Abstract
Hazelnut oil (HO) is important in terms of fatty acid composition and bioactive substances. Although there are a few studies on hazelnut oil encapsulation, there is limited research on the investigation of different wall material combinations for hazelnut oil microencapsulation and oxidative stability of the microcapsules. This study aimed to evaluate the effects of different wall material combinations (gum Arabic, sodium caseinate, whey protein, gelatine, modified starch “Em-Cap” and pea protein with maltodextrin at a ratio of 1:9,w/w, respectively) on the oxidation degree of hazelnut oil microcapsules produced by spray drying. The feed emulsions used for microcapsule production were analyzed for emulsion stability (ES) and particle droplet size. The encapsulation efficiency (EE), moisture content, bulk density, particle size and total yield of hazelnut oil microcapsules were analyzed. Peroxide and p-Anisidine values were investigated during 15 days of storage at 50 °C. While the highest encapsulation efficiency, emulsion and oxidative stability were obtained for modified starch/maltodextrin (Em-Cap/MD), the lowest yeild was obtained for gelatine/maltodextrin (GE/MD) with the highest oxidation rate. Among the six wall material combinations evaluated, the modified starch (Em-Cap) performed best, with the highest encapsulation efficiency and lowest lipid oxidation rate. The results showed that the oxidative stability of hazelnut oil microcapsules was enhanced by combining different wall materials to increase the shelf life, which is reflected at the level of the food industry.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Turkey is by far the largest producer of hazelnuts, accounting for nearly 70% of the total world production of hazelnuts. Hazelnut contain 41.96–63.73% oil (dry basis), and the oil is consisting of large amounts of unsaturated fatty acids such as oleic (65.5–82.5%) and linoleic (14.0–23.3%) acids, and low amounts of palmitic (4.5–5.9%), stearic (0.5–2.8%), and linolenic (0.1–0.2%) acids [1]. In the study conducted with 20 different varieties, the fatty acid content of hazelnut oil was found to be: palmitic acid 4.39–8.85%; stearic acid 1.67–3.18%; oleic acid 73.48–81.57%; linoleic acid 10.46–14.95%; linolenic acid 0.02–0.34% and myristic acid 0.01–0.16% [2].
In addition, bioactive substances such as tocopherols and phytosterols of hazelnut oil can positively influence human health or prevent diseases such as cancer and cardiovascular diseases [3]. The content of bioactive substances in conventional hazelnut oil is as follows: total tocopherol 64.62 mg/100 g, vitamin E 57.57 mg/100 g and total sterols 115.34 mg/100 g [4].
Because of the high amount of oil in hazelnuts (about 60%), methods of extracting the oil from hazelnuts inevitably cause rapid lipid oxidation, which reduces the shelf life of hazelnut oil by causing rancidity. By preventing the oxidation of unsaturated fatty acids in hazelnut oil, long-term quality is ensured and product loss due to rancidity is prevented. Microencapsulation can extend the shelf life of hazelnut oil, a widely used technique to extend the shelf life of essential oils and flavorings [5].
Microencapsulation is defined as a physico-chemical and mechanical process that aims to keep active ingredients, which may be solid, liquid or gaseous, in a protective layer that protects them from chemical and physical damage, which are generally used in different fields such as pharmaceutical, chemical, cosmetic and food industries [6, 7]. The microencapsulation method is widely used, which can eliminate the negative effects of environmental factors, such as the presence of light, oxygen and extreme pH values, in order to stabilize the compounds during production and storage [8]. In addition, microencapsulation increases the oxidative stability of the oils and prevents the formation of a negative taste due to oxidation [9]. The oil encapsulation process is a two-step process in the form of emulsifying the core material with a thick solution of the wall material and drying or cooling the subsequently formed emulsions [10]. The type of bio-polymer as a wall material, the ratio of core substrate to wall material, the method of encapsulation and the storage conditions affect the stability of the matrix and thus play a role in the stability of the encapsulated active substance [11, 12]. The most widely used and oldest method for encapsulating oils is spray drying. In addition to encapsulation, the product converts to a more stable state by reducing the water activity of the product [13].
There are many existing coating agents used in microencapsulation. Since the ability to keep the core material in the capsule is one of the most important parameters affecting the efficiency of the process, the choice of wall material is very crucial [14]. Carbohydrates are used as wall materials in the microencapsulation of flavors and oils, such as maltodextrin, hydrolyzed starch, modified starch, cyclodextrin and gums, moreover proteins such as milk proteins, whey proteins and soy proteins are being used [12, 15]. Maltodextrin is a widely used wall material because it meets the necessary properties, such as bland taste, cheap, low viscosity at high solids content, good preservation against oxidation [16]. Due to its high ability to be dissolved in water, low stickiness and perfect emulsifying features, gum Arabic is considered one of the suitable bio-polymer used in spray drying. Sodium caseinate is another preferred wall material in encapsulation with its amphiphilic properties and emulsifying ability [16]. Due to their high surface activity, proteins are mainly used as emulsifiers and stabilizers. Pea proteins have high nutritional value, non-allergenic properties and good functional properties [17]. Gum Arabic also provides stable emulsions over a wide pH range. Modified starches have similar wall material properties to gum Arabic, and are less expensive than gum Arabic [18, 19]. To date, there are few studies on the encapsulation of hazelnut oil. The effect of the temperature of air inlet on the microencapsulation of hazelnut oil (HO) by spray drying was evaluated with maltodextrin as the wall material [5]. In another study, skimmed milk powder was used as wall material for encapsulation of hazelnut oil and microcapsules were added to cake products [20].
In this study, it was aimed to investigate the effect of different plant and animal based wall materials such as modified starch, gum Arabic, whey protein concentrate, gelatine, pea protein and sodium caseinate together with maltodextrin for the microencapsulation of hazelnut oil. Emulsions and microcapsules were characterized in terms of emulsion stability, particle size, encapsulation efficiency, moisture content, tapped bulk density, total yield, and morphology. In addition, the oxidative stability that encapsulation will provide was evaluated by measuring the peroxide and p-Anisidine values.
2 Materials and methods
All chemicals were of analytical grade and purchased from Sigma Aldrich, Germany. Gum Arabic, sodium caseinate, whey protein, gelatine, modified starch (EmCap) and maltodextrin were supplied from Cargill Co., Istanbul, Turkey. Pea protein was supplied from Pulsin Ltd., Gloucester, UK. Hazelnut oil was purchased from a local store in İstanbul, Turkey.
2.1 Quality properties of hazelnut oil
The acid and peroxide values of hazelnut oil were determined according to the official standard methods (AOAC, 2005).
2.2 Fatty acid composition of hazelnut oil
The fatty acid profile was determined using the procedures described by Zahran and Tawfeuk [21]. The fatty acid methyl esters (FAMEs) of oil samples were analyzed for its constituents by GLC-FID (HP 6890 Gas chromatography occupied with flame ionization detector, Hewlett Packard, USA). The injection volume was 1μL and splitting ratio at 100:1. A capillary column Supelco™ SP-2380 (60 m × 0.25 mm × 0.20 μm, Sigma-Aldrich, USA) was used, and the detector and injector temperatures were set as 250 °C. The carrier gas was helium at a flow rate of 1.2 mL/min.
2.3 Emulsion preparation
Maltodextrin and other selected bio-polymers (gum Arabic, sodium caseinate, whey protein, gelatine, modified starch “EmCap” and pea protein) were mixed at a ratio of 9:1, w/w, respectively. The hydrated solutions of wall materials (with concentrations of 30% "w/w on wet basis" and 70% distilled water), once prepared, were stirred at room temperature (25 °C) for 2 h to confirm a full saturation of the polymer particles [22]. The emulsion was prepared by blending hazelnut oil (at concentration of 20% respect to the wall materials) and the solution was homogenized using a T18 digital Ultra Turrax® homogenizer (IKA, Germany), at a speed of 15 × 103 rpm for 5 min.
2.4 Emulsion characterization
2.4.1 Emulsion stability
Directly after preparation of the emulsion, 25 mL of emulsion of each sample were relocated into graduated cylinders. The cylinders were wrapped and kept at room temperature, and the volume of the upper phase was measured at zero time, and after 6, 24 h, as well as after 7 days. The emulsion stability was calculated according to the percentage of separation and expressed as:
where Ho represents the emulsion initial height, and H1 is the upper layer height [19].
2.4.2 Emulsion droplet size
The distribution of droplets was analyzed by using a laser light diffraction instrument, Mastersizer S (Malvern Instruments, Malvern, UK). A slight amount of sample was suspended in water using magnetic agitation, and the droplet size distribution was monitored during each measurement until consecutive readings became constant. The emulsion droplet size was expressed as D32, the Sauter mean diameter [23].
2.5 Microencapsulation by spray drying
Spray drying process was performed using a laboratory scale spray dryer (Mini Spray Dryer B-290, BÜCHI Labortechnik AG, Flawil, Switzerland), with a nozzle atomization system with 1.5 mm diameter and 100% aspirator capacity. The emulsions were introduced into the main chamber through a peristaltic pump and the feed flow rate was controlled by the pump rotation speed. The conditions of spray drying were as follows: Inlet temperature at 140 °C, airflow rate at 0.439 m3/h, pump rate at 4.5 mL/min [19].
2.6 Powder analysis
Microcapsules were characterized for moisture content, bulk density, total yield, encapsulation efficiency and morphology.
2.6.1 Moisture content
Determination of moisture content was performed by using a moisture analyzer (Precisa XM 50, Novatron Scientific Ltd., Novatron House, UK).
2.6.2 Bulk density
Bulk density was determined by transferring 2 g of powder to a 50 mL graduated cylinder. Packed bulk density was calculated from the height of powder in the cylinder after being tapped by hand on a bench 50 times from a height of 10 cm [22].
2.6.3 Encapsulation efficiency (EE)
Encapsulation efficiency was measured using the method described by Carneiro et al. [19]. Fifteen millilitres of hexane were added to 2 g of powder in a glass jar with a lid, which was shaken by hand for the extraction of free oil for 2 min at room temperature. The mixture was filtered through a Whatman® No. 1 filter paper, and the powder collected on the filter was washed three times with 20 mL of solvent. Then, the hexane was left to vaporize at room temperature and subsequently at 60 °C until constant weight. The surface oil (non-encapsulated oil) was determined by mass difference among the initial clean flask and that containing the removed oil residue [24]. Total oil was expected to be equal to the initial oil, since preliminary tests revealed that all the initial oil was retained, which was expected, since hazelnut oil is not volatile. Encapsulation efficiency (EE) was calculated by using the following equation:
where To is the total oil content and So is the surface oil content.
2.6.4 Microencapsulation yield
Microencapsulation yield in the encapsulation process is defined as the ratio of core material in the final dried microcapsules to that in the emulsion [22]. The resulted powder (microcapsules) was collected, weighed and proportioned to the solid components of the emulsion, and calculated as follows:
2.6.5 Morphology and size of microparticles
Scanning electron microscopy (SEM) analysis was conducted with a TESCAN microscope (TESCAN VEGA3™, Kohoutovice, Czech Republic) at an accelerating voltage of 20.00 kV and a working distance of 9.00 mm. Samples were sputter-coated with a gold–palladium mixture under vacuum prior to the examination. Particle diameters were measured from the SEM micrographs in their original magnification using the image software. Size distributions were obtained from a minimum of 200 measurements [24].
2.7 Oxidative stability of microcapsule during storage
For the stability tests, the microcapsules (5 g of powder) were sealed in individuals glass vials (20 mL), and stored at 50 °C in order to accelerate the oxidation process. During storage period, microcapsules were evaluated for oxidation by two distinct methods (peroxide and p-anisidin values) at time zero (right after drying) and over two weeks of storage. Hazelnut oil was extracted from the microcapsules by chloroform/methanol (1:1, v/v), with vortexing for 3 min, then the samples were filtered and the organic solvent was evaporated using rotary evaporator at 35 °C under vacuum. The samples were flushed by N2 to remove the solvent residues.
2.7.1 Peroxide and p-anisidine values
Briefly, the peroxide value and p-anisidine value analysis was carried out to determine the primary and secondary oxidation products, respectively, according to AOCS official methods [25].
2.7.2 Total oxidation (TOTOX) value
The total oxidation value (TOTOX) was used to estimate the oxidative deterioration of lipids [26]. TOTOX value is defined as the sum of both values (PV and p-AV) to total oxidation and was calculated according to the following formula:
2.8 Statistical analysis
The obtained results were statistically analyzed using the SPSS statistical package (Version 22.0, 2013). Analysis of variance (ANOVA) and Duncan’s multiple range test were chosen to determine any significant difference at p < 0.05 according to Steel et al. [27].
3 Results and discussion
3.1 Quality properties and fatty acid composition of hazelnut oil
The quality analysis indicated that the free fatty acid content and peroxide value (PV) of the hazelnut oil were found to be at acceptable levels (0.74% as oleic acid and 1.23 mEq. O2/kg, respectively) set by the Codex Alimentarius Commission [28].
In terms of fatty acid composition as seen in Table 1, unsaturated fatty acid (UFA) content was found to be 91.89%, whereas oleic acid (18:1 n9) was predominant UFA found at 84.70% consistent with the results of other researchers [29].
3.2 Emulsion characterization
The droplet size and emulsion stability observed with emulsions with different types of wall materials are listed in Table 2. The size of the emulsion droplets varied depending on the wall material used. The emulsion was prepared with MD/GE had the largest droplet size compared to the others, while the emulsion was prepared with MD/SC had the smallest droplet size. The largest drop in MD/GE had poor stability and after 6 h the emulsion was degraded. Therefore, it was not possible to measure stability for 24 h and 7 days. Although the emulsion prepared with MD/SC with the smallest droplet size remained stable for 7 days, it did not reach the highest percentage of stability. MD/WPC, MD/GE and MD/PP emulsions could not be measured because the emulsions were not stable for 7 days. There are many wall materials that can be used as oil encapsulating agents. The choice of wall material is critical because it affects the properties of the emulsion before drying, the oil retention during the process and also the storability of the encapsulated powder after drying. These properties depend on the emulsifying properties of the biopolymers [22].
The emulsions characteristics affected significantly (p < 0.05) by use of different wall materials (Table 1). The use of MD/GE in emulsion preparation yielded the largest size of droplets, when compared to the other wall materials, while the feed emulsion produced from the MD/SC mixture yielded the smallest ones. The obtained results could be related to the highest thikness resulted by the MD/GE emulsion, hence, that reverse in a greater durability to droplets movement, avoidance coalescence, and consequencing in smaller diameters [19].
3.3 Powder characterization
Microcapsules obtained after spray drying were analyzed for moisture load, bulk density, encapsulation yield, encapsulation efficiency, morphology and particle size.
3.3.1 Particle characterization
Table 3 shows the characteristics of particles prepared with different wall materials. The moisture content of the microcapsules ranged from a minimum of 2.47% to a maximum of 4.05%. In a study on soybean oil encapsulated by spray drying the moisture content ranged between 1 and 3% [30]. In general, the analyzed samples did not revers significant differences in moisture load, when different bio-polymers were used. The bulk density values of the samples ranged from 0.31 to 0.45 g/cm3. The advantage of produced powders with higher density, could be stored in high amounts into a little containers, when compared to products with lower densities. In addition, a higher bulk density may marked a lower amount of air clogged in the anti-particle spaces, which may help to prevent lipid deterioration . The obtained results were in accordance with the values obtained for the encapsulated hazelnut oil [5]. The highest moisture load and bulk density were observed for the sample prepared by MD:GE. Mean particle diameters ranged from 3.71 to 20.39 μm. The microcapsules formed by maltodextrin and gelatine was larger in size, probably due to their higher emulsion density. The total yield ranged between 74.76 and 90.52% with the highest belonging to MD/Em-Cap. In a study on microencapsulation of rapeseed oil using spray drying, the oil microencapsulation yield in the powders was ranged at a level from 71 to 94% [31].
Although all combinations of wall materials led to different particle size allocation, the powders made with the MD/GE blend showed a wider allocation, meaning that these particles were minimal identical. The lower stability of the maltodextrin and gelatine as feed emulsions may be also referred to differences in particle size.
3.3.2 Encapsulation efficiency (EE)
The obtained results in Table 3, showed the type of wall material used significantly affected the encapsulation efficiency of the samples. Encapsulation efficiency values ranged from 75.8 to 96.7%. The highest efficiency was observed in the emulsions produced by MD/Em-Cap, which resulted in particles with significant differences of lower surface oil than those produced with other emulsions, as shown in Fig. 1. The more stable the emulsion, which has small droplets size, the higher of encapsulation efficiency. While, the lowest efficiency was observed in the emulsion prepared with MD/GE, with significantly differences among other wall materials, which had the largest emulsion droplet size as in Table 2 and the highest surface oil content as in Fig. 1. Many studies show that the smaller the oil droplet size, the longer the active material is retained, which results in increased stability [17].
In a study examined the effect of air inlet temperature on hazelnut oil microencapsulation by spray drying, encapsulation efficiencies ranged from 74.70 to 81.6%, with a 2:1 and 4:1 MD/HPMC ratio [5]. In our work, better encapsulation efficiency was achieved with combinations of wall materials other than MD/GE.
The result of a study by Tonon et al. [32], in which linseed oil was encapsulated with modified starch, gum Arabic and whey protein wall materials, the highest encapsulation efficiency was observed for modified starch and the lowest efficiency was observed for whey protein, which were similar to the results of our study.
3.3.3 Morphology of microparticles
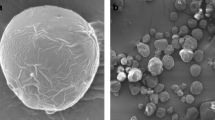
The morphology of microcapsules is shown in Fig. 2. According to the scanning electron micrograph of the surface, of the microcapsules had spherical shapes. Powder particles prepared with MD/SC, MD/Em-cap starch, MD/PP and MD/WPC had fewer surface dents and shrinkage than others. These are in agreement with the reported data of Jafari et al. [22] in which they indicated that WPC powder particles had minimum recesses. Accordingly, to the morphology of particles size, in the emulsion environment the mechanism of different wall materials behave a flocculation ripening of particles, except for MD/GE follows the coalescence behavior.
SEM micrographs of capsules prepared with different wall materials. A MD:gum Arabic, B MD:Em-Cap starch, C MD:gelatine, D MD:pea protein, E MD:sodium caseinate, F MD:whey protein. MD, maltodextrin; Em-Cap, modified starch; GA, gum Arabic; WPC, whey protein concentrate; GE, gelatine; PP, pea protein; SC, sodium caseinate
3.4 Oxidative stability
In this work, the progress of lipid oxidation was monitored by measuring peroxide and p-anisidine value. The TOTOX value is used to determine the total oxidative stability of the oils by including primary and secondary oxidation measurements.
3.4.1 Peroxide value
The variations of the peroxide number of the encapsulated hazelnut oil with different bio-polymers as a wall materials are given in Fig. 3. At the time of zero, all samples presented a low degree of oxidation with 1.23–1.27 mEq. O2/kg oil. The unencapsulated oil oxidized rapidly, reaching the highest peroxide values at 81.41 mEq. O2/kg oil at the end of the storage period. The peroxide value of the samples did not differ significantly from each other up to 9 days (p < 0.05). Sample prepared with MD/GE had the highest peroxide value, reaching to 15.05 mEq. O2/kg oil at the end of the 15th day. This may be well explained by the lowest encapsulation efficiency resulting with more prone to oxidation. Low encapsulation efficiency means that high amount of oil is available for oxidation in the particle surface [33].
In a previous study by Jimenez et al. [34] for encapsulation of the conjugated linoleic acid (CLA) by using polymeric matrices (as wall materials) the oxidative stability was checked by various methods, including the PV. The whey protein concentrate (WPC) was used with maltodextrin and gum Arabic (as wall materials), in addition mentioned that WPC was more efficient than AG in the prevention of lipid against oxidation.
3.4.2 p-Anisidine value
The variations in the P-Anisidine values of encapsulated hazelnut oil using different bio-polymers as a wall materials were shown in Fig. 3. At the time of zero, all samples reversed a low degree of oxidation which ranged from 4.13 to 6.01. The un-encapsulated oil oxidized rapidly, which resulting the highest p-anisidine values. At the end of day 15, the un-encapsulated sample reached to 29.36 and the p-anisidine value of the sample prepared with MD/GE had the highest p-anisidine value among the encapsulated samples (15.03). The reported data for p-anisidine values of all samples referred to significantly (p < 0.05) changes with increasing storage time. As we know, no data was available for hazelnut oil, in a study on wheat germ oil (WGO) which investigated the effect of roasting on the oxidative stability by Zou et al. [35], the p-anisidine and peroxide values of encapsulated WGO were higher than those found in our study for hazelnut oil, when stored at 60 °C for 15 days. The p-anisidine values of the microencapsulated HO, significant differences were observed (p < 0.05) for bulk HO throughout the storage time. These results were in agreement with the result of Zhang et al. [36] who mentioned that, with increasing storage time, the secondary oxidation product of buttermilk microencapsulated algal oil was increased. Considering both p-anisidine and peroxide values, our findings revealed that the HO encapsulation using different wall materials in particular MD/Em-cap modified starch was able to delay the oxidation rate of HO.
Based on the analysis results of PV and p-AV values, the total oxidation value was calculated to estimate the oxidative deterioration as shown in Fig. 3. The highest TOTOX value was calculated for the bulk oil at the end of the 15th day. Among the encapsulated samples, the highest TOTOX value was calculated in the sample prepared with MD/GE, the lowest TOTOX value was calculated in the sample prepared with MD/Em-Cap. There is a correlation between peroxide, p-anisidine value and TOTOX value. Therefore, values with high TOTOX values belong to samples with high peroxide and p-anisidine values.
4 Conclusion
This study describes the use of six combinations of wall materials with maltodextrin and gum Arabic, sodium caseinate, whey protein, gelatine and modified starch to microencapsulate hazelnut oil by spray drying and the effect of them to the oxidation state of the microencapsulation. The wall material used in the encapsulation affects emulsion stability, encapsulation efficiency, surface oil content and oxidation stability. Emulsions prepared with MD/Em-Cap resulted in the best emulsion stability of 98.7% after 7 days. Therefore, the highest encapsulation efficiency of 96.7% was observed for emulsions prepared with MD/Em-Cap with minimal surface oil content. Therefore, the lowest peroxide, p-Anisidine and TOTOX values were observed for microcapsules with MD/Em-Cap. Maltodextrin and modified starch, relatively inexpensive, wall material has good emulsifying properties. Successful results on encapsulation efficiency and oxidation stability suggest that a combination of maltodextrin and modified starch for hazelnut oil can be used to increase shelf life.
Data availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
Abbreviations
- HO:
-
Hazelnut oil
- MD:
-
Maltodextrin
- GE:
-
Gelatine
- GA:
-
Gum Arabic
- SC:
-
Sodium caseinate
- PP:
-
Pea protein
- WPC:
-
Wey protein concentrate
- WGO:
-
Wheat germ oil
- FAME:
-
Fatty acids methyl esters
- GLC:
-
Gas liquid chromatography
- EE:
-
Encapsulation efficiency
- SEM:
-
Scanning electron microscopy
- TOTOX:
-
Total oxidation value
- PV:
-
Peroxide value
- p-AV:
-
Para-anisidine value
- AV:
-
Acid value
- ANOVA:
-
Analysis of variance
- UFA:
-
Unsaturated fatty acid
- PUFA:
-
Poly unsaturated fatty acid
- FA:
-
Fatty acid
- mL:
-
Millilitre
- µL:
-
Microliter
- d:
-
Day
- h:
-
Hour
- min:
-
Minute
- s:
-
Second
- rpm:
-
Round per minute
- g:
-
Gram
References
Cui N, Wang G, Ma Q, Zhao T, Li R, Liang L. Effect of cold-pressed on fatty acid profile, bioactive compounds and oil oxidation of hazelnut during oxidation process. LWT. 2020;129: 109552. https://doi.org/10.1016/j.lwt.2020.109552.
Balta MF, Yarılgaç T, Aşkın MA, Kuçuk M, Balta F, Özrenk K. Determination of fatty acid compositions, oil contents and some quality traits of hazelnut genetic resources grown in eastern Anatolia of Turkey. J Food Comp Anal. 2006;19:681–6. https://doi.org/10.1016/j.jfca.2005.10.007.
Durmaz G, Gökmen V. Effect of refining on bioactive composition and oxidative stability of hazelnut oil. Food Res Int. 2019;116:586–91. https://doi.org/10.1016/j.foodres.2018.08.077.
Karaosmanoğlu H. Lipid characteristics, bioactive properties and mineral content in hazelnut grown under different cultivation systems. J Food Proc Pres. 2022. https://doi.org/10.1111/jfpp.16717.
Kalkan F, Vanga SK, Murugesan R, Orsat V, Raghavan V. Microencapsulation of hazelnut oil through spray drying. Drying Tech. 2017;35:527–33. https://doi.org/10.1080/07373937.2016.1182549.
Zahran H, Mabrouk AM, Salama HH. Evaluation of yoghurt fortified with encapsulated echium oil rich in stearidonic acid as a low-fat dairy food. Egypt J Chem. 2022;65(4):29–41. https://doi.org/10.21608/ejchem.2021.99859.4642.
Soliman TN, Farrag AF, Zahran HA, El-Salam ME. Preparation and properties nano-encapsulated wheat germ oil and its use in the manufacture of functional labneh cheese. Pak J Bio Sci. 2019;22:318–26. https://doi.org/10.3923/pjbs.2019.318.326.
Matos-Jr FE, Di Sabatino M, Passerini N, Favaro-Trindade CS, Albertini B. Development and characterization of solid lipid microparticles loaded with ascorbic acid and produced by spray congealing. Food Res Int. 2015;67:52–9. https://doi.org/10.1016/j.foodres.2014.11.002.
Başyiğit B, Sağlam H, Kandemir Ş, Karaaslan A, Karaaslan M. Microencapsulation of sour cherry oil by spray drying: evaluation of physical morphology, thermal properties, storage stability, and antimicrobial activity. Powder Tech. 2020;364:654–63. https://doi.org/10.1016/j.powtec.2020.02.035.
Umaña M, Turchiuli C, Rosselló C, Simal S. Addition of a mushroom by-product in oil-in-water emulsions for the microencapsulation of sunflower oil by spray drying. Food Chem. 2021;343: 128429. https://doi.org/10.1016/j.foodchem.2020.128429.
Farrag AF, Zahran H, Al-Okaby MF, El-Sheikh MM, Soliman TN. Physicochemical properties of white soft cheese supplemented with encapsulated olive phenolic compounds. Egypt J Chem. 2020;63:2921–31. https://doi.org/10.21608/ejchem.2020.23381.2388.
Hamed SF, Elshafei K, El-Sayed HS, Abo-Elwafa GA, Afifi SM, Zahran H. Formulation of multi-functional omega-3 oil rich microcapsules by spray drying methodology. Egypt J of Chem. 2021;63:5117–36. https://doi.org/10.21608/ejchem.2020.43946.2891.
Luna-Guevara JJ, Ochoa-Velasco CE, Hernández-Carranza P, Guerrero-Beltrán JA. Microencapsulation of walnut, peanut and pecan oils by spray drying. Food Str. 2017;12:26–32. https://doi.org/10.1016/j.foostr.2017.04.001.
Tarone AG, Cazarin CB, Junior MR. Anthocyanins: new techniques and challenges in microencapsulation. Food Res Int. 2020;133: 109092. https://doi.org/10.1016/j.foodres.2020.109092.
Wang S, Shi Y, Han L. Development and evaluation of microencapsulated peony seed oil prepared by spray drying: oxidative stability and its release behavior during in-vitro digestion. J Food Eng. 2018;231:1–9. https://doi.org/10.1016/j.jfoodeng.2018.03.007.
Chew SC, Tan CP. Nyam KL (2018) Microencapsulation of refined kenaf (Hibiscus cannabinus L.) seed oil by spray drying using β-cyclodextrin/gum arabic/sodium caseinate. J Food Eng. 2018;237:78–85. https://doi.org/10.1016/j.jfoodeng.2018.05.016.
Aberkane L, Roudaut G, Saurel R. Encapsulation and oxidative stability of PUFA-rich oil microencapsulated by spray drying using pea protein and pectin. Food Bio Tech. 2014;7(5):1505–17. https://doi.org/10.1007/s11947-013-1202-9.
Premi M, Sharma HK. Effect of different combinations of maltodextrin, gum arabic and whey protein concentrate on the encapsulation behavior and oxidative stability of spray dried drumstick (Moringa oleifera) oil. Int J Bio Macro. 2017;105:1232–40. https://doi.org/10.1016/j.ijbiomac.2017.07.160.
Carneiro HC, Tonon RV, Grosso CR, Hubinger MD. Encapsulation efficiency and oxidative stability of flaxseed oil microencapsulated by spray drying using different combinations of wall materials. J Food Eng. 2013;115:443–51. https://doi.org/10.1016/j.jfoodeng.2012.03.033.
Emlek BO, Çevik K, Alaşalvar H, Erinç H, Yalçin H, Yildirim Z, Özbey A. Effect of hazelnut oil and microencapsulated hazelnut oil usage on physicochemical and textural properties of cake. J Inst Sci Tech. 2021;11:281–9. https://doi.org/10.21597/jist.724667.
Zahran HA, Tawfeuk HZ. Physicochemical properties of new peanut (Arachis hypogaea L.) varieties. OCL. 2019;26:19. https://doi.org/10.1051/ocl/2019018.
Jafari SM, Assadpoor E, He Y, Bhandari B. Encapsulation efficiency of food flavours and oils during spray drying. Drying Tech. 2008;26:816–35. https://doi.org/10.1080/07373930802135972.
Silva VM, Vieira GS, Hubinger MD. Influence of different combinations of wall materials and homogenisation pressure on the microencapsulation of green coffee oil by spray drying. Food Res Int. 2014;61:132–43. https://doi.org/10.1016/j.foodres.2014.01.052.
Abozed SS, Elaraby GM, Zahran HA. Application of spray-dried microcapsules of purslane (Portulaca oleracea L.) seed oil enhances quality of mango juice. Open Agric. 2021;15:1–9. https://doi.org/10.2174/1874331502115010001.
AOAC. Official methods of analysis. 19th ed. Washington: Association of Official Analytical Chemists; 2005.
De Abreu DP, Losada PP, Maroto J, Cruz JM. Evaluation of the effectiveness of a new active packaging film containing natural antioxidants (from barley husks) that retard lipid damage in frozen Atlantic salmon (Salmo salar L.). Food Res Int. 2010;43:1277–82. https://doi.org/10.1016/j.foodres.2010.03.019.
Steel RGD, Torrie JH, Dickey DA. Principles and procedures of statistics: a biometrical approach. 3rd ed. New York: McGraw Hill; 1997.
Masson-Matthee MD. The codex alimentarius commission and its standards. The Hague: TMC Asser Press; 2007.
Turan A. Effect of drying methods on fatty acid profile and oil oxidation of hazelnut oil during storage. Eur Food Res Tech. 2018;244:2181–90. https://doi.org/10.1007/s00217-018-3128-y.
Hogan SA, McNamee BF, O’Riordan ED, O’Sullivan M. Emulsification and microencapsulation properties of sodium caseinate/carbohydrate blends. Int Dairy J. 2001;11(3):137–44. https://doi.org/10.1016/s0958-6946(01)00091-7.
Domian E, Wasak I. Microencapsulation of rapeseed oil based on the spray drying method. Polish J Food Nutr Sci. 2008. https://doi.org/10.1515/pjfns-2015-0015.
Tonon RV, Grosso CR, Hubinger MD. Influence of emulsion composition and inlet air temperature on the microencapsulation of flaxseed oil by spray drying. Food Res Int. 2011;44(1):282–9. https://doi.org/10.1016/j.foodres.2010.10.018.
Tonon RV, Pedro RB, Grosso CR, Hubinger MD. Microencapsulation of flaxseed oil by spray drying: effect of oil load and type of wall material. Drying Tech. 2012;30:1491–501. https://doi.org/10.1080/07373937.2012.696227.
Jimenez M, Garcia HS, Beristain CI. Spray-dried encapsulation of conjugated linoleic acid (CLA) with polymeric matrices. J Sci Food Agric. 2006;86(14):2431–7. https://doi.org/10.1002/jsfa.2636.
Zou Y, Gao Y, He H, Yang T. Effect of roasting on physico-chemical properties, antioxidant capacity, and oxidative stability of wheat germ oil. LWT. 2018;90:246–53. https://doi.org/10.1016/j.lwt.2017.12.038.
Zhang Y, Pang X, Zhang S, Liu L, Ma C, Lu J, Lyu J. Buttermilk as a wall material for microencapsulation of omega-3 oils by spray drying. LWT. 2020;127: 109320. https://doi.org/10.1016/j.lwt.2020.109320.
Acknowledgements
Hamdy Zahran acknowledges the Scientific and Technological Research Council of Turkey (TUBİTAK), BIDEB 2221-Fellowship program for Visiting Scientists and Scientists on Sabbatical Leave for the fellowship. We acknowledge Cargill Co., Turkey for kindly providing modified starch and maltodextrin for this study.
Funding
The research paper supported and funding by National Research Center, Egypt.
Author information
Authors and Affiliations
Contributions
HZ: conceptualization, methodology, formal analysis, investigation, writing—original draft; HB: writing—original draft, writing—review and editing; NŞ-Y: conceptualization, methodology, formal analysis, investigation, writing—original draft, writing—review and editing, supervision, project administration. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zahran, H., Bat, H. & Şahin-Yeşilçubuk, N. Influence of wall material combination on the lipid oxidation of the hazelnut oil microcapsules. Discov Food 2, 17 (2022). https://doi.org/10.1007/s44187-022-00018-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44187-022-00018-4