Abstract
Background
Pregnant and postpartum women are at high risk of depression due to hormonal and biological changes. Antenatal depression is understudied compared to postpartum depression and its predictors remain highly controversial.
Aim
To estimate the prevalence of depressive symptoms during pregnancy and investigate factors associated with this condition including vitamin D, folate and Vitamin B12 among participants in the Kuwait Birth Study.
Methods
Data collection occurred as part of the Kuwait Birth Cohort Study in which pregnant women were recruited in the second and third trimester during antenatal care visits. Data on antenatal depression were collected using the Edinburgh Postnatal Depression Scale (EPDS), considering a score of ≥ 13 as an indicator of depression. Logistic regression was used to investigate factors associated with depressive symptoms in pregnant women.
Results
Of 1108 participants in the Kuwait Birth Cohort study, 1070(96.6%) completed the EPDS. The prevalence of depressive symptoms was 21.03%(95%CI:18.62–23.59%) and 17.85%(95%CI:15.60-20.28%) as indicated by an EPDS ≥ 13 and EPDS ≥ 14 respectively. In the multivariable analysis, passive smoking at home, experiencing stressful life events during pregnancy, and a lower level of vitamin B12 were identified as predisposing factors. Conversely, having desire for the pregnancy and consumption of fruits and vegetables were inversely associated with depressive symptoms.
Conclusion
Approximately, one fifth of pregnant women had depressive symptoms indicating the need to implement screening program for depression in pregnant women, a measure not systematically implemented in Kuwait. Specifically, screening efforts should focus on pregnant women with unintended pregnancies, exposure to passive smoking at home, and recent stressful live events.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Antenatal depression is a common health condition with between 10 and 20% of women experiencing depressive symptoms during pregnancy [1,2,3,4]. This is even higher among adolescent mothers [2, 5, 6] and those with a history of depression before becoming pregnant [7]. Depression during pregnancy is usually overlooked primarily because its symptoms can imitate those of normal pregnancy [8] and the fact that healthcare providers are usually focused on physical health aspects of pregnancy [9]. The substantial hormonal changes associated with pregnancy, particularly steroid hormones that influence hypothalamic pituitary adrenal and hypothalamic pituitary gonadal axes is linked to mood disorders, which may explain why women during pregnancy and the postpartum period are extremely vulnerable to depressive symptoms [10].
Studies have shown that antenatal depression has negative consequences on both mothers and offspring. During pregnancy, depression may increase the risk of preeclampsia [11], failure to seek optimal antenatal care [12], miscarriage or preterm birth [13, 14], failure to have optimal diet required for the mother and the fetus [12], risky behavior such as smoking or drug abuse [12], risk of suicide [15], delivery by elective cesarean section [16], risk of postpartum depression [17, 18], having difficulty bonding with the child [19, 20], decreased breast feeding [21], and prolonged sick leave [12, 16]. With respect to the impact of antenatal depression on the offspring, studies have shown that maternal depression during pregnancy may increase the risk of preterm birth [13, 21], low birth weight [13, 22], and poor cognitive function [23, 24]. Unfortunately, the treatment of depression during pregnancy may also carry some adverse effects as most antidepression medications cross the placenta [25, 26]. Adverse effects of antidepression treatment in offspring include prenatal antidepressant exposure syndrome [27], autism spectrum disorder [28, 29] and attention-deficit/hyperactivity disorder [29] although these are not confirmed and remained under rigorous debate [30, 31].
Compared to postpartum depression, antenatal depression has received much less attention [32]. Identifying modifiable risk factors for antenatal depression is a prerequisite for interventions that aim to reduce the burden on mothers and their children. However, the evidence remains inconclusive about the link between antenatal depression and several potential risk factors including the lack of physical activity [33], pre-pregnancy obesity [34], and nutritional factors [35, 36] indicating the need for high quality primary research. In high-income Arab countries in the Gulf region, there is a lack of data on the prevalence of antenatal depression as well as factors predisposing to this condition. The few studies that have estimated the prevalence of depressive symptoms during pregnancy in these countries showed heterogenous estimates ranging from 17 to 57.5% [37,38,39,40,41]. This study aimed to estimate the prevalence of depressive symptoms during pregnancy and investigate its association with several potential risk factors including the lack of physical activity, pre-pregnancy obesity, and dietary pattern as well as several nutritional biological markers such as vitamin D, folate, and Vitamin B12.
2 Methods
2.1 Study Design and Study Participants
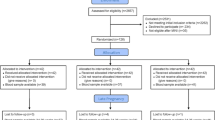
Participants in this study were recruited through the Kuwait Birth Cohort Study in which pregnant women were recruited in the second or third trimester during antenatal care visits. Ethical approval of this study was granted by the research ethics committee at the Ministry of Health, Kuwait (Ref: project 173/2014; date: February 14th, 2017), as well as the Institutional Review Board at Old Dominion University (Ref: 1,517,949). Prior to enrollment, written informed consent was obtained from all study participants.
2.2 Data Collection
2.2.1 Measuring Depression (the Dependent Variable)
Data on depressive symptoms were collected using the Edinburgh Postnatal Depression Scale (EPDS) [42], which has been validated in our setting [43]. The EPDS is used to screen for depression in women during pregnancy and postpartum period, but it is not considered a diagnostic tool. It contains 10 items, each is scored from 0 to 3, hence the total score is between 0 and 30. The higher the score, the greater the level of depression symptoms. Several cutoff points have been used to define depression including EPDS score ≥ 10, EPDS score ≥ 13 and EPDS score ≥ 14. In this study, we considered EPDS score ≥ 13 as indicative of depression. A recent validation study of the Arabic version of EPDS showed that this score has 87% sensitivity and 90% specificity [43].
2.2.2 Measuring Explanatory Variables
Socio-demographic and lifestyle data were obtained through face-to-face interviews with pregnant women, conducted by a trained data collector. The data collection form was created, and pilot tested on 30 pregnant women who were not part of the main study. Dietary data were collected through questions sourced from the Kuwait Nutritional Surveillance System and WHO stepwise approach to noncommunicable disease risk factors surveillance [44]. Data regarding physical activity were gathered using the pregnancy physical activity questionnaire [45], which has been previously validated in a similar context [46]. Participants self-reported their pre-pregnancy weight and height, while current weight and height measurements were taken in a standardized manner. Clinical data, including comorbidity data, was extracted from medical records by a healthcare professional.
2.3 Laboratory Methods
Blood samples were collected and assessed in the laboratory of Al Sabah Maternity Hospital, where these laboratory investigations are routinely carried out with strict adherence to quality control standards. Vitamin B12, folate, and 25-Hydroxyvitamin D were examined using the Cobas e601 analyzer manufactured by Roche Diagnostics GmbH in Mannheim, Germany. Serum vitamin B12 was quantified using the Roche commercial kit (Catalog #7,212,771,190), whereas the total folate in hemolyzed whole blood was measured using the Roche commercial kit (Catalog # 7,559,992,190). Finally, 25-Hydroxyvitamin D levels were determined using the Roche commercial kit (Catalog # 9,038,078,190). Vitamin D status was classified as deficiency/insufficiency (25-Hydroxyvitamin D < 75 nmol/L) and sufficiency (25-Hydroxyvitamin D ≥ 75 nmol/L) using acceptable cut-off points [47].
2.4 Statistical Methods
Data were entered in specially designed database using EpiData and were analyzed by STATA Release 17. The total score from EPDS was calculated by adding together the scores for each of the ten items. As per most studies in the region [37, 41, 48], those with EPDS score of ≥ 13 were considered as having depression. It has been suggested that this score has 87% sensitivity and 90% specificity in our setting [43]. We also recategorized the score as per other cutoff points and reported the results in the text as a sensitivity analysis. For example, we used EPDS score ≥ 14, which has been associated with greater certainty of depression [49] and evaluated the impact of this on our findings. We used logistic regression models to investigate factors associated with depressive symptoms calculating the crude and adjusted odds ratio and their 95% confidence intervals (CI). Factors that showed p < 0.05 were deemed to be statistically significant. In multivariable analysis, variables with p < 20% were grouped based on the stress process model framework [50], which has been used before in antenatal depression [51, 52] and postpartum depression [53]. The first group included stressors (listed in Tables (1), (2) and (4)) and the second group included mediators (listed in Tables (3) and (5)). Variables from these groups were modeled separately with models assessed using the Pearson χ2 goodness-of-fit test.
3 Results
Of 1108 participants in Kuwait Birth Cohort study, 1070 (96.6%) completed EPDS. The mean (SD) age was 31.46 (5.28) years, and the majority (76.31%) were non-Kuwaiti. Most women were housewives (52.91%) and reported having no specific monthly income (54.99%). Other socio-demographic factors of the study participants can be gleaned from Table (1). The median (IQR) of EPDS score was 6 (11), while 14 (1.31%) pregnant women thought of harming themselves (chose responses other than “never” in item 10 in EPDS). Of those who completed the EPDS, 225 (21.03%; 95%CI: 18.62–23.59%) had depressive symptoms as defined by EPDS score ≥ 13, which was significantly higher in non-Kuwaiti compared to Kuwaiti mothers (22.77% vs. 15.42%; p = 0.012). The prevalence of depressive symptoms as define by EPDS score ≥ 14 was 17.85% (95%CI: 15.60-20.28%).
Table (1) shows the association between depressive symptoms and socio-demographic factors in univariable analysis. Demographic factors that were found to be associated with depressive symptoms included nationality (p = 0.012), age (p = 0.026), husband being married to another women (p = 0.007), and type of housing (p = 0.011). Similarly, women who wished to be pregnant were less likely to have depressive symptoms, crude odds ratio (COR) 0.57 (95%CI: 0.42–0.77); p < 0.001; while women who became pregnant while taking contraception were more likely to have depressive symptoms, COR 1.66 (95%CI: 0.99–2.78); p = 0.049 (Table 2). Additionally, women who were exposed to passive smoking at home were more likely to have depressive symptoms, COR 1.56 (95%CI: 1.16–2.11); p = 0.003 (Table 2). Of the dietary and lifestyle factors, taking supplements during pregnancy were inversely associated with depressive symptoms, COR 0.44 (95%CI: 0.25–0.76); p = 0.003; while consumption of fresh fruits or fresh vegetables were inversely associated with depressive symptoms (p < 0.001) (Table 3). Surprisingly, the total physical activity was positively associated with depressive symptoms (p < 0.001) Table (3). None of the comorbidities during pregnancy showed an association with depressive symptoms but having a stressful life event such as death of a relative or close friend was significantly associated with depressive symptoms, COR 2.61(95%CI: 1.86–3.67); p < 0.001 (Table 4). Apart from vitamin B12, which showed inverse association with depressive symptoms (p = 0.009), none of the laboratory factors including iron (p = 0.884), folate (p = 0.962) or vitamin D (p = 0.093) showed significant association with depressive symptoms (Table 5).
In multivariable analysis, out of the stressors’ group, the desire to get pregnant was negatively associated with depressive symptoms, adjusted odds ratio (AOR) 0.67(95%CI: 0.47–0.96); p = 0.028 (Table 6). Both passive smoking at home and stressful life events during pregnancy were positively associated with depressive symptoms, AOR 1.52(95%CI: 1.10–2.10); p = 0.011 and 2.20 (95%CI: 1.53–3.17); p < 0.001, respectively. Weekly consumption of each fresh fruits and fresh vegetables were inversely associated with depressive symptoms; p = 0.021 and p = 0.023, respectively. Surprisingly, physical activity was positively associated with depressive symptoms with those in the highest tertile being more likely to report symptoms of depression compared to those in the lowest tertile, AOR 2.04(95%CI: 1.38–3.02); p = 0.001. Finally, vitamin B12 was inversely associated with depressive symptoms, p = 0.038 (Table 6). The analysis above was repeated with the depressive symptoms defined as EPDS score ≥ 14, which show some differences in the multivariable model but minimal changes in our conclusions (See supplementary Tables 1–6). For example, passive smoking and fruit consumption lost statistical significance, (p = 0.087) and (p = 0.070), respectively. Also, vitamin B12 was no longer associated with depressive symptoms (p = 0.407) and instead current consumption of supplements (p = 0.001), and meat consumption (p = 0.004) were significantly associated with depressive symptoms. Finally, pre-pregnancy Body Mass Index (BMI) was significantly associated with antenatal depressive symptoms with women who were overweight being more likely to have depressive symptoms (p = 0.039).
4 Discussion
This study aimed to estimate the prevalence of depressive symptoms during pregnancy in Kuwait and investigated factors associated with these symptoms during pregnancy. In particular, we aimed to investigate the association between depressive symptoms during pregnancy and socio-demographic, reproductive, and clinical factors as well as dietary pattern and biological nutritional markers such as vitamin D, folate and vitamin B12. We found that around 21% of pregnant women had depressive symptoms as per the EPDS score ≥ 13 while 18% of pregnant women had depressive symptoms defined as an EPDS score ≥ 14. Several factors were found to be positively associated with depressive symptoms during pregnancy including having stressful life events, and passive smoking at home as well as lower levels of vitamin B12. Factors that were found to be negatively associated with depressive symptoms during pregnancy included planned pregnancy and consumptions of fresh fruits and fresh vegetables.
The prevalence of depressive symptoms during pregnancy was found to be 21% (EPDS score ≥ 13) that became 18% with an EPDS score ≥ 14, which is similar to that reported from Oman (24%) [37]. However, it is lower than the estimates reported from Saudi Arabia, where small studies showed that between 32% [48] and 57.5% [41] of pregnant women had depressive symptoms as well as other low-income countries in the region such as Egypt (63%) [54] and Jordan (57%) [55]. It is also lower than that reported from low- or middle-income countries such as China (28.5%) [32], Ethiopia (25%) [56], Tanzania (33.8%) [57], Brazil (35.6%) [58], and Iran (37%) [59]. An earlier study in Kuwait estimated the prevalence of depressive symptoms during pregnancy to be 20% based on a lower cutoff point of EPDS score ≥ 10 to define depression [39]. Using this cutoff point in our study would yield a prevalence of 31.87%. The difference between our estimate and earlier estimate could be due to the fact that we recruited women from a hospital, while the earlier study recruited their participants from the general population. Our findings highlight the need to implement systematic screening for depression during pregnancy in Kuwait, as per the recommendation by the American College of Obstetricians and Gynecologists as well as the US preventive services taskforce (USPSTF) [60, 61]. It is important that pregnant women with positive screening are evaluated further for diagnosis and, if appropriate, are referred for evidence-based mental healthcare services as screening per say would not provide benefit beyond self-awareness.
In univariable analysis, several factors in the first conceptual domain of stress process model [50] were found to be associated with antenatal depression. Being non-Kuwaiti was positively associated with depressive symptoms which may be explained by the fact that they are away from their extended families in their own countries in addition to the advantages that Kuwaiti citizens enjoy compared to non-Kuwaiti such as free healthcare, education, housing, as well as subsidized food items. Of note is the positive association between having a husband married to another women and depressive symptoms, which is another major stressor. Although only a small number of women reported a husband married to another women, this particular group of women need to be screened for antenatal depression. Unlike an earlier study in Saudi Arabia [41], which showed association between depressive symptoms in pregnant women and the number of children or the number of daughters, we found no association between depressive symptoms during pregnancy and these factors in univariable or multivariable analysis.
In multivariable analysis, women who wanted to get pregnant were less likely to have depressive symptoms, which is similar to that reported from other studies that showed unplanned pregnancy [56, 62] or unintended pregnancy [63] as a significant predictor for antenatal depression. Having a stressful life event during pregnancy was significantly associated with depressive symptoms in our study, which is similar to the conclusion of a systematic literature review that showed stressful live events as a significant predictor for depressive symptoms in pregnant women [63]. Similarly, passive smoking at home was positively associated with depressive symptoms in our setting, which is similar to that reported from other settings [48, 64]. A recent literature review on this issue, concluded that secondhand smoking is associated with a significant increase in the odds of antenatal depression [65]. These findings are highly valuable to help identify pregnant women at risk for antenatal depression in our setting.
It was surprising to find that pregnant women who are at the highest tertile of physical activity are more likely to have depressive symptoms compared to those in the lowest tertile as the opposite was expected. A recent literature review of 11 studies on the association of physical activity during pregnancy and postpartum depression highlighted the heterogeneous results with one study showing positive association-like ours- and nine studies showing no association, although the authors concluded that physical activity may prevent postpartum depression [33]. Another literature review of the trials on yoga-based exercises or other defined exercises during pregnancy concluded that physical activity during pregnancy may reduce depressive symptoms in the perinatal period [66]. It should be clear that most of the physical activity in our setting was reported in the domain of household work rather than regular physical exercises or yoga-based exercise. The higher level of physical activity among our participants reflects more repetitive house chore (e.g., taking care of children) as outlined in the physical activity questionnaire, which may be an added burden that may predispose women to antenatal depression.
We found no evidence for the association between pre-pregnancy self-reported BMI and depressive symptoms during pregnancy. Studies that investigated the association between pre-pregnancy BMI and antenatal or postnatal depression have shown controversial findings (reviewed recently by Dachew et al. [34]). Although most reviews concluded that obesity before pregnancy is a significant risk factor for antenatal depression despite the high degree of conflicting results from individual studies [34, 67], there is strong believe that this association appeared because of unmeasured cofounders or residual confounding [34]. It has been suggested that the lack of association between pre-pregnancy obesity and antenatal depression could be explained by the fact that women during pregnancy may view high body weight as a positive sign of normal pregnancy [68, 69]. Women are more likely to think this way in the second or third trimester, which may explain our findings. Unfortunately, none of the reviews stratified the analysis by trimester despite the fact that the association may become weaker during the second or third trimester. Nevertheless, pre-pregnancy BMI was significantly associated with depressive symptoms when we used EPDS score ≥ 14 as a cutoff point (Supplementary Table 6).
The role of nutrition in developing depression in general and during pregnancy has attracted a lot of attention. The theoretical plausibility that can explain how several nutrients may affect synthesis or regulation of some neurotransmitters hence regulate mood have been presented in the literature [70,71,72]. This created strong motivation to demonstrate the impact of nutrition on depression using epidemiological studies, particularly during pregnancy, a period in which nutritional requirements increases significantly. Overall, the evidence for the link between dietary patterns and depression is limited and inconsistent [35, 36]. In our study, we collected data on dietary pattern and measured multiple biological nutritional markers including iron, folate, vitamin D, and vitamin B12. We found consumption of fresh fruits and vegetables as a significant predictor for depressive symptoms in pregnant women in univariable and multivariable analyses (Tables 3 and 6). In a recent small trial, a higher consumption of fruits showed an inverse association with postpartum depression [73]. Also, a brief intervention with diet that includes fruits and vegetable has been shown to reduce depressive symptoms in adults [74]. An earlier literature review showed that dietary pattern characterized by a high intake of fruit, vegetables, whole grain, fish, olive oil, low-fat dairy, and antioxidants; and low intakes of animal foods was associated with a decreased risk of depression in general adults [36]. Consumption of fruits and vegetables provides a wide range of nutrients, which may explain this association in our study. As we collected dietary data and measured antenatal depression at the same time, it is possible that the association may reflect a reverse causality (women with depression became less likely to take care of their nutrition hence consume less fruits and vegetables compared to women without depression).
One of the major strengths of our study is measuring multiple biological nutritional biomarkers such as vitamin B12, vitamin D and folate. As mentioned above, we found no association between folate and depressive symptoms during pregnancy in univariable or multivariable analysis, which is consistent with a recent review that concluded that there is no association between folic acid intervention and EDPS score during and post pregnancy [75]. Our finding showed that low vitamin B12 levels are associated with depressive symptoms in univariable and multivariable analysis (Tables 5 and 6). Despite the biological plausibility that can explain the impact of vitamin B12 on mental health, epidemiological studies do not generally support the link between vitamin B12 and antenatal depression. An analysis of National Health and Nutrition Examination Survey (NHANES) data showed that vitamin B12 levels were associated with antenatal depression in univariable and multivariable analysis although depression was measured using the Patient Health Questionnaire-9 [76]. In the birth cohort of Singapore (n = 709), plasma vitamin B12 concentrations were not associated with perinatal depression [77]. A recent literature review showed that only 5 studies addressed this issue and that there was no association between vitamin B12 deficiency or insufficiency and depression during pregnancy [78]. Finally, a recent literature review of 11 randomized control trials (six trials on vitamin B12 alone and five for vitamin B complex) concluded that vitamin B12 supplementation is ineffective in improving depressive symptoms in patients without advanced neurological disorder [79]. It is worth noting that we found no association between vitamin B12 and depressive symptoms when we used EPDS score ≥ 14 as a cutoff point (Supplementary Table 6).
Vitamin D was not significantly associated with depressive symptoms during pregnancy in our study. Despite the biological plausibility that can explain the link between vitamin D and antenatal depression, a recent literature review on this topic showed that the evidence is poor and inconclusive [80]. In fact, multiple reviews over the past decade have consistently called for better quality of research on this topic [80,81,82,83]. One of the major weaknesses of observational studies on this issue is the lack of adjusting the association between vitamin D and antenatal depression for season in which blood samples were taken. We adjusted for the season in which blood sample was taken and there was no change in our findings, AOR 0.76(95%CI: 0.52–1.10) (p = 0.141). This analysis, however, showed that women recruited in Summer or Spring had higher prevalence of depressive symptoms compared to those recruited in Fall or Winter (p = 0.013).
This study has several strengths including the large sample size and utilization of EPDS, a validated tool that has been widely used to measure depressive symptoms in numerous studies. Nonetheless, it is essential to recognize that EPDS is merely a screening tool for depression in pregnant and postpartum women, and by no mean can be considered sufficient for the diagnosis of depression, which requires clinical assessment. The other limitation of this study is the lack of data on social support. Also, despite the fact that our study was birth cohort, this analysis was based on data collected at baseline hence the data on the risk factors and antenatal depression were collected at that same time, which makes us less certain about the temporal relationship between some risk factors and depressive symptoms. For example, pregnant women with depression may neglect their diet hence eat less fruits and vegetable compared to women without depression, which makes it difficult to assume that the association between diet and depressive symptoms is causal.
5 Conclusion
In conclusion, about one fifth of pregnant women had depressive symptoms based on the EPDS, which suggest the need to implement screening program for depression among pregnant women in Kuwait. Despite the well-accepted recommendation of screening for depression during pregnancy [60, 61], this is not systematically implemented in Kuwait. Pregnant women with positive screening should be evaluated for diagnosis and, if appropriate, are referred for evidence-based mental healthcare services. Pregnant women with unintended pregnancy, passive smoking at home, and stressful life events should be targeted by the screening program for antenatal depression. Finally, further studies are recommended to elucidate the impact of lifestyle factors such as physical activity and dietary pattern on the risk of antenatal depression.
Data Availability
Data analyzed in this study will be available from the corresponding authors upon reasonable request.
Abbreviations
- EPDS:
-
Edinburgh Postnatal Depression Scale
- CI:
-
Confidence Interval
- SD:
-
Standard Deviation
- IQR:
-
Interquartile Range
- COR:
-
Crude Odds Ratio
- AOR:
-
Adjusted Odds Ratio
- BMI:
-
Body Mass Index
References
Marcus SM, Flynn HA, Blow FC, Barry KL. Depressive symptoms among pregnant women screened in obstetrics settings. J Womens Health (Larchmt). 2003;12(4):373–80.
Dietz PM, Williams SB, Callaghan WM, Bachman DJ, Whitlock EP, Hornbrook MC. Clinically identified maternal depression before, during, and after pregnancies ending in live births. Am J Psychiatry. 2007;164(10):1515–20.
Melville JL, Gavin A, Guo Y, Fan MY, Katon WJ. Depressive disorders during pregnancy: prevalence and risk factors in a large urban sample. Obstet Gynecol. 2010;116(5):1064–70.
Van Niel MS, Payne JL. Perinatal depression: a review. Cleve Clin J Med. 2020;87(5):273–7.
Figueiredo B, Pacheco A, Costa R. Depression during pregnancy and the postpartum period in adolescent and adult Portuguese mothers. Arch Womens Ment Health. 2007;10(3):103–9.
Viguera AC, Tondo L, Koukopoulos AE, Reginaldi D, Lepri B, Baldessarini RJ. Episodes of mood disorders in 2,252 pregnancies and postpartum periods. Am J Psychiatry. 2011;168(11):1179–85.
Stewart DE. Clinical practice. Depression during pregnancy. N Engl J Med. 2011;365(17):1605–11.
Sidebottom AC, Harrison PA, Godecker A, Kim H. Validation of the Patient Health Questionnaire (PHQ)-9 for prenatal depression screening. Arch Womens Ment Health. 2012;15(5):367–74.
Strass P, Billay E. A public health nursing initiative to promote antenatal health. Can Nurse. 2008;104(2):29–33.
Brummelte S, Galea LA. Postpartum depression: etiology, treatment and consequences for maternal care. Horm Behav. 2016;77:153–66.
Qiu C, Williams MA, Calderon-Margalit R, Cripe SM, Sorensen TK. Preeclampsia risk in relation to maternal mood and anxiety disorders diagnosed before or during early pregnancy. Am J Hypertens. 2009;22(4):397–402.
Gentile S. Untreated depression during pregnancy: short- and long-term effects in offspring. A systematic review. Neuroscience. 2017;342:154–66.
Grote NK, Bridge JA, Gavin AR, Melville JL, Iyengar S, Katon WJ. A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight, and intrauterine growth restriction. Arch Gen Psychiatry. 2010;67(10):1012–24.
Dhobale MV, Pisal HR, Mehendale SS, Joshi SR. Differential expression of human placental neurotrophic factors in preterm and term deliveries. Int J Dev Neurosci. 2013;31(8):719–23.
Gausia K, Fisher C, Ali M, Oosthuizen J. Antenatal depression and suicidal ideation among rural Bangladeshi women: a community-based study. Arch Womens Ment Health. 2009;12(5):351–8.
Andersson L, Sundström-Poromaa I, Wulff M, Aström M, Bixo M. Implications of antenatal depression and anxiety for obstetric outcome. Obstet Gynecol. 2004;104(3):467–76.
Leung BM, Kaplan BJ, Field CJ, Tough S, Eliasziw M, Gomez MF, McCargar LJ, Gagnon L. Prenatal micronutrient supplementation and postpartum depressive symptoms in a pregnancy cohort. BMC Pregnancy Childbirth. 2013;13:2.
Harvey ST, Pun PK. Analysis of positive Edinburgh depression scale referrals to a consultation liaison psychiatry service in a two-year period. Int J Ment Health Nurs. 2007;16(3):161–7.
O’Dea GA, Youssef GJ, Hagg LJ, Francis LM, Spry EA, Rossen L, Smith I, Teague SJ, Mansour K, Booth A, et al. Associations between maternal psychological distress and mother-infant bonding: a systematic review and meta-analysis. Arch Womens Ment Health. 2023;26(4):441–52.
Tichelman E, Westerneng M, Witteveen AB, van Baar AL, van der Horst HE, de Jonge A, Berger MY, Schellevis FG, Burger H, Peters LL. Correlates of prenatal and postnatal mother-to-infant bonding quality: a systematic review. PLoS ONE. 2019;14(9):e0222998.
Grigoriadis S, VonderPorten EH, Mamisashvili L, Tomlinson G, Dennis CL, Koren G, Steiner M, Mousmanis P, Cheung A, Radford K, et al. The impact of maternal depression during pregnancy on perinatal outcomes: a systematic review and meta-analysis. J Clin Psychiatry. 2013;74(4):e321–341.
Diego MA, Field T, Hernandez-Reif M, Schanberg S, Kuhn C, Gonzalez-Quintero VH. Prenatal depression restricts fetal growth. Early Hum Dev. 2009;85(1):65–70.
Gentile S, Galbally M. Prenatal exposure to antidepressant medications and neurodevelopmental outcomes: a systematic review. J Affect Disord. 2011;128(1–2):1–9.
Bjørnebekk A, Siqveland TS, Haabrekke K, Moe V, Slinning K, Fjell AM, Walhovd KB. Development of children born to mothers with mental health problems: subcortical volumes and cognitive performance at 4½ years. Eur Child Adolesc Psychiatry. 2015;24(1):115–8.
Heikkinen T, Ekblad U, Laine K. Transplacental transfer of Amitriptyline and nortriptyline in isolated perfused human placenta. Psychopharmacology. 2001;153(4):450–4.
Loebstein R, Lalkin A, Koren G. Pharmacokinetic changes during pregnancy and their clinical relevance. Clin Pharmacokinet. 1997;33(5):328–43.
Gentile S. On categorizing gestational, birth, and neonatal complications following late pregnancy exposure to antidepressants: the prenatal antidepressant exposure syndrome. CNS Spectr. 2010;15(3):167–85.
Gentile S. Prenatal antidepressant exposure and the risk of autism spectrum disorders in children. Are we looking at the fall of gods? J Affect Disord. 2015;182:132–7.
Morales DR, Slattery J, Evans S, Kurz X. Antidepressant use during pregnancy and risk of autism spectrum disorder and attention deficit hyperactivity disorder: systematic review of observational studies and methodological considerations. BMC Med. 2018;16(1):6.
Sujan AC, Öberg AS, Quinn PD, D’Onofrio BM. Annual Research Review: maternal antidepressant use during pregnancy and offspring neurodevelopmental problems - a critical review and recommendations for future research. J Child Psychol Psychiatry. 2019;60(4):356–76.
Stein A, Pearson RM, Goodman SH, Rapa E, Rahman A, McCallum M, Howard LM, Pariante CM. Effects of perinatal mental disorders on the fetus and child. Lancet. 2014;384(9956):1800–19.
Zeng Y, Cui Y, Li J. Prevalence and predictors of antenatal depressive symptoms among Chinese women in their third trimester: a cross-sectional survey. BMC Psychiatry. 2015;15:66.
Nakamura A, van der Waerden J, Melchior M, Bolze C, El-Khoury F, Pryor L. Physical activity during pregnancy and postpartum depression: systematic review and meta-analysis. J Affect Disord. 2019;246:29–41.
Dachew BA, Ayano G, Betts K, Alati R. The impact of pre-pregnancy BMI on maternal depressive and anxiety symptoms during pregnancy and the postpartum period: a systematic review and meta-analysis. J Affect Disord. 2021;281:321–30.
Sparling TM, Henschke N, Nesbitt RC, Gabrysch S. The role of diet and nutritional supplementation in perinatal depression: a systematic review. Matern Child Nutr 2017, 13(1).
Li Y, Lv MR, Wei YJ, Sun L, Zhang JX, Zhang HG, Li B. Dietary patterns and depression risk: a meta-analysis. Psychiatry Res. 2017;253:373–82.
Al-Azri M, Al-Lawati I, Al-Kamyani R, Al-Kiyumi M, Al-Rawahi A, Davidson R, Al-Maniri A. Prevalence and risk factors of Antenatal Depression among Omani women in a primary care Setting: cross-sectional study. Sultan Qaboos Univ Med J. 2016;16(1):e35–41.
Al Rawahi A, Al Kiyumi MH, Al Kimyani R, Al-Lawati I, Murthi S, Davidson R, Al Maniri A, Al Azri M. The Effect of Antepartum Depression on the outcomes of pregnancy and development of Postpartum Depression: a prospective cohort study of Omani women. Sultan Qaboos Univ Med J. 2020;20(2):e179–86.
Pampaka D, Papatheodorou SI, AlSeaidan M, Al Wotayan R, Wright RJ, Buring JE, Dockery DW, Christophi CA. Depressive symptoms and comorbid problems in pregnancy - results from a population based study. J Psychosom Res. 2018;112:53–8.
Burgut FT, Bener A, Ghuloum S, Sheikh J. A study of postpartum depression and maternal risk factors in Qatar. J Psychosom Obstet Gynaecol. 2013;34(2):90–7.
Bawahab JA, Alahmadi JR, Ibrahim AM. Prevalence and determinants of antenatal depression among women attending primary health care centers in Western Saudi Arabia. Saudi Med J. 2017;38(12):1237–42.
Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh postnatal depression scale. Br J Psychiatry. 1987;150:782–6.
Naja S, Al-Kubaisi N, Chehab M, Al-Dahshan A, Abuhashem N, Bougmiza I. Psychometric properties of the arabic version of EPDS and BDI-II as a screening tool for antenatal depression: evidence from Qatar. BMJ Open. 2019;9(9):e030365.
The WHO STEPwise. approach to noncommunicable disease risk factor surveillance (STEPS) [https://www.who.int/publications/m/item/standard-steps-instrument].
Chasan-Taber L, Schmidt MD, Roberts DE, Hosmer D, Markenson G, Freedson PS. Development and validation of a pregnancy physical activity questionnaire. Med Sci Sports Exerc. 2004;36(10):1750–60.
Papazian T, El Osta N, Hout H, Chammas DE, El Helou N, Younes H, Abi Tayeh G, Rabbaa Khabbaz L. Pregnancy physical activity questionnaire (PPAQ): translation and cross cultural adaption of an arabic version. PLoS ONE. 2020;15(3):e0230420.
Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM. Evaluation, treatment, and prevention of vitamin D deficiency: an endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–30.
Al-Hejji Z, Al-Khudhair M, Al-Musaileem M, Al-Eithan M. Prevalence and associated risk factors of antenatal depression among women attending antenatal clinics in primary health care centers in the Ministry of Health in Al-Ahsa City, Saudi Arabia. J Family Med Prim Care. 2019;8(12):3900–7.
Chaudron LH, Szilagyi PG, Tang W, Anson E, Talbot NL, Wadkins HI, Tu X, Wisner KL. Accuracy of depression screening tools for identifying postpartum depression among urban mothers. Pediatrics. 2010;125(3):e609–617.
Pearlin L. The stress process revisited. In: Aneshensel CS, Phelan JC, editors. Handbook of the sociology of mental health. Boston: Springer; 1999.
Li Y, Zeng Y, Zhu W, Cui Y, Li J. Path model of antenatal stress and depressive symptoms among Chinese primipara in late pregnancy. BMC Pregnancy Childbirth. 2016;16(1):180.
Dadi AF, Miller ER, Woodman R, Bisetegn TA, Mwanri L. Antenatal depression and its potential causal mechanisms among pregnant mothers in Gondar town: application of structural equation model. BMC Pregnancy Childbirth. 2020;20(1):168.
Liu S, Yan Y, Gao X, Xiang S, Sha T, Zeng G, He Q. Risk factors for postpartum depression among Chinese women: path model analysis. BMC Pregnancy Childbirth. 2017;17(1):133.
Abdelhai R, Mosleh H. Screening for antepartum anxiety and depression and their association with domestic violence among Egyptian pregnant women. J Egypt Public Health Assoc. 2015;90(3):101–8.
Abujilban SK, Abuidhail J, Al-Modallal H, Hamaideh S, Mosemli O. Predictors of antenatal depression among Jordanian pregnant women in their third trimester. Health Care Women Int. 2014;35(2):200–15.
Biratu A, Haile D. Prevalence of antenatal depression and associated factors among pregnant women in Addis Ababa, Ethiopia: a cross-sectional study. Reprod Health. 2015;12:99.
Rwakarema M, Premji SS, Nyanza EC, Riziki P, Palacios-Derflingher L. Antenatal depression is associated with pregnancy-related anxiety, partner relations, and wealth in women in Northern Tanzania: a cross-sectional study. BMC Womens Health. 2015;15:68.
Batalha MA, Dos Reis Costa PN, Ferreira ALL, Freitas-Costa NC, Figueiredo ACC, Shahab-Ferdows S, Hampel D, Allen LH, Pérez-Escamilla R, Kac G. Maternal Mental Health in late pregnancy and longitudinal changes in Postpartum serum vitamin B-12, Homocysteine, and milk B-12 concentration among Brazilian women. Front Nutr. 2022;9:923569.
Moshki M, Cheravi K. Relationships among depression during pregnancy, social support and health locus of control among Iranian pregnant women. Int J Soc Psychiatry. 2016;62(2):148–55.
Kendig S, Keats JP, Hoffman MC, Kay LB, Miller ES, Moore Simas TA, Frieder A, Hackley B, Indman P, Raines C, et al. Consensus Bundle on Maternal Mental Health: perinatal depression and anxiety. Obstet Gynecol. 2017;129(3):422–30.
O’Connor E, Rossom RC, Henninger M, Groom HC, Burda BU. Primary care screening for and treatment of Depression in pregnant and Postpartum women: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;315(4):388–406.
Mohammad KI, Gamble J, Creedy DK. Prevalence and factors associated with the development of antenatal and postnatal depression among Jordanian women. Midwifery. 2011;27(6):e238–245.
Lancaster CA, Gold KJ, Flynn HA, Yoo H, Marcus SM, Davis MM. Risk factors for depressive symptoms during pregnancy: a systematic review. Am J Obstet Gynecol. 2010;202(1):5–14.
Weng SC, Huang JP, Huang YL, Lee TS, Chen YH. Effects of tobacco exposure on perinatal suicidal ideation, depression, and anxiety. BMC Public Health. 2016;16:623.
Suzuki D, Wariki WMV, Suto M, Yamaji N, Takemoto Y, Rahman MM, Ota E. Association of secondhand smoke and depressive symptoms in nonsmoking pregnant women: a systematic review and meta-analysis. J Affect Disord. 2019;245:918–27.
Jarbou NS, Newell KA. Exercise and yoga during pregnancy and their impact on depression: a systematic literature review. Arch Womens Ment Health. 2022;25(3):539–59.
Molyneaux E, Poston L, Ashurst-Williams S, Howard LM. Obesity and mental disorders during pregnancy and postpartum: a systematic review and meta-analysis. Obstet Gynecol. 2014;123(4):857–67.
Loth KA, Bauer KW, Wall M, Berge J, Neumark-Sztainer D. Body satisfaction during pregnancy. Body Image. 2011;8(3):297–300.
Carter AS, Baker CW, Brownell KD. Body mass index, eating attitudes, and symptoms of depression and anxiety in pregnancy and the postpartum period. Psychosom Med. 2000;62(2):264–70.
Kaplan BJ, Crawford SG, Field CJ, Simpson JS. Vitamins, minerals, and mood. Psychol Bull. 2007;133(5):747–60.
Rechenberg K, Humphries D. Nutritional interventions in depression and perinatal depression. Yale J Biol Med. 2013;86(2):127–37.
Leung BM, Kaplan BJ. Perinatal depression: prevalence, risks, and the nutrition link–a review of the literature. J Am Diet Assoc. 2009;109(9):1566–75.
Flor-Alemany M, Migueles JH, Alemany-Arrebola I, Aparicio VA, Baena-García L. Exercise, Mediterranean Diet adherence or both during pregnancy to prevent Postpartum Depression-GESTAFIT Trial secondary analyses. Int J Environ Res Public Health 2022, 19(21).
Francis HM, Stevenson RJ, Chambers JR, Gupta D, Newey B, Lim CK. A brief diet intervention can reduce symptoms of depression in young adults - a randomised controlled trial. PLoS ONE. 2019;14(10):e0222768.
Jin X, Cheng Z, Yu X, Tao Q, Huang R, Wang S. Continuous supplementation of folic acid in pregnancy and the risk of perinatal depression-A meta-analysis. J Affect Disord. 2022;302:258–72.
Peppard L, Oh KM, Gallo S, Milligan R. Risk of depression in pregnant women with low-normal serum vitamin B12. Res Nurs Health. 2019;42(4):264–72.
Chong MF, Wong JX, Colega M, Chen LW, van Dam RM, Tan CS, Lim AL, Cai S, Broekman BF, Lee YS, et al. Relationships of maternal folate and vitamin B12 status during pregnancy with perinatal depression: the GUSTO study. J Psychiatr Res. 2014;55:110–6.
Ramadan EF, Grisdale M, Morais M. Maternal vitamin B(12) levels during pregnancy and their effects on maternal neurocognitive symptoms: a systematic review. J Obstet Gynaecol Can. 2022;44(4):390–e394393.
Markun S, Gravestock I, Jäger L, Rosemann T, Pichierri G, Burgstaller JM. Effects of vitamin B12 supplementation on cognitive function, depressive symptoms, and fatigue: a systematic review, Meta-analysis, and Meta-Regression. Nutrients 2021, 13(3).
Gould JF, Gibson RA, Green TJ, Makrides M. A systematic review of vitamin D during pregnancy and postnatally and symptoms of Depression in the Antenatal and Postpartum Period from Randomized controlled trials and observational studies. Nutrients 2022, 14(11).
Wang J, Liu N, Sun W, Chen D, Zhao J, Zhang W. Association between vitamin D deficiency and antepartum and postpartum depression: a systematic review and meta-analysis of longitudinal studies. Arch Gynecol Obstet. 2018;298(6):1045–59.
Janet T, Matias Costa V, Jaqueline L, Fernanda R, Lucilla P, Dharmintra P, Gilberto K. A systematic review of the associations between maternal nutritional biomarkers and depression and/or anxiety during pregnancy and postpartum. J Affect Disord. 2018;232:185–203.
Aghajafari F, Letourneau N, Mahinpey N, Cosic N, Giesbrecht G. Vitamin D Deficiency and Antenatal and Postpartum Depression: a systematic review. Nutrients 2018, 10(4).
Funding
This study was funded and supported by Kuwait University: Research Project No. MC01/15.
Author information
Authors and Affiliations
Contributions
RSB: contributed to the study design as well as application for fund and drafted the paper. AAT: designed the study, applied for fund, analyzed the data, and helped drafting the paper. AHZ: contributed to the data analysis and revised the manuscript. SAK: contributed to the study design and application for fund in addition to revising the manuscript. MSH: contributed to the study design, application for fund, and data collection/interpretation in addition to revising the manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
This study was conducted according to the guidelines of the Declaration of Helsinki and approved by the ethics committee at the Ministry of Health, Kuwait (Ref: project 173/2014; date: February 14th, 2017) and the Institutional Review Board at Old Dominion University Ref: 1517949). Written informed consent was taken from each study participant before recruitment.
Consent for Publication
All authors have read and approved the manuscript for publication.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Al-Sabah, R., Al-Taiar, A., Ziyab, A.H. et al. Antenatal Depression and its Associated Factors: Findings from Kuwait Birth Cohort Study. J Epidemiol Glob Health (2024). https://doi.org/10.1007/s44197-024-00223-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44197-024-00223-7