for categorical variables. Pearson’s chi-squared test was used to compare the distribution of thyroid diseases, and Student’s t-test was used to assess the associations between disease activity and thyroid diseases.
3 Results
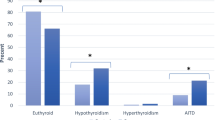
A total of 151 adult patients (141 women and 10 men) with SLE were reviewed. The female to male ratio in our study was 14.1:1. The mean age at diagnosis of SLE was 34 ± 10 years. The main clinical manifestations in our cohort consisted of skin rash in 80 (53%), photosensitivity in 69 (46%), mucosal ulcers in 46 (30.5%) and hair loss 45 (29.8%) patients. Other demographic features in our patients are summarized in Table 1. ANA and anti-dsDNA were positive in 92% and 60.7% of patients, respectively. Anti-phospholipid antibodies were detected in 31 patients (27.7%). Twenty-six patients (17.2%) had thyroid dysfunction. Eleven patients (7.3%) had subclinical hypothyroidism, and seven patients (4.6%) had hypothyroidism. Five patients (3.3%) had subclinical hyperthyroidism, and one patient (0.7%) had hyperthyroidism. Two patients (1.3%) had sick euthyroid status (Table 1). Anti-thyroid antibodies, both TPO and Tg, were present in four patients (57.14%) with hypothyroidism. Neither TPO nor Tg was positive in any patients with hyperthyroidism.
A total of 82% of patients had a positive ANA. The mean value of anti-dsDNA was 91 IU/ml. The mean value of C3 was low, at 0.7. Inflammatory markers revealed a high mean erythrocyte sedimentation rate (51) with normal mean C-reactive protein levels (4.4). Most patients had anemia (73.5%), leukopenia (25.8%) or thrombocytopenia (18.5%). The average 24-h urine protein was unremarkable, at 0.224 g/l. Elevated creatinine was found in 10.6% of patients.
Thyroid status and the prevalence of thyroid auto-antibodies are shown in Table 2. Four of seven (57%) patients with hypothyroidism had positive Tg and TPO. One patient with hyperthyroidism tested negative for thyroid auto-antibodies.
Fourteen patients (53.8%) had severe flares, and 12 patients (46.2%) had mild to moderate flares. Fourteen patients (53.8%) had anti-dsDNA positivity.
No association was observed between thyroid dysfunction and SLE disease activity, specifically the SELENA-SLEDAI score, anti-dsDNA level, C3 level and 24-h urine protein, with P-values of 0.206, 0.775, 0.729 and 0.366, respectively (Table 3).
4 Discussion
Thyroid dysfunction is fairly common in patients with SLE. Several studies have shown that the prevalence of thyroid disorders is higher among patients with SLE than in the general population, and is associated with SLE activity [3, 8,9,10,11].
Growing evidence indicates a genetic link between SLE and thyroid disorders. Concurrent thyroid disease and SLE tend to be encountered in patients with the R620W polymorphism in the PTPN22 gene encoding a T-cell protein [12]. Moreover, in a study of families with both SLE and thyroid disease, a site on chromosome 5 (5q14.3–15) has been reported to be a susceptibility gene shared by patients with SLE and patients with autoimmune thyroid diseases [13].
Data exploring the prevalence of thyroid dysfunction in patients with SLE in Saudi Arabia are lacking. In this study, 17.2% of patients with SLE had thyroid dysfunction, 7.3% had subclinical hypothyroidism, and 4.6% had hypothyroidism. We found subclinical hyperthyroidism in 3.3%, hyperthyroidism in 0.7% and sick euthyroid in 1.3% of patients. Our findings are in line with those in previous studies, in which the rate of hypothyroidism has been reported to be 3.9–17.4%, and that of hyperthyroidism has been reported to be 0.5–8% in patients with SLE [1,2,3, 5, 9, 13,14,15,16,17,18]. The high variability among studies is probably due to differences in sample size, ethnic groups and study design.
In our study, subclinical hypothyroidism was the most frequent thyroid disorder; this finding is supported by a recent meta-analysis of 10,500 patients with SLE and 44,170 controls, which has reported that subclinical hypothyroidism is more common in patients with SLE than controls (OR = 5.67, 95% CI = 3.50–9.18) [8]. In contrast, other studies have described primary hypothyroidism as a common thyroid dysfunction in patients with lupus (15–19%) [9, 14, 18]. This discrepancy might have been due to a lack of evaluation of subclinical hypothyroidism [9, 16], or to differences in the study design and populations [14].
Regarding anti-thyroid antibodies in lupus patients, autoimmunity is responsible for approximately 80% of primary hypothyroidism pathogenesis [18]. Moreover, ATA levels are associated with certain SLE antibodies, such as anti-dsDNA, RNP and anti-Smith (Sm) antibody [21]. Antonelli et al. have reported anti-thyroid antibodies in more than two-thirds of patients with SLE with hypothyroidism. Moreover, hypothyroidism has been found to be higher in patients with than without SLE [14].
Pan et al. have detected higher levels of Tg and TPO in patients with SLE than controls [20]. Further subgroup analysis by geographic location has indicated that participants with SLE from American and European populations are more likely to be Tg positive, whereas those from Asian and African populations are less likely. Regarding TPO, a positive association has been observed between TPO positivity and SLE in African and European populations, but not in Asian populations or American populations, thus suggesting a possible role of geography-driven differences in genetic backgrounds and living environments [20].
In our cohort, anti-thyroid antibodies were detected in 57% of patients who had hypothyroidism, a value greater than previously reported in some studies [5]. One explanation for this dissimilarity is the low number of patients with hypothyroidism in our study.
With respect to the relationship between thyroid disorders and SLE activity, our data did not show a significant association between thyroid disease and SLE disease activity in terms of SELENA-SLEDAI (P = 0.20), anti-ds-DNA (P = 0.77), C3 levels (P = 0.72) or 24-h urine for protein > 500 mg/day (P = 0.36). Our findings are consistent with those in a previous report by Mader et al. [2], who did not find an association between the SLEDAI score and ATA positivity. By contrast, one study in 167 patients with SLE has reported that patients with SLE with hypothyroidism had a statistically significantly higher Systemic Lupus Activity Measure (SLAM) and SLEDAI score than patients with euthyroid and subclinical hyperthyroidism [21]. This finding is inconsistent with our study outcome and may be explained by differences in the study populations.
Another study in 63 patients with SLE has revealed that Hashimoto's thyroiditis is associated with disease activity [22]. Moreover, Liu et al. have demonstrated a high risk of renal and CNS involvement in patients with SLE with thyroid diseases (3).
Notably, our study has several limitations. First, because it was a retrospective single-center study, selection bias could not be completely avoided. Moreover, the lack of some information regarding demographic data among patients who were diagnosed with thyroid dysfunction, in addition to the lack of a control group, might have influenced the conclusions.
5 Conclusion
Our study suggests that subclinical and overt hypothyroidism is common in patients with SLE, but SLE activity is not associated with thyroid disorders. Physicians should be aware of the possibility of thyroid disorder when treating patients with SLE.
Data Availability Statement
Raw data were generated at King Fahad Medical City. Derived data supporting the findings of this study are available from the corresponding author on request.
Abbreviations
- ANA:
-
Anti-nuclear antibodies
- Anti-dsDNA:
-
Anti-double stranded deoxyribonucleic acid
- C3 and C4:
-
Complement 3 and 4
- SELENA-SLEDAI:
-
Safety of Estrogens in Lupus National Assessment–Systemic Lupus Erythematosus Disease Activity Index
- SLE:
-
Systemic lupus erythematosus
- T3:
-
Free triiodothyronine
- T4:
-
Free thyroxine
- TFT:
-
Thyroid function test
- Tg:
-
Anti-thyroglobulin antibodies
- TPO:
-
Anti-thyroid peroxidase antibodies
References
Kumar K, Kole AK, Karmakar PS, Ghosh A. The spectrum of thyroid disorders in systemic lupus erythematosus. Rheumatol Int. 2012;32(1):73–8.
Mader R, Mishail S, Adawi M, Lavi I, Luboshitzky R. Thyroid dysfunction in patients with systemic lupus erythematosus (SLE): relation to disease activity. Clin Rheumatol. 2007;26(11):1891–4.
Liu YC, Lin WY, Tsai MC, Fu LS. Systemic lupus erythematosus and thyroid disease—experience in a single medical center in Taiwan. J Microbiol Immunol Infect. 2019;52(3):480–6.
Rasaei N, Shams M, Kamali-Sarvestani E, Nazarinia MA. The prevalence of thyroid dysfunction in patients with systemic lupus erythematosus. Iran Red Crescent Med J. 2015. https://doi.org/10.5812/ircmj.17298.
Boey ML, Fong PH, Lee JSC, Ng WY, Thai AC. Autoimmune thyroid disorders in SLE in Singapore. Lupus. 1993;2(1):51–4.
Pyne D, Isenberg DA. Autoimmune thyroid disease in systemic lupus erythematosus. Ann Rheum Dis. 2002;61(1):70–2.
Weetman AP, Walport MJ. The association of autoimmune thyroiditis with systemic lupus erythematosus. Br J Rheumatol. 1987;26(5):359–61.
Luo W, Mao P, Zhang L, Yang Z. Association between systemic lupus erythematosus and thyroid dysfunction: a meta-analysis. Lupus. 2018;27(13):2120–8.
Watad A, Cohen AD, Comaneshter D, Tekes-Manova D, Amital H. Hyperthyroidism association with SLE, lessons from real-life data—a case-control study. Autoimmunity. 2016;49(1):17–20.
Franco JS, Amaya-Amaya J, Molano-González N, et al. Autoimmune thyroid disease in Colombian patients with systemic lupus erythematosus. Clin Endocrinol (Oxf). 2015;83(6):943–50.
Posselt RT, Coelho VN, Skare TL. Hashimoto thyroiditis, anti-thyroid antibodies and systemic lupus erythematosus. Int J Rheum Dis. 2018;21(1):186–93.
Domingues SL, Gonçalves FT, Jorge MLMP, Limongi JE, Ranza R, Jorge PT. High prevalence of hypothyroidism in systemic lupus erythematosus patients without an increase in circulating anti-thyroid antibodies. Endocr Pract. 2017;23(11):1304–10.
Watad A, Mahroum N, Whitby A, et al. Hypothyroidism among SLE patients: case-control study. Autoimmun Rev. 2016;15(5):484–6.
Antonelli A, Fallahi P, Mosca M, et al. Prevalence of thyroid dysfunction in systemic lupus erythematosus. Metabolism. 2010;59(6):896–900.
Chan AT, Al-Saffar Z, Bucknall RC. Thyroid disease in systemic lupus erythematosus and rheumatoid arthritis. Rheumatology (Oxford). 2001;40(3):353–4.
Tsai RT, Chang TC, Wang CR, Chuang CY, Chen CY. Thyroid disorders in Chinese patients with systemic lupus erythematosus. Rheumatol Int. 1993;13(1):9–13.
Lin WY, Chang CL, Fu LS, Lin CH, Lin HK. Systemic lupus erythematosus and thyroid disease: a 10-year study. J Microbiol Immunol Infect. 2015;48(6):676–83.
Klionsky Y, Antonelli M. Thyroid disease in lupus: an updated review. ACR Open Rheumatol. 2020;2(2):74–8.
Konstadoulakis MM, Kroubouzos G, Tosca A, et al. Thyroid autoantibodies in the subsets of lupus erythematosus: correlation with other autoantibodies and thyroid function. Thyroidology. 1993;5(1):1–7.
Pan XF, Gu JQ, Shan ZY. Patients with systemic lupus erythematosus have higher prevalence of thyroid autoantibodies: a systematic review and meta-analysis. PLoS One. 2015;10(4): e0123291.
Liu H, Yang LH, Yin G, Xie QB. Correlation of thyroid autoantibodies, system lupus erythematosus immunologic indicators and disease activity in SLE with HT. Sichuan Da Xue Xue Bao Yi Xue Ban. 2018;49(2):179–82.
Pillai S, Velayudhan G. Thyroid dysfunction in SLE and association of thyroid antibody levels with disease activity in sSLE. J Evolut Med Dental Sci. 2017;6:2684–8.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
IA and RA contributed to study conceptualization. RA and SA contributed to data collection and data analysis. IA, RA, SA and AM participated in drafting the article.
Corresponding author
Ethics declarations
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Ethical Approval
Ethical approval for the current study was obtained from the ethics committee at King Fahad Medical City.
Informed Consent
Informed consent is not applicable, because this was a retrospective study.
Consent for Publication
The authors agreed to publish the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
AL-Homood, I.A., Alkhathami, R.A., Alenazi, S.K. et al. Thyroid Dysfunction Among Patients with Systemic Lupus Erythematosus in Saudi Arabia. Dr. Sulaiman Al Habib Med J 4, 169–173 (2022). https://doi.org/10.1007/s44229-022-00017-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s44229-022-00017-8