Abstract
Background
Rheumatoid Arthritis (RA) and autoimmune thyroid disease (AITD) are the two most prevalent coexisting autoimmune diseases due to their similar pathogenesis. Considering the potential effect of AITD on the severity of RA disease, this study aimed to determine the association between thyroid dysfunction, anti-thyroid peroxidase (anti-TPO) positivity, AITD, and RA disease severity in the Iranian population.
Methods
Three hundred and fifty RA patients who presented to Shahid Beheshti tertiary care center, Qom, Iran, were included in this cross-sectional study. The data were collected through the patient’s medical records, interviews, physical examinations, and laboratory tests. The RA disease activity score in 28 joints for RA with erythrocyte sedimentation rate (DAS-28-ESR) was used to divide patients into three subgroups, remission (DAS-28-ESR ⩽ 2.6), mild-to-moderate (2.6 < DAS-28-ESR ⩽ 5.1), and severe disease activity (DAS-28-ESR > 5.1).
Results
Using the aforementioned method, 111, 96, and 138 patients were put into remission, mild-to-moderate, and severe disease activity groups, respectively. Anti-TPO antibody positivity rate was 2.93 times more prevalent among patients with severe disease compared to the remission subgroup (OR: 2.93; P-value < 0.001). Patients suffering from a more severe disease were almost 2.7 times more probable to have AITD (OR = 2.71; P-value < 0.001) and they were 82% more likely to have thyroid dysfunction compared to patients in remission (OR = 1.82; P-value = 0.006).
Conclusions
It was demonstrated that thyroid dysfunction, anti-TPO antibody positivity, and AITD were significantly more common among RA patients with more severe disease activity.
Similar content being viewed by others
Background
Rheumatoid arthritis (RA) is a chronic systemic autoimmune disease that damages small symmetrical joints. Pathogenesis of the disease is complex and consists of environmental stimulations and the immune system malfunction in genetically susceptible individuals [1]. Although the hallmark of RA is the articular involvement, it is considered a systemic disease with a multitude of extra-articular involvements [2], Such as subcutaneous nodules, interstitial lung disease, neuropathy, glomerulonephritis, ischemic heart diseases, etc., primarily observed in longstanding patients [3].
Autoimmune thyroid disease (AITD), primarily Graves’ disease and Hashimoto’s thyroiditis, are the leading causes of goiters in non-iodine deficient regions with a prevalence of up to 5% of the general population. Various autoantibodies are produced in AITD, each targeting specific points in thyroid hormone synthesis process [4]. AITDs and other systematic autoimmune diseases such as Systemic Lupus Erythematous, Sjögren’s syndrome, Systemic sclerosis, and RA frequently coexist due to apparent pathogenesis [5].
Thyroid dysfunction also seems to be more prevalent in many autoimmune diseases, including RA [6]. Previous studies within different demographics and geographic locations have demonstrated various rates of AITD prevalence among RA patients [7]. Although the mechanism of AITDs and RA affecting each other is not yet well defined, several studies have proposed autoimmunity as one of the leading causes of their coexistence and influence on each other [4]. Considering the potential impact of thyroid abnormality on the severity of RA disease, and few studies taking racial diversity into account, it is imperative for further studies to be conducted. Therefore, this study has aimed to investigate the association between RA disease severity and thyroid dysfunction, AITD, and anti-thyroid peroxidase (anti-TPO) positivity in the Iranian population.
Materials and methods
Study design and participants
In this observational, cross-sectional study, 350 RA patients registered at Shahid Beheshti tertiary care center, Qom, Iran, from April 2021 to March 2022, were included consecutively.
Patients were diagnosed utilizing the American College of Rheumatology and the European League Against Rheumatism (ACR/EULAR) 2010 criteria for RA [8]. All patients were older than 18, and none suffered from concurrent diabetes mellitus, chronic renal or liver disease, malignancy, current infection, and collagen vascular disease except RA. In addition, patients receiving medications with the potential to interfere with thyroid test and possibly cause false positive or negative results (e.g., dopamine agonists, interferon-α, anticonvulsant drugs, amiodarone, lithium, and somatostatin analogs) were excluded from the study. Pregnant women were also excluded.
Before enrollment, all patients gave informed consent, according to the Declaration of Helsinki, and the protocol was approved by the ethics committee residing at the medical university of Qom (7/23/2021; IR.MUQ.REC.1400.042).
Clinical assessments
The clinical data were obtained through the patient’s medical record, interview, and physical examination. The data included Patients’ demographic features, prior history of thyroid function disease, duration of RA and thyroid disease, current medications, and the number of tender and swollen joints.
Laboratory tests including rheumatoid factor (RF), erythrocyte sedimentation rate (ESR) using the Westergren method, C-reactive protein (CRP) titer based on Biotec method, mutated citrullinated vimentin autoantibodies (anti-MCV) and anti-cyclic-citrullinated peptide antibodies (anti-CCP) based on an enzyme-linked immunosorbent assay (ELISA) method, Thyroid function tests based on chemiluminescent immunoassay (CLIA) method, and anti-TPO antibody using radioimmunoassay were checked. Anti-CCP and anti-MCV values were considered positive for serum levels of more than 5 U/ml and 20 U/ml, respectively. Thyroid function tests included thyroid stimulating hormone (TSH), free thyroxin (FT4), and free triiodothyronine (FT3) were normal in the range of 0.3–3.6 mIU/L, 0.7–1.8 ng/dl, 2.57–4.43 pg/mL, respectively. Anti-TPO antibody was considered positive for serum levels of more than 50 IU/mL.
Thyroid dysfunction is defined as abnormal levels of TSH with or without abnormal FT4 or FT3 [9]. AITD is diagnosed by endocrinologist as current or prior coexistence of thyroid dysfunction with anti-TPO antibody positivity. Thyroid ultrasound was also performed in cases with suspicious findings in the history of physical examinations [7, 10].
The disease activity score in 28 Joints for RA with ESR (DAS-28-ESR), which is a tool for measuring the severity of the disease, was calculated with the following formula: [11]
DAS-28-ESR = 0.56 × √ (TJC-28) + 0.28 × √ (SJC-28) + 0.014 × VAS + 0.70 × ln (ESR).
Where TJC and SJC stand for the tender joint count and swollen joint count, respectively, and the visual analog scale (VAS) represents the patient’s global health assessment of disease activity and his/her overall health is scored subjectively ranging from 0 as the healthiest to 100 as least healthy.
DAS-28-ESR values were categorized as follows:
-
Remission: DAS-28-ESR ⩽ 2.6.
-
mild disease activity: 2.6 < DAS-28-ESR ⩽ 3.2.
-
Moderate disease activity: 3.2 < DAS-28-ESR ⩽ 5.1.
-
severe disease activity: DAS-28-ESR > 5.1.
Statistical analyses
Quantitate and Qualitative variables were described as mean ± standard deviation (SD) and frequency (percentage), respectively. Kolmogorov-Smirnov test, Q–Q, and P-P plots were used to assess the normality of quantitative variables, in which age and FT3 were normal, and all others were not normal. One-way ANOVA test was recruited for normally distributed data and degrees of freedom, the F value (F) was reported. Additionally, Kruskal–Wallis test was recruited for non-normally distributed data and Kruskal-Wallis H (H) was also reported. The Chi-square test and ordinal regression were used to compare and analyze categorical variables between different types of disease activity; moreover, the 95% confidence interval (CI) was reported. Bivariant correlation analysis was used to determine the correlation between quantitative disease activity parameters and thyroid dysfunction test levels. The deletion method was applied where participants had a missing field which was less than 1%. To demonstrate independent factors associated with disease activity, ordinal regression was used. The data analysis was conducted using IBM SPSS Statistics version 26, and the significance of all tests was considered if the P-value < 0.05.
Results
Out of 350 patients with RA disease, 345 patients were included in this study and 5 patients were excluded due to the lack of sufficient data to calculate DAS-28-ESR. Based on DAS-28-ESR values, 111, 10, 86, and 138 patients were categorized in remission, mild, moderate, and severe phases of disease activity, respectively. Afterwards, patients were sorted into three subsequent groups of 111 patients in remission, 96 patients with mild-to-moderate, and 138 patients with severe disease activity.
The demographic feature and laboratory findings of all patients are presented in Table 1. Among the 112 patients suffering from thyroid dysfunction, 73 individuals had been already diagnosed with thyroid dysfunction prior to the study which all except for two had overt hypothyroidism, but 39 patients were considered new cases and were diagnosed during the study. ESR, CRP, anti-CCP, and anti-MCV were positive in 11 (9.9%), 14 (12.7%), 60 (54.1%), and 39 (35.5%) patients in remission, 45 (46.9%), 54 (56.3%), 55 (57.9%), and 46 (51.7%) patients with mild-to-moderate disease activity, and 106 (76.8%), 102 (73.9%), 91 (65.9%), and 98 (72.1%) patients with severe disease activity, respectively. As it is shown in Table 1, there was a statistically significant difference between remission, mild-to-moderate, and severe groups for FT3 [F (2, 340) = 35.49, P-value < 0.001] and anti-TPO (H = 15.74, P-value < 0.001).
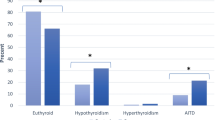
Anti-TPO antibody was positive in 18 (16.2%) patients in remission, 25 (26.9%) patients with mild-to-moderate disease activity, and 58 (43.6%) patients with severe disease activity. Among patients with severe disease activity, it was 2.93 times more probable to detect anti-TPO positive value than the patients in remission (OR = 2.93, 95% CI: 1.86–4.62; P-value < 0.001). The number of patients suffering from thyroid dysfunction and AITD were 27 (24.3%) and 13 (11.7%) in remission, 27 (28.1%), and 10 (10.4%) mild-to-moderate disease activity, and 55 (40.1%) and 37 (28.0%) with severe disease activity, respectively. Hence, the patients with higher disease activity were 82% more likely to have thyroid dysfunction (OR = 1.82, 95% CI: 1.19–2.77; P-value = 0.006) and 2.7 times more probable to have AITD than the remission patients (OR = 2.71, 95% CI = 1.57–4.62; P-value < 0.001).
DAS-28-ESR level was positively correlated with anti-TPO (r = 0.192; P-value < 0.001) and FT3 (r = 0.388; P-value < 0.001), but there was no significant correlation with TSH nor FT4. The dose of Methotrexate and Hydroxychloroquine was only correlated with FT3 (r = 0.371; p-value < 0.001 and r = 0.121; p-value = 0.024, respectively). Yet, Prednisolone dosage was positively correlated with FT3 (r = 0.404; p-value < 0.001) and anti-TPO (r = 0.236; p-value < 0.001) levels but did not have any significant correlation with FT4 and TSH levels.
The parameters that may impact the disease severity in the presence of other accompanying factors are shown in Table 2 to indicate independent predictors affecting RA disease severity. As it is demonstrated higher levels of thyroid function tests, anti-TPO, and female gender are independent predictors which are significantly associated with more severe RA disease activity.
Discussion
The coexistence of RA with AITD has been long debated due to similar pathogenesis and the possible effect of RA treatment regiment on supression of thyroid hormones. However, a consensus on this matter is not yet achieved [12, 13]. Some studies have shown that Arthropathies and connective tissue diseases such as, RA are the most common autoimmuine disease coexisting with AITD [14, 15]. Moreover, several studies have established a higher prevalence of thyroid dysfunction, particularly overt hypothyroidism, anti-TPO positivity, and AITD among RA patients [16, 17]. In addition, recent studies have also suggested that AITD comorbidity can exacerbate RA severity and activity [18]. On the contrary, Joshi et al. found high RA disease activity was more common among patients with hypothyroidism, though it was not statistically significant [19]. Also, some studies have found no association between thyroid disease and RA disease activity which is in contrast with the findings of the aforementioned studies [20].
Due to conflicting results on this matter, we aimed to assess the association between RA severity and thyroid dysfunction, anti-TPO positivity, and AITD. In our study, 345 RA patients were included and were divided into three subgroups based on disease severity which was determined by the DAS-28-ESR score using. In each group, the clinical and paraclinical factors determining the severity of RA were analyzed regarding serum levels of thyroid function tests and thyroid autoantibodies. Our study’s data analysis yielded a significant association between RA disease severity and thyroid dysfunction, AITD, and anti-TPO positivity. Higher serum levels of TSH, FT3, FT4, and anti-TPO and the female gender could also influence the severity of RA disease.
Recent studies have shown that RA patients with hypothyroidism or positive anti-TPO had higher DAS-28-ESR levels [21].In addition, Koszarny et al., found a positive correlation between DAS-28-ESR and anti-thyroid antibodies, including anti-TPO levels [22]. However, Atzeni et al. found no correlation between anti-thyroid antibodies and RA disease activity parameters [23]. In our study, DAS-28-ESR was positively correlated with FT3 and anti-TPO serum levels.
Elattar et al. demonstrated that TSH level was positively correlated with other RA disease activity parameters, including Methotrexate dosage [18]. Although in our study, no correlation was found between the dosage of Methotrexate and TSH serum level, but positive correlation between Methotrexate and Hydroxychloroquine dosage and FT3 serum level were found. Consumption dosage of other medications such as Prednisolone was also positively correlated with FT3 and anti-TPO serum levels, but no significant correlation with other thyroid function tests was found. The aforementioned results demonstrate that FT3 may be a better measure of RA severity among the thyroid function tests.
Noteworthy, our study revealed that the patient’s age had no significant impact on disease activity which may have been due to the insufficient sample size. Another shortcoming of this study which leaves room for further studies investigations, is that thyroid ultrasound was only requested by the endocrinologist in patients with a suspicious history or physical examination findings in this study due to limited availability. In addition, other thyroid autoantibodies such as anti-thyrgobulin antibody were not measured due to the expensive costs and lack of availability, but it is noteworthy that the rate of isolated anti-thyroglobulin antibody positivity (presence of anti-thyroglobulin antibody without the presence of anti-TPO) seems to be minute [24, 25]; therefore, the impact of this matter on the eventual conclusion of the study seems to be insignificant. In conclusion, thyroid abnormality, including thyroid dysfunction and AITD comorbidity with RA disease, had a considerable impact on the RA disease severity, which can affect the quality of life and prognosis of the disease and widens the scope of approaching RA management and its numerous complications.
Data Availability
The datasets generated and analyzed during the current study are not publicly available due to restrictions in ethical and data management approvals. Still, they are available from the corresponding authors upon reasonable request.
Abbreviations
- RA:
-
Rheumatoid arthritis
- AITD:
-
autoimmune thyroid disease
- anti-TPO:
-
anti-thyroid peroxidase
- ACR/EULAR:
-
American College of Rheumatology and the European League against Rheumatism
- RF:
-
rheumatoid factor
- ESR:
-
erythrocyte sedimentation rate
- CRP:
-
C-reactive protein
- anti-MCV:
-
anti-mutated-citrullinated vimentin autoantibodies
- anti-CCP:
-
anti-cyclic-citrullinated peptide antibodies
- ELISA:
-
enzyme-linked immunosorbent assay
- RF:
-
rheumatoid factor
- CLIA:
-
chemiluminescent immunoassay
- TSH:
-
thyroid stimulating hormone
- FT4:
-
free thyroxin
- FT3:
-
free triiodothyronine
- DAS-28-ESR:
-
disease activity score in 28 Joints for RA with ESR
- VAS:
-
visual analog scale
- SD:
-
standard deviation
- OR:
-
odds ratio
- CI:
-
confidence interval
References
Smolen JS, Aletaha D, Barton A, Burmester GR, Emery P, Firestein GS, et al. Rheumatoid arthritis. Nat Rev Dis Primers. 2018;4:18001. https://doi.org/10.1038/nrdp.2018.1.
Turesson C, O’fallon W, Crowson C, Gabriel S, Matteson E. Extra-articular disease manifestations in rheumatoid arthritis: incidence trends and risk factors over 46 years. Ann Rheum Dis. 2003;62(8):722–7. https://doi.org/10.1136/ard.62.8.722.
Young A, Koduri G. Extra-articular manifestations and complications of rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2007;21(5):907–27. https://doi.org/10.1016/j.berh.2007.05.007.
Lazúrová I, Jochmanová I, Benhatchi K, Sotak Å. Autoimmune thyroid disease and rheumatoid arthritis: relationship and the role of genetics. Immunol Res. 2014;60(2–3):193–200. https://doi.org/10.1007/s12026-014-8598-9.
Bourji K, Gatto M, Cozzi F, Doria A, Punzi L. Rheumatic and autoimmune thyroid disorders: a causal or casual relationship? Autoimmun rev. 2015;14(1):57–63. https://doi.org/10.1016/j.autrev.2014.10.007.
Mahagna H, Caplan A, Watad A, Bragazzi NL, Sharif K, Tiosano S, et al. Rheumatoid arthritis and thyroid dysfunction: a cross-sectional study and a review of the literature. Best Pract Res Clin Rheumatol. 2018;32(5):683–91. https://doi.org/10.1016/j.berh.2019.01.021.
Cárdenas Roldán J, Amaya-Amaya J, Castellanos-de la Hoz J, Giraldo-Villamil J, Montoya-Ortiz G, Cruz-Tapias P, et al. Autoimmune thyroid disease in rheumatoid arthritis: A Global Perspective. Arthritis. 2012;2012:1–15. https://doi.org/10.1155/2012/864907.
Aletaha D, Neogi T, Silman A, Funovits J, Felson D, Bingham C III, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Ann Rheum Dis. 2010;69:1580–8. https://doi.org/10.1002/art.27584.
Criteria for thyroid abnormalities. according to the Dutch national healthcare consensus committee. Https://www.dk.cvz.nl.
Prevalence of thyroid diseases. And antithyroid antibodies in women with rheumatoid arthritis. 2009. https://doi.org/10.4314/eamj.v85i9.117086.
Prevoo ML, van’t Hof MA, Kuper HH, et al. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38(1):44–8. https://doi.org/10.1002/art.1780380107.
Goadsby PJ, Lipton RB, Ferrari MD. Migraine—current understanding and treatment. N Engl J Med. 2002;346(4):257–70. https://doi.org/10.1056/NEJMra010917.
Nazary K, Hussain N, Ojo RO, Anwar S, Kadurei F, Hafizyar F, et al. Prevalence of thyroid dysfunction in newly diagnosed rheumatoid arthritis patients. Cureus. 2021;13(9). https://doi.org/10.7759/cureus.18204.
Ruggeri RM, Trimarchi F, Giuffrida G, Certo R, Cama E, Campennì A, Alibrandi A, De Luca F, Wasniewska M. Autoimmune comorbidities in Hashimoto’s thyroiditis: different patterns of association in adulthood and childhood/adolescence. Eur J Endocrinol. 2017;176(2):133–41. https://doi.org/10.1530/EJE-16-0737.
Boelaert K, Newby PR, Simmonds MJ, Holder RL, Carr-Smith JD, Heward JM, et al. Prevalence and relative risk of other autoimmune diseases in subjects with autoimmune thyroid disease. Am J Med. 2010;123(2):183. e9.
Medrea I, Christi S. Chronic migraine–evolution of the concept and clinical implications. Headache: The Journal of Head and Face Pain. 2018;58(9):1495–500. https://doi.org/10.1111/head.13380.
Bagherzadeh-Fard M, Yazdanifar MA, Aghaali M, Masoumi M. The prevalence of thyroid dysfunction and autoimmune thyroid disease in patients with rheumatoid arthritis. 2022. https://doi.org/10.1186/s41927-021-00227-x.
Elattar EA, Younes TB, Mobasher SA. Hypothyroidism in patients with rheumatoid arthritis and its relation to disease activity. Egypt Rheumatol Rehabilitation. 2014;41(2):58–65. https://doi.org/10.4103/1110-161X.132458.
Joshi P, Agarwal A, Vyas S, Kumar R. Prevalence of hypothyroidism in rheumatoid arthritis and its correlation with disease activity. Trop Doct. 2017;47(1):6–10. https://doi.org/10.1177/0049475515627235.
Emamifar A, Hangaard J, Hansen IMJ. Thyroid disorders in patients with newly diagnosed rheumatoid arthritis is associated with poor initial treatment response evaluated by disease activity score in 28 joints-C-reactive protein (DAS28-CRP): an observational cohort study. Medicine. 2017;96(43). https://doi.org/10.1097/MD.0000000000008357.
Conigliaro P, D’Antonio A, Pinto S, Chimenti MS, Triggianese P, Rotondi M, et al. Autoimmune thyroid disorders and rheumatoid arthritis: a bidirectional interplay. Autoimmun Rev. 2020;19(6):102529. https://doi.org/10.1016/j.autrev.2020.102529.
Koszarny A, Majdan M, Suszek D, Wielosz E, Dryglewska M. Relationship between rheumatoid arthritis activity and antithyroid antibodies. Pol Arch Med Wewn. 2013;123(7–8):394–400. https://doi.org/10.20452/pamw.1829.
Atzeni F, Atzeni F, Doria A, Ghirardello A, Turiel M, Batticciotto A, et al. Anti-thyroid antibodies and thyroid dysfunction in rheumatoid arthritis: prevalence and clinical value. Autoimmunity. 2008;41(1):111–5. https://doi.org/10.1080/08916930701620100.
Atzeni F, Doria A, Ghirardello A, Turiel M, Batticciotto A, Carrabba M, et al. Anti-thyroid antibodies and thyroid dysfunction in rheumatoid arthritis: prevalence and clinical value. Autoimmunity. 2008;41(1):111–5. https://doi.org/10.1080/08916930701620100.
Kuria J, Amayo A. Prevalence of anti-thyroid antibodies in patients with primary thyroid disorders. East Afr Med J. 2008;85(9):459–62. https://doi.org/10.4314/eamj.v85i9.117086.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
MM and MB designed the study. MAY and MBF wrote the proposal, and all authors contributed to preparing the final draft. MAY, MM, and MAH collected the data. MBF and MV analyzed the data. MAY, MBF, and MAH wrote the manuscript, and all authors critically revised the manuscript and approved the final manuscript for publication.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Qom University of Medical Sciences (7/23/2021; IR.MUQ.REC.1400.042). Before enrollment, Informed consent was obtained from all individual participants included in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yazdanifar, M.A., Bagherzadeh-Fard, M., Habibi, M.A. et al. The association between thyroid dysfunction, autoimmune thyroid disease, and rheumatoid arthritis disease severity. BMC Endocr Disord 23, 212 (2023). https://doi.org/10.1186/s12902-023-01473-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12902-023-01473-5