Abstract
Introduction
Multiple studies investigated the endurance and occurrence of symptoms three months after SARS-CoV-2 infection. This study examines the possible effects of COVID-19 vaccination on the persistence of post-recovery symptoms.
Patients and Methods
A cross-sectional survey was conducted in Saudi Arabia to evaluate 14 prevalent long COVID-19 symptoms among vaccinated individuals. Patients self-reported their acute COVID-19 experience, demographic information, chronic conditions, vaccine history, and persistent symptoms.
Results
Of the 484 patients, four respondents were excluded from the study as they had not received the vaccine, and 111 (23.1%) were vaccinated but did not get infected and were also excluded. The remaining 369 (76.9%) reported COVID-19 and a vaccination and thus they were included in the study. The occurrence of post-COVID-19 symptoms was reported in 59 (16.1%) for ≤ 3 months, 202 (54.8%) experienced persistent symptoms 3–6 months, and 108 (29.1%) reported symptoms lasting > 6 months. In relation to age group, persistent symptoms 3–6 months after recovery was more common in those > 50 years and symptoms lasting > 6 months were more common in 30–50 years of age (p < 0.001). Persistence of symptoms for 3-6 months was more common in those who were infected prior to vaccination compared to those who were infected after vaccination (P < 0.001). Of the included patients, 323 (87.5%) rated their health as good, 41 (11.1%) considered it fair, and 5 (1.4%) described their well-being as poor or terrible.
Conclusion
The study provides information of persistent symptoms in vaccinated individuals who had recovered from COVID-19 and highlights the need for targeted interventions to alleviate post-COVID-19 symptoms. The study is limited by its reliance on self-reported data and potential selection bias. Future research is needed to understand the mechanisms underlying persistent symptoms in vaccinated individuals and to identify effective interventions for long COVID.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is responsible for the global pandemic of Coronavirus Disease-19 (COVID-19), declared by the World Health Organization on March 11, 2020 [1]. As of April 2023, approximately 68.4% of the global population has received at least one dose of a COVID-19 vaccine [2]. While the daily vaccination rate worldwide is around 4.25 million, only 24% of individuals in low-income countries have received a minimum of one dose [3].
COVID-19 vaccines have demonstrated effectiveness in preventing severe illness, hospitalization, and death [4]. Vaccines also significantly reduce the likelihood of hospitalization, and vaccinated individuals who do contract SARS-CoV-2 tend to experience milder symptoms compared to the unvaccinated [5]. In one study, vaccination has been shown to decrease the severity and long-term impact of persistent symptoms, commonly known as long COVID, at 120 days [6]. Long COVID, or the persistence of symptoms following acute SARS-CoV-2 infection, is increasingly recognized [7]. Common symptoms include fatigue, breathlessness, myalgia, and insomnia [8]. Studies conducted in the UK on participants aged 18 to 69 years showed that the first COVID-19 vaccination reduced the likelihood of self-reported long COVID by 13%, and the second dose further decreased the likelihood by 9% [9].
The prevalence of long COVID after SARS-CoV-2 infection has been reported to be six times higher than post-other viral diseases, ranging from 9 to 63% [10]. Another study showed that the prevalence of symptoms varies from 35% to 90.5% [11]. A previous study conducted in Saudi Arabia between May and June 2021 reported that approximately half of the surveyed COVID-19 patients experienced post-COVID symptoms. However, the study did not consider the impact of COVID-19 vaccination on reducing long COVID symptoms [12]. Another study demonstrated that the rate of post-COVID-19 symptoms decreased as the number of vaccine doses increased. The rates of the condition were 41.8% (95% CI, 37.0%-46.7%), 30.0% (95% CI, 6.7%-65.2%), 17.4% (95% CI, 7.8%-31.4%), and 16.0% (95% CI, 11.8%-21.0%) for individuals who received 0, 1, 2, and 3 doses of the vaccine, respectively [13]. In this study, we aim to evaluate the occurrence and types of long COVID-19 among vaccinated individuals who had infection.
2 Patients and Methods
2.1 Study Design
In this study, we examined the effects of various vaccines on the occurence of 14 post-COVID-19 symptoms to identify potential patterns. The questionnaire was circulated through WhatsApp groups, including those for healthcare professionals and the general public, between August and October 2022. Post-COVID-19 symptoms refer to a range of symptoms persisting or developing three months following infection and lasting at least two months with no other explanation [14]. Specifically, symptoms may begin as early as one month following infection and can last for an extended period, with some patients experiencing symptoms beyond six months. We distinguished between different stages of persistence: short-term (< 3 month), medium-term (3–6 months), and long-term (lasting more than six months). We limited the survey to patients who tested positive via PCR or antigen tests, and thus confirming positive cases. The collected data included the type and timing of the vaccine received, the date and severity of the initial illness, pre-existing chronic conditions, and demographic information. Before participating, respondents were asked to consent to their involvement in the study. The study protocol was approved by the Ethics Review Board for Human Studies at the Faculty of Medicine, Umm Al-Qura University (Approval no. HAPO-02-K-012–2022-09–1180), in compliance with the guidelines set forth by the Saudi National Committee for Bioethics (HABO-02-K-012).
The survey collected essential data, such as age, gender, nationality (Saudi and non-Saudi citizens), area of residence, chronic disease status, hospitalization history (severity of illness, length of hospital stays, need for respiratory support, and requirement for intensive care unit [ICU]), duration since symptom onset, and persistence of symptoms. Patients self-reported information regarding their acute COVID-19 experience. The severity of the disease can be mild to moderate, severe, or critical. Patient symptoms were grouped by system: general (fatigue, myalgia), respiratory (chest pain, cough, wheezing), cardiovascular (palpitations), neuropsychiatric (headache, hypersomnia, depression, anxiety), dermatological (hair loss), and gastrointestinal (diarrhea, constipation). The severity of symptoms was determined based on the patients’ reported outcomes and graded from none to severe. The study considered various COVID-19 vaccines, including Pfizer/BioNTech (BNT162b2), Oxford-AstraZeneca (ChAdOx1 nCoV-19), Moderna (mRNA-1273), and Janssen (Johnson & Johnson; JNJ-78436735 or Ad26.COV2.S). Vaccination status and vaccine doses either before or after SARS-CoV-2 infection were also obtained.
2.2 Statistical Analysis
All analyses were conducted using the SPSS software (version 21). Socio-demographic characteristics of the study sample were expressed as total percentages, means, and standard deviations. Descriptive statistics were reported as mean ± standard deviation (SD) or medians along with quartiles (25th–75th percentile) for continuous variables that lacked a normal distribution, and as frequency and percentages for categorical variables. A t-test was utilized to assess continuous variables with normally distributed data. A p-value below 0.05 was considered statistically significant.
3 Results
3.1 Patients Demographic Characteristics
A total of 484 individuals participated in the study through an online questionnaire circulated among various WhatsApp groups. All patients had recovered from COVID-19 and tested positive via PCR or antigent test. Four respondents were excluded from the study as they had not received the vaccine, and 111 (23.1%) were vaccinated but did not get infected and were also excluded. The remaining 369 (76.9%) reported COVID-19 and a vaccination and were included in the final analysis. Among the 369, 192 (52%) were female and 177 (48%) were male; and there were 206 (55.8%) Saudis and 163 (44.2%) non-Saudis (Table 1). There were 232 (63%) healthcare workers, 130 (35.4%) were employed in other professions, and 7 (1.6%) were not working. Of the included patients, 288 (76%) reported no comorbidities, and 81 (24%) had at least one comorbidity such as hypertension (10.5%), diabetes mellitus (6.7%), and heart diseases (1%) (Fig. 1).
Of the included patients, 70 (19%) were 18–29 years old, 233 (63%) were 30–50 years old, and 66 (18%) were ≥ 50 years old. In a multivariate analysis, co-morbidity status showed a significant difference across the age groups (p < 0.001) (Table 2). Most of patients without co-morbidities were in the 30–50 age group (70.5%), while most (53.1%) patients with one or more co-morbidities were over 50 years of age. Vaccination type and dosage revealed significant differences across age groups (p < 0.05). For Pfizer (BNT162b2) and Oxford vaccines (ChAdOx1 nCoV-19), the largest percentage of patients receiving first, second, and booster doses were in the 30–50 age group. In relation to age group, persistent symptoms 3–6 months after recovery was more common in those > 50 years and symptoms lasting > 6 months were more common in 30–50 years of age (p < 0.001) (Table 2).
3.2 Description of COVID-19 Vaccination Among Study Individuals
Of the total of 369 patients, the first dose was administered as early as December 2020 and 327 (88.8%) had a booster dose more than three months after the initial two doses and 41 (11.2%) had two does but not a booster dose. In terms of vaccine type distribution, 239 (64.7%), 278 (75.3%), and 274 (74.3%) patients received the Pfizer vaccine (BNT162b2) for the first, second, and booster doses, respectively. In addition, (43.1%), 90 (24.4%), and 4 (3.4%) had the Oxford-Astrazeneca vaccine (ChAdOx1 nCoV-19) for the first, second and third doses, respectively. A small portion, 16 (4.5%), received the Moderna vaccine (mRNA-1273) as the booster (third) dose. However, 66 (18.1%) of the respondents were uncertain about the type of booster dose they had received (Fig. 2). The time between the first COVID-19 vaccine and subsequent infection for the vaccinated group was at least three months.
3.3 Characteristics of Post-COVID-19 Short- and Long-Term Symptoms
Post-COVID-19 symptoms occurred in 59 (16.1%) for ≤ 3 months, and 202 (54.8%) experienced persistent symptoms 3–6 months, and 108 (29.1%) reported symptoms lasting > 6 months. Of the included individuals, 237 (64.3%) had COVID-19 after receiving three doses of the vaccine, among them 234 (98.7%) were not hospitalized and were isolated at home and did not require oxygen supplement. However, only 3 (1.3%) were hospitalized for less than seven days and none required ICU admission. On the other hand, 102 (27.6%) were infected before vaccination and of them 8 7.8%) were hospitalized (P = 0.007); the remaining 30 (8.1%) of the total cases were unsure whether their infection occurred before or after vaccination.
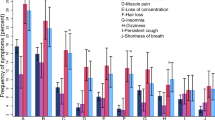
Post-COVID-19 symptoms were weight loss (34.7%), abdominal pain (32.5%), palpitations (32.2%), loss of smell and taste (31.7%), and headaches (29.2%). Persistent symptoms lasting more than 14 days encompassed cough (5.7%), fatigue and myalgia (5.1%), and headaches and anxiety (3.2% each). In general, respiratory symptoms, such as cough, wheezing, and chest pain, persisted for one month in 20.3% of patients, alongside weight loss in 7.5% (Fig. 3).
Of the included patients, 108 (29.1%) reported symptoms lasting > 6 months and these symptoms included neuropsychiatric symptoms like concentration or memory deficits (7.8%) and headaches (2.1%). Anxiety and depression were reported by 5.9% of patients. Additional reported symptoms were dizziness (6.1%), itchiness (1.6%), fever (1.8%), and hair loss (1%).
In addition to post-COVID-19 symptoms, the survey assessed patients' perceptions of their general well-being after recovery. A majority (323; 87.5%) of the patients rated their health as good, 41 (11.1%) considered it fair, and 5 (1.4%) described their well-being as poor or terrible.
3.4 Correlation of Post-COVID-19 Symptoms and the Relationship Between Vaccination and the Occurrence of Infection
Table 3 shows a multivariate analysis of the relation between vaccination and infection status and the development of post-COVID-19 symptoms. In terms of co-morbidities, the majority (82.3%) of those infected after full vaccination had no comorbidities, with a similar observation of 77.5% in those infected pre-vaccination. However, in the uncertain group, 53.3% had at least one co-morbidity and (46.7%) had no co-morbidities (p < 0.001).
Of the 237 patients who were infected after receiving three doses of the vaccine, 234 (98.7%) described their illness as mild to moderate (were isolated at home without the need to have oxygen supplementation); only 3 (1.3%) required admission for less than seven days; and none required ICU admission. On the other hand, of the 102 individuals infected before vaccination, 94 (92.2%) did not require hospitalization and 8 (7.8%) required hospitalization. These rates were higher among those infected prior to vaccination compared to those infected after receiving three doses of the vaccination (p-value < 0.00001) (Table 3).
Persistence of symptoms for > 3–6 months was more common in those who were infected prior to vaccination compared to those who were infected after vaccination (P < 0.001) (Table 3).
Table 4 presents the results of a regression analysis examining predictors of the development of post-COVID-19 symptoms. The analysis showed significant relationships between post-COVID-19 symptoms 3–6 months and the following predictors: gender, presence of co-morbidities, vaccination status, and severity of symptoms. Gender demonstrated a statistically significant association (95% CI = 0.12–0.94, P < 0.001) with females being less at risk compared to males. The presence of co-morbidities also significantly was associated with development of symptoms. Specifically, each additional co-morbidity was linked to a 0.23 increase in the odds of less recovery (95% CI = 0.15–0.31, P < 0.001). The vaccination status also significantly influenced recovery (95% CI = 0.33–0.73, P < 0.05). Lastly, severity of symptoms significantly affected recovery, there was an associated 0.33 decrease in the odds of recovery 3–6 months post-COVID-19 (95% CI = 0.05–0.65, P < 0.001) with each unit increase in symptom severity.
4 Discussion
This study explores variations in the probability of experiencing persistent symptoms post-COVID-19, and the study was conducted at the time when there were 826,009 confirmed cases of COVID-19 including 9,477 deaths in Saudi Arabia, from 3 January 2020 to 9 December 2022. We evaluated the occurrence and features of short and long term post-COVID-19 symptoms among 369 patients in Saudi Arabia.
The majority 237 (64.3%) had COVID-19 after receiving three doses of the vaccine, among them only 3 (1.3%) were hospitalized. These findings align with previous research suggesting that the majority of COVID-19 cases are mild to moderate in severity among vaccinated individuals and in the latter waves of the pandemic [15, 16]. The high number of hospitalizations (7.8%) among the individuals infected before vaccination contrasts sharply with the low hospitalization rate among those infected after receiving three doses of the vaccine (1.3%). The difference in hospitalization rates indicates the protective effect of vaccination against severe illness requiring hospital care, as was shown in a previous study from Saudi Arabia [17] and other studies.
The vaccination impact on individuals with existing long-COVID-19 symptoms is emerging and data showed changes in symptoms severity while others show no correlation between symptoms and vaccination status [18]. As known, low level of evidence (grade III, case-controls, cohort studies) indicates that vaccination before COVID-19 infection may reduce the risk of post-COVID-19 symptoms [18,19,20]. A previous study showed that vaccination before SARS-CoV-2 acute infection reduced long COVID-19 symptoms [18]. The vaccination impact on the development of long-COVID symptoms was variable in a scoping review of 16 studies [21]. An additional study showed that COVID-19 vaccination reduced long-COVID-19 symptoms at 6 weeks after illness onset from 79.1% among unvaccinated to 60.6% among vaccinated patients [22]. In a study of 1832 adults, the risk of symptoms > 28 days after SARS-CoV-2 symptoms was higher in unvaccinated at the time of infection [23] while Al-Aly et al. showed that two doses of COVID-19 vaccines would be more effective in reducing post-COVID-19 symptoms with certain vaccines better than others [24]. The current study showed that the rate of post-OCVID-19 was lower at 3–6 months among vaccinated individuals but was higher at > 6 months. This finding is intriguing and could be explained by recollection of symptoms, variability in severity of disease, or the occurrence of subsequent infections that had not been diagnosed. In one study, the occurrence of post-COVID-19 symptoms was 40.2% at 6 months after infection [25] and another study showed 29.6% had at least one symptom 6–12 months after testing compared to 13% of all test-negative patients [26]. However, these studies did not address the impact of vaccination on post-COVID-19 and further studies are waranted to explore this finding further.
Persistent symptoms included weight loss, abdominal pain, palpitations, loss of smell and taste, and headaches, while long-term symptoms encompassed cough, fatigue, myalgia, and neuropsychiatric symptoms. These findings are consistent with prior research reporting a wide range of persistent symptoms following COVID-19 infection [27,28,29,30,31]. The findings of this study showed that the majority of patients (61%) who reported symptoms after recovering from COVID-19 belonged to the 30–50 age group. This difference in symptom prevalence among different age groups was statistically significant (p < 0.001), in alignment with a previous study [32]. Additionally, other studies have reported the persistence of symptoms in vaccinated individuals as well [27] and age-related aspects of the findings echo similar results from other investigations. A recent meta-analysis emphasizes that middle-aged adults are more prone to post-COVID-19 symptoms [33]. This age group's vulnerability might be linked to a combination of immune response, pre-existing health conditions, and other socio-economic factors, though definitive reasons remain elusive [34, 35].
Interestingly, gender difference in post-COVID-19 observed in our study had also been reported, although we observed less long-COVID-19 among females. Previous studies reported gender differences in the occurrence of long COVID-19 as well as differences in the COVID-19 impact [24, 25]. However, the current study showed less impact on females and this finding might be related to the sample size or the recall of occurrence of symptoms. Females were more likely to report long COVID-19, a pattern that has been found in various cohorts and geographical regions [36]. However, the underlying biological or sociological reasons for this gender disparity need further exploration [37, 38]. The exact reasons why some individuals experience ongoing symptoms while others do not remain unclear, but it is believed to be associated with factors such as initial infection's severity, age, and presence of underlying health conditions [39]. In addition, this is a significant area for future research, as gendered insights into long COVID -19 could tailor public health measures and individual care.
The fact that 29% of participants in our study experienced persistent symptoms for more than six months highlights the potential long-term health implications of COVID-19. Other studies also reported the presence of neuropsychiatric symptoms, such as difficulties with concentration or memory, headaches, anxiety, and depression [23, 40]. These symptoms can have a significant impact on patients' quality of life and deserve further investigation [41,42,43]. Interestingly, despite the high prevalence of persistent symptoms, the majority of recovered patients (87.5%) rated their overall health as good. This suggests that, for many individuals, recovery from COVID-19 is associated with a generally positive perception of their health [44]. These findings are consistent with a study by Garrigues et al., which observed that most patients who had recovered from COVID-19 reported an overall improvement in their health status, with a significant decrease in symptom severity over time [45]. Additionally, another study found that more than half of the patients who had recovered from COVID-19 reported an enhancement in their quality of life, despite the presence of some persistent symptoms [46, 47]. These findings support the idea that many individuals maintain a positive outlook on their health following recovery from COVID-19, even in the presence of persistent symptoms. This aligns with the observation that the majority of recovered patients rated their health as good, indicating a generally positive perception of health post-recovery [48].
The lower hospitalization rates and the lack of ICU admissions in our study population, particularly among vaccinated individuals, align with global data reflecting the successful reduction in severe disease following vaccination [49]. Nevertheless, the persistence of symptoms in vaccinated individuals emphasizes the importance of a nuanced understanding of immunity, viral dynamics, and host factors. The precise mechanisms remain to be fully explored, and comprehensive studies are essential to inform clinical practice [50, 51]. Furthermore, the broad spectrum of persistent symptoms, including neuropsychiatric manifestations, aligns with the growing body of evidence that COVID-19 has multifaceted impacts on various systems [52, 53]. This suggests that a multidisciplinary approach to patient care is warranted, involving specialists in neurology, psychiatry, internal medicine, and other fields [54].
Our study contributes to the growing understanding of long COVID-19, offering valuable insights into the persistence of symptoms in relation to gender, age, vaccination status, and infection status. These findings should encourage a multidimensional and patient-centered approach to managing long COVID-19, integrating clinical care, research, and public health strategies. Continued collaboration between healthcare providers, researchers, and policymakers is essential to mitigate the ongoing and potentially long-lasting effects of this global health challenge.
It is important to acknowledge the limitations of our study. Firstly, the study sample was obtained through an online questionnaire distributed among various WhatsApp groups, which may introduce selection bias and limit the generalizability of our findings to the broader population. Secondly, our study design is cross-sectional, which makes it challenging to establish causal relationships between variables. Thirdly, the reliance on self-reported data in our study introduces the possibility of recall bias or the potential for participants to over or underreport their symptoms. Fourthly, the absence of a control group consisting of non-COVID-19-infected individuals makes it difficult to differentiate between post-recovery symptoms and symptoms related to comorbidities or other illnesses. Lastly, our study did not investigate the impact of vaccination on the severity of COVID-19 infection or hospitalization rates, which could have provided valuable insights into the effectiveness of the vaccine.
Despite these limitations, our study offers important insights into the persistence of symptoms in vaccinated individuals who have recovered from COVID-19. This information can contribute to the development of targeted interventions aimed at alleviating post-COVID-19 symptoms. It is crucial for healthcare providers to be aware of the potential for post-recovery symptoms in vaccinated individuals and to monitor their patients accordingly. Further research is needed to gain a better understanding of the underlying mechanisms behind persistent symptoms in vaccinated individuals and to identify effective interventions for long COVID.
Availability of Data and Materials
Data are available upon a reasonable request.
Abbreviations
- COVID-19:
-
Coronavirus diseases 2019
- 95% CI:
-
95% Confidence interval
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
- PASC:
-
Post-acute sequelae of SARS-CoV-2 infection
References
Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–33. https://doi.org/10.1056/nejmoa2001017.
Office for National Statistics. People, population and community n.d. https://www.ons.gov.uk/peoplepopulationandcommunity (Accessed June 2, 2023).
Oxford Martin School. Coronavirus (COVID-19) Vaccinations - Our World in Data n.d. https://ourworldindata.org/covid-vaccinations?country=OWID_WRL (Accessed January 13, 2023).
Centers for Disease Control and Prevention (CDC). Long COVID or Post-COVID Conditions n.d. https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.html (Accessed June 2, 2023).
Tran V-T, Perrodeau E, Saldanha J, Pane I, Ravaud P. Efficacy of first dose of covid-19 vaccine versus no vaccination on symptoms of patients with long covid: target trial emulation based on ComPaRe e-cohort. BMJ Med. 2023;2:e000229. https://doi.org/10.1136/BMJMED-2022-000229.
Tran VT, Riveros C, Clepier BCDS, Desvarieux M, Collet C, Yordanov Y, et al. Development and validation of the long coronavirus disease (COVID) symptom and impact tools: a set of patient-reported instruments constructed from patients’ lived experience. Clin Infect Dis. 2022;74:278–87. https://doi.org/10.1093/CID/CIAB352.
Glynne P, Tahmasebi N, Gant V, Gupta R. Long COVID following mild SARS-CoV-2 infection: characteristic T cell alterations and response to antihistamines. J Investig Med. 2022;70:61–7. https://doi.org/10.1136/jim-2021-002051.
Sudre CH, Murray B, Varsavsky T, Graham MS, Penfold RS, Bowyer RC, et al. Attributes and predictors of long COVID. Nat Med. 2021;27:626–31. https://doi.org/10.1038/S41591-021-01292-Y.
Antonelli M, Penfold RS, Merino J, Sudre CH, Molteni E, Berry S, et al. Risk factors and disease profile of post-vaccination SARS-CoV-2 infection in UK users of the COVID symptom study app: a prospective, community-based, nested, case-control study. Lancet Infect Dis. 2022;22:43–55. https://doi.org/10.1016/S1473-3099(21)00460-6.
Lippi G, Sanchis-Gomar F, Henry BM. COVID-19 and its long-term sequelae: what do we know in 2023? Polish Arch Intern Med. 2023. https://doi.org/10.20452/pamw.16402.
Abdel-Gawad M, Zaghloul MS, Abd-elsalam S, Hashem M, Lashen SA, Mahros AM, et al. Post-COVID-19 syndrome clinical manifestations: a systematic review. Antiinflamm Antiallergy Agents Med Chem. 2022;21:115–20. https://doi.org/10.2174/1871523021666220328115818.
Mahmoud MH, Alghamdi FA, Alghamdi GA, Alkhotani LA, Alrehaili MA, El-Deeb DK. Study of Post-COVID-19 syndrome in Saudi Arabia. Cureus. 2021. https://doi.org/10.7759/CUREUS.17787.
Azzolini E, Levi R, Sarti R, Pozzi C, Mollura M, Mantovani A, et al. Association between BNT162b2 vaccination and long COVID after infections not requiring hospitalization in health care workers. JAMA. 2022;328:676–8. https://doi.org/10.1001/jama.2022.11691.
World Health Organization. Post COVID-19 condition (Long COVID) 2022. https://www.who.int/europe/news-room/fact-sheets/item/post-covid-19-condition (Accessed June 2, 2023).
Kobayashi M, Miyamoto A, Watanabe T, Sawa K, Sato K, Yamada K, et al. COVID-19 vaccination benefits in preventing severe disease in mild-to-moderate cases: an analysis in the first specialized hospital for COVID-19 in Japan. Respir Investig. 2023;61:230–9. https://doi.org/10.1016/J.RESINV.2022.12.011.
Ferdinands JM, Rao S, Dixon BE, Mitchell PK, Desilva MB, Irving SA, et al. Waning of vaccine effectiveness against moderate and severe covid-19 among adults in the US from the VISION network: test negative, case-control study. BMJ. 2022. https://doi.org/10.1136/BMJ-2022-072141.
AlBahrani S, AlBarrak A, Al-Musawi T, AlGubaisi NA, Almalki M, Hakami FH, et al. COVID-19 vaccine had a significant positive impact on patients with SARS-COV-2 during the third (Omicron) wave in Saudi Arabia. J Infect Public Health. 2022;15:1169–74. https://doi.org/10.1016/j.jiph.2022.09.005.
Byambasuren O, Stehlik P, Clark J, Alcorn K, Glasziou P. Effect of covid-19 vaccination on long covid: systematic review. BMJ Med. 2023;2:e000385. https://doi.org/10.1136/BMJMED-2022-000385.
Notarte KI, Catahay JA, Velasco JV, Pastrana A, Ver AT, Pangilinan FC, et al. Impact of COVID-19 vaccination on the risk of developing long-COVID and on existing long-COVID symptoms: a systematic review. EClinicalMedicine. 2022. https://doi.org/10.1016/j.eclinm.2022.101624.
AlBahrani S, AlBarrak A, AlGubaisi N, Alkurdi H, Alburaiki D, AlGhamdi A, et al. Self-reported long COVID-19 symptoms are rare among vaccinated healthcare workers. J Infect Public Health. 2023;16:1276–80. https://doi.org/10.1016/j.jiph.2023.05.037.
Mumtaz A, Sheikh AAE, Khan AM, Khalid SN, Khan J, Nasrullah A, et al. COVID-19 vaccine and long COVID: a scoping review. Life. 2022. https://doi.org/10.3390/life12071066.
Mohr NM, Plumb ID, Harland KK, Pilishvili T, Fleming-Dutra KE, Krishnadasan A, et al. Presence of symptoms 6 weeks after COVID-19 among vaccinated and unvaccinated US healthcare personnel: a prospective cohort study. BMJ Open. 2023. https://doi.org/10.1136/bmjopen-2022-063141.
Richard SA, Pollett SD, Fries AC, Berjohn CM, Maves RC, Lalani T, et al. Persistent COVID-19 symptoms at 6 months after onset and the role of vaccination before or after SARS-CoV-2 infection. JAMA Netw Open. 2023;6:e2251360. https://doi.org/10.1001/jamanetworkopen.2022.51360.
Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594:259–64. https://doi.org/10.1038/S41586-021-03553-9.
Peghin M, Palese A, Venturini M, De Martino M, Gerussi V, Graziano E, et al. Post-COVID-19 symptoms 6 months after acute infection among hospitalized and non-hospitalized patients. Clin Microbiol Infect. 2021;27:1507–13. https://doi.org/10.1016/j.cmi.2021.05.033.
Sørensen AIV, Spiliopoulos L, Bager P, Nielsen NM, Hansen JV, Koch A, et al. Post-acute symptoms, new onset diagnoses and health problems 6 to 12 months after SARS-CoV-2 infection: a nationwide questionnaire study in the adult Danish population. MedRxiv. 2022. https://doi.org/10.1101/2022.02.27.22271328.
Carfì A, Bernabei R, Landi F. Persistent symptoms in patients after acute COVID-19. JAMA - J Am Med Assoc. 2020;324:603–5. https://doi.org/10.1001/jama.2020.12603.
Behnood SA, Shafran R, Bennett SD, Zhang AXD, O’Mahoney LL, Stephenson TJ, et al. Persistent symptoms following SARS-CoV-2 infection amongst children and young people: a meta-analysis of controlled and uncontrolled studies. J Infect. 2022;84:158–70. https://doi.org/10.1016/J.JINF.2021.11.011.
Stavem K, Ghanima W, Olsen MK, Gilboe HM, Einvik G. Persistent symptoms 1.5–6 months after COVID-19 in non-hospitalised subjects: a population-based cohort study. Thorax. 2021;76:405–7. https://doi.org/10.1136/thoraxjnl-2020-216377.
Goërtz YMJ, Van Herck M, Delbressine JM, Vaes AW, Meys R, Machado FVC, et al. Persistent symptoms 3 months after a SARS-CoV-2 infection: the post-COVID-19 syndrome? ERJ Open Res. 2020;6:1–10. https://doi.org/10.1183/23120541.00542-2020.
Whitaker M, Elliott J, Chadeau-Hyam M, Riley S, Darzi A, Cooke G, et al. Persistent COVID-19 symptoms in a community study of 606,434 people in England. Nat Commun. 2022. https://doi.org/10.1038/S41467-022-29521-Z.
Tenforde MW, Kim SS, Lindsell CJ, Billig Rose E, Shapiro NI, Files DC, et al. Symptom Duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a multistate health care systems network—United States, March–June 2020. MMWR Morb Mortal Wkly Rep. 2020;69:993–8. https://doi.org/10.15585/mmwr.mm6930e1.
O’Mahoney LL, Routen A, Gillies C, Ekezie W, Welford A, Zhang A, et al. The prevalence and long-term health effects of long covid among hospitalised and non-hospitalised populations: a systematic review and meta-analysis. EClinicalMedicine. 2022. https://doi.org/10.1016/J.ECLINM.2022.101762.
Monod M, Blenkinsop A, Xi X, Hebert D, Bershan S, Tietze S, et al. Age groups that sustain resurging COVID-19 epidemics in the United States. Science. 2021;371:6536. https://doi.org/10.1126/SCIENCE.ABE8372.
Tang IW, Vieira VM, Shearer E. Effect of socioeconomic factors during the early COVID-19 pandemic: a spatial analysis. BMC Public Health. 2022. https://doi.org/10.1186/s12889-022-13618-7.
Davis HE, McCorkell L, Vogel JM, Topol EJ. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. 2023;21:133–46. https://doi.org/10.1038/s41579-022-00846-2.
Fisher AN, Ryan MK. Gender inequalities during COVID-19. Gr Process Intergr Relations. 2021;24:237–45. https://doi.org/10.1177/1368430220984248.
Doerre A, Doblhammer G. The influence of gender on COVID-19 infections and mortality in Germany: Insights from age- and gender-specific modeling of contact rates, infections, and deaths in the early phase of the pandemic. PLoS One. 2022;17:e0268119. https://doi.org/10.1371/journal.pone.0268119.
Huang L, Yao Q, Gu X, Wang Q, Ren L, Wang Y, et al. 1-year outcomes in hospital survivors with COVID-19: a longitudinal cohort study. Lancet. 2021;398:747–58. https://doi.org/10.1016/S0140-6736(21)01755-4.
Ghosn J, Piroth L, Epaulard O, Le Turnier P, Mentré F, Bachelet D, et al. Persistent COVID-19 symptoms are highly prevalent 6 months after hospitalization: results from a large prospective cohort. Clin Microbiol Infect. 2021;27:1041.e1-1041.e4. https://doi.org/10.1016/j.cmi.2021.03.012.
Khodeir MM, Shabana HA, Rasheed Z, Alkhamiss AS, Khodeir M, Alkhowailed MS, et al. COVID-19: post-recovery long-term symptoms among patients in Saudi Arabia. PLoS One. 2021;16:e0260259. https://doi.org/10.1371/JOURNAL.PONE.0260259.
Qamar MA, Martins RS, Dhillon RA, Tharwani A, Irfan O, Suriya QF, et al. Residual symptoms and the quality of life in individuals recovered from COVID-19 infection: a survey from Pakistan. Ann Med Surg. 2022. https://doi.org/10.1016/J.AMSU.2022.103361.
Jabali MA, Alsabban AS, Bahakeem LM, Zwawy MA, Bagasi AT, Bagasi HT, et al. Persistent symptoms post-COVID-19: an observational study at King Abdulaziz Medical City, Jeddah. Saudi Arabia Cureus. 2022. https://doi.org/10.7759/cureus.24343.
Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27:601–15. https://doi.org/10.1038/s41591-021-01283-z.
Garrigues E, Janvier P, Kherabi Y, Le Bot A, Hamon A, Gouze H, et al. Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J Infect. 2020;81:e4-6. https://doi.org/10.1016/J.JINF.2020.08.029.
Garout MA, Saleh SAK, Adly HM, Abdulkhaliq AA, Khafagy AA, Abdeltawab MR, et al. Post-COVID-19 syndrome: assessment of short- and long-term post-recovery symptoms in recovered cases in Saudi Arabia. Infection. 2022;50:1431–9. https://doi.org/10.1007/s15010-022-01788-w.
Leth S, Gunst JD, Mathiasen V, Hansen K, Søgaard O, Østergaard L, et al. Persistent symptoms in patients recovering from COVID-19 in Denmark. Open Forum Infect Dis. 2021. https://doi.org/10.1093/ofid/ofab042.
Regunath H, Goldstein NM, Guntur VP. Long COVID: where are we in 2023? Mo Med. 2023;120:102–5.
Rahman MS, Harun MGD, Sumon SA, Mohona TM, Abdullah SAHM, Khan MNH, et al. Hospitalization and mortality by vaccination status among COVID-19 patients aged ≥ 25 years in Bangladesh: results from a multicenter cross-sectional study. Vaccines. 2022. https://doi.org/10.3390/vaccines10121987.
Pang NYL, Pang ASR, Chow VT, Wang DY. Understanding neutralising antibodies against SARS-CoV-2 and their implications in clinical practice. Mil Med Res. 2021. https://doi.org/10.1186/s40779-021-00342-3.
Akbar SMF, Al Mahtab M, Khan S. Cellular and molecular mechanisms of pathogenic and protective immune responses to SARS-CoV-2 and implications of COVID-19 vaccines. Vaccines. 2023. https://doi.org/10.3390/vaccines11030615.
Mohandas S, Jagannathan P, Henrich TJ, Sherif ZA, Bime C, Quinlan E, et al. Immune mechanisms underlying COVID-19 pathology and post-acute sequelae of SARS-CoV-2 infection (PASC). Elife. 2023. https://doi.org/10.7554/eLife.86014.
Sherif ZA, Gomez CR, Connors TJ, Henrich TJ, Reeves WB. Pathogenic mechanisms of post-acute sequelae of SARS-CoV-2 infection (PASC). Elife. 2023. https://doi.org/10.7554/elife.86002.
Bailey J, Lavelle B, Miller J, Jimenez M, Lim PH, Orban ZS, et al. Multidisciplinary center care for long covid syndrome—a retrospective cohort study. Am J Med. 2023. https://doi.org/10.1016/j.amjmed.2023.05.002.
Funding
The authors: Dr. Heba M. Adly and Dr. Saleh A.K. Saleh extend their appreciation to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia for funding their work through the project number: IFP22UQU4240001DSR010.
Author information
Authors and Affiliations
Contributions
All authors similarly contributed to the study conceptualization and design, data analysis and interpretation; initial article drafting; and final article review and approval.
Corresponding author
Ethics declarations
Conflict of Interest
None of the authors has any conflict of interest to declare.
Ethical Approval
The study protocol was approved by the Ethics Review Board for Human Studies at the Faculty of Medicine, Umm Al-Qura University. Approval no. HAPO-02-K-012-2022-09-1180 in accordance with Saudi National Committee for Bioethics HABO-02-K-012.
Consent to Participate
Patients indicated acceptance to answer questionnaires at the beginning of the survey.
Consent for Publication
Not applicable.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Adly, H.M., Saleh, S.A.K., Garout, M.A. et al. Post COVID-19 Symptoms Among Infected Vaccinated Individuals: A Cross-Sectional Study in Saudi Arabia. J Epidemiol Glob Health 13, 740–750 (2023). https://doi.org/10.1007/s44197-023-00146-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s44197-023-00146-9