Abstract
Heat stress, which is caused by global warming, threatens crops yield and quality across the world. As a kind of post-translation modification, SUMOylation involves in plants heat stress response with a rapid and wide pattern. Here, we identified small ubiquitin modifiers (SUMO), which affect drought tolerance in apple, also participated in thermotolerance. Six isoforms of SUMOs located on six chromosomes in apple genome, and all the SUMOs were up-regulated in response to heat stress condition. The MdSUMO2 RNAi transgenic apple plants exhibited higher survival rate, lower ion leakage, higher catalase (CAT) activity, and Malondialdehyde (MDA) content under heat stress. MdDREB2A, the substrate of MdSUMO2 in apple, was accumulated in MdSUMO2 RNAi transgenic plants than the wild type GL-3 at the protein level in response to heat stress treatment. Further, the inhibited SUMOylation level of MdDREB2A in MdSUMO2 RNAi plants might repress its ubiquitination, too. The accumulated MdDREB2A in MdSUMO2 RNAi plants further induced heat-responsive genes expression to strengthen plants thermotolerance, including MdHSFA3, MdHSP26.5, MdHSP18.2, MdHSP70, MdCYP18-1 and MdTLP1. In summary, these findings illustrate that interfering small ubiquitin modifiers (SUMO) in apple improves plants thermotolerance, partly by facilitating the stability and activity of MdDREB2A.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Global climate change is a big threat for the balance of terrestrial ecosystem, including the continuously increased temperature of ocean and continent, unpredictable rainfall or drought patterns, and extreme weather (Li et al., 2022a; Niu et al. 2022; Smith and Gregory 2013; Wheeler and von Braun 2013). Among the aforementioned climate changes, greenhouse gas-induced global warming poses a significant threat to agriculture production (Geng et al. 2020; Walters et al. 2022; White et al. 2006). Previous studies found that heat stress contributes to overall yield losses of about 40% in wheat (White et al. 2006), about 1–1.7% loss in maize for every raise in temperature above 30 °C (Lobell et al. 2011a), and a 3–7% loss of corn and soybeans with every degree of increased heat. From 1980 to 2008, the reduction in the yield of maize and wheat were 3.8% and 5.5%, respectively, due to global warming (Huang et al. 2019; Lobell et al. 2011b). Additionally, higher temperature and drought events usually coincide more often due to nowadays climate change, that cause the plants more vulnerable to insects and disease infections and finally magnify the yield loss (Dai 2013; Kumar et al. 2019). According to the records of NOAA (National Oceanic and Atmospheric Administration, www.noaa.gov/climate), earth’s temperature has risen by 0.08 °C per decade since 1880 and the last nine years from 2013 to 2021 ranked among the warmest years. While, July 2022 was among the top 10 warmest Julys on record for several continents, and almost the whole world is undergoing the heat-waves for now. So, the discovery of the thermotolerant genes for laying the breeding foundation of heat-tolerant cultivars is urgent need of current research.
Apple is a perennial temperate woody plant, which can survive through high temperature (Huo et al. 2021, 2020). Despite this, the extreme high temperatures cause a significant loss of economic benefit for apple orchards, including the loss of fruit quality and quantity. Direct sunlight can easily destroy the apple fruits due to high temperature, that is known as sunburn with tissue discoloration, yellowing, browning, and necrosis (Torres et al. 2013). Heat stress also affects the quality parameters of apple fruits, including fruit shape, texture, water content, and sugar concentration (Franco et al. 1992; Torres et al. 2017). Apart from the effects on fruit, heat stress can also lead to the unavoidably biochemical and physiological changes in apple trees, including accumulation of ROS (Reactive Oxygen Species) and MDA (malonaldehyde), activated antioxidant enzyme activities, elevated leaf electrolyte leakage, changed stomatal movement, decreased photosynthesis capacity, phytohormones homeostasis, anthocyanin metabolism and enhanced autophagic activity (Dong et al. 2021; Fang et al. 2019; Huo et al. 2021, 2020; Torres et al. 2017). Additionally, the roles of various genes associated with the apple heat stress response have been revealed; and the HSFs (heat shock transcription factors) are the most investigated of these genes. In apple genome, 25 HSFs have been identified and proved to participate in heat stress response (Giorno et al. 2012). Especially, MdHSF3b and MdHSF4a, two apple positive regulators under high temperature condition, bind to the heat shock cis-element of MdCOL4 promoter. Then, the induced MdCOL4 enhances its inhibitory effect to regulate the expression of genes involved in anthocyanin biosynthesis under high temperature conditions (Fang et al. 2019). MdNup62, an interacted protein of MdHSFs, negatively regulates the heat stress by inhibiting the expression of HSPs (Zhang et al. 2022). Moreover, the VQ motif-containing protein-coding gene (MdVQ37) is a negative regulator of apple basal thermotolerance by affecting salicylic acid homeostasis and heat related genes expression (Dong et al. 2021). Furthermore, apple basal thermotolerance can also be affected by autophagy, and overexpression of MdATG18a enhanced the apple thermotolerance by decreasing chloroplasts damage (Huo et al. 2020). Since the vital roles of autophagy played in apple heat stress response, MdATG8i also has been proved to be a positive regulator under high temperature environment, and the same role of the MdATG8i-interacting protein, MdHARBI1 (Huo et al. 2021). However, research on apple heat stress response is still limited, and more research is needed on the gene functions involved in apple thermotolerance.
SUMO, a small protein of approximately 100–115 amino acids, usually attached to a protein for participating in plants biotic and abiotic stress response (Castro et al. 2012; Chang and Yeh 2020; Hay 2005). The attachment of SUMO to the substrates is known as a kind of protein post-translation modification—SUMOylation (Morrell and Sadanandom 2019; Tempe et al. 2008; Wang et al. 2020). SUMOylation is a rapid and efficient manner for plants biotic and abiotic stress response by affecting the functions of target proteins, including stability, transcription activity, subcellular trafficking, genome integrity, and chromatin-remodeling (Castro et al. 2012; Guerra et al. 2015; Miller et al. 2010; Miller and Vierstra 2011). Previous studies have found various potential target substrates of SUMO under heat stress condition, like HSPs, HSFs, DREB2A, WRKYs, TPL, SUVH9, and histone H2B (Castro et al. 2012; Guerra et al. 2015; Miller et al. 2010; Miller and Vierstra 2011; Rytz et al. 2018). However, the impact of SUMOylation of these target proteins is largely unresolved, with the exception of the transcription factors HSFA2 and DREB2A. Arabidopsis HSFA2 is a positive regulator of plants acquired thermotolerance, which is SUMOylated at K315. The transcriptional activation of HSFA2 on the down-stream HSPs promoters is repressed by overexpression of AtSUMO1 (Cohen-Peer et al. 2010). DREB2A is another positive regulator of heat stress, and its SUMOylation enhances the protein stability to confer plants acquired thermotolerance (Wang et al. 2020). Additionally, the DREB2A is also known as a target substrate of SUMOs in apple and its SUMOylation responds to drought stress condition via a fine-tunning manner (Li et al., 2022a), that is in contrast with Arabidopsis. However, how SUMOs participate in apple heat stress has not been reported yet.
In this study, we found that interfering SUMO expression could improve thermotolerance of transgenic apple plants. The MdSUMO2 RNAi plants showed enhanced capability of scavenging H2O2 and maintaining integrity of plasma membranes. Furthermore, we found that the SUMO target substrate, MdDREB2A was accumulated more in the MdSUMO2 RNAi plants compared to the wild type GL-3 under heat stress condition. Inhibited SUMOylation of MdDREB2A also suppressed its ubiquitination and then facilitated the protein stability of MdDREB2A. Since the positive role of MdDREB2A in plants heat stress response, we concluded that the accumulated MdDREB2A partly contributed to the thermotolerance of MdSUMO2 RNAi plants. Moreover, the elevated expression of heat-responsive genes acted as the down-stream of MdDREB2A might be another reason for the improved thermotolerance of MdSUMO2 RNAi plants. Taken together, we have characterized the improved thermotolerance of MdSUMO2 RNAi plants by enhancing the protein stability of MdDREB2A and activating the down-stream heat-responsive genes expression.
Results
Apple small ubiquitin modifiers are involved in heat stress
Previous study found that there were six small ubiquitin modifiers (SUMO) in apple genome, and due to the whole genome duplication, these SUMOs located on six chromosomes shared very similar sequences (Li et al., 2022a). Apple MdSUMOs modulate drought stress response with a fine-tuning manner. To further explore whether MdSUMOs function under heat stress in apple, firstly, GL-3 apple plants were subjected to heat exposure (45 °C) for 2 h. Due to the almost identical coding sequences, we separated apple SUMOs to MdSUMO2A (on chromosome 3 and 11), MdSUMO2B (on chromosome 7 and 17), and MdSUMO2C (on chromosome 5 and 10). The qRT-PCR results showed that three MdSUMO2s were up-regulated with varying degrees under heat stress condition (Fig. 1), implying the similar roles of MdSUMO2 involved in heat stress.
Interfering small ubiquitin modifiers (SUMO) improves thermotolerance of apple
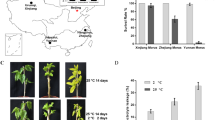
We generated apple transgenic plants of MdSUMO2 RNAi, which knocked down the expression of all SUMOs mentioned in the previous study (Li et al., 2022a). Initially, one-month-old GL-3 and MdSUMO2 RNAi apple seedlings were used as experimental materials. When exposed to 45 °C for 8 h, almost all the leaves of the wild type—GL-3 plants wilted and turned brown. In contrast, scorching of the MdSUMO2 RNAi leaves partially occurred with symptoms like edges dry out and turn brown (Fig. 2A). Two weeks later, more than 20% MdSUMO2 RNAi transgenic seedlings recovered with new leaves, however, only 5% of the wild GL-3 survived and recovered (Fig. 2B). Similar phenotypes were also found in three-month-old GL-3 and MdSUMO2 RNAi transgenic apple seedlings exposed to the outdoor high temperature environment for 7 days. At 40 °C, the leaves of the GL-3 plants mostly displayed the symptoms of dehydration and shriveled, nevertheless, the leaves of the MdSUMO2 RNAi transgenic plants remained green and vigorous (Fig. 2C). Additionally, eight-month-old GL-3 and MdSUMO2 RNAi plants raised in a greenhouse suffered from high temperature at noon when the temperature reached to 50 °C within a short time. A short exposure of heat resulted the serious injury of new leaves of GL-3 (about 28.6%), however few new leaves of MdSUMO2 RNAi plants exhibited yellowing and dehydration (Fig. 2D).
The MdSUMO2 RNAi transgenic apple plants are more tolerance than the wild type (GL-3) under heat stress condition. A, Morphology of one-month-old MdSUMO2 RNAi transgenic apple plants and GL-3 under heat stress for 8 h. B, The survival rate in A. C, Morphology of 3-month-old MdSUMO2 RNAi transgenic apple plants and GL-3 under 40 °C for 7 days. D, The new leaves of GL-3 and transgenic plants under heat stress (up, the morphology), and the statistical data (down, the injury rate of new leaves). Error bars indicate SD, n = 24 in A and B, and n = 10 in D. Asterisks indicate significant differences between MdSUMO2 RNAi transgenic apple plants and GL-3 plants in each group (control and heat treatment). Student’s t test was performed and statistically significant differences were indicated by *P ≤ 0.05, **P ≤ 0.01
Knock-down of the MdSUMO2s in apple enhanced the stability of plasma membranes
Previously, it has been reported that cell plasma membranes are considered as heat stress sensors (Török et al. 2014), so we further investigated the stability of plasma membranes of GL-3 and MdSUMO2 RNAi plants under heat stress condition. As Fig. 3A showed, apple plants could suffer high temperature at 35 °C. While the leaves of both GL-3 and MdSUMO2 RNAi plants started ion leakage at 39 °C. The electrolytic leakage aggravated when the temperature reached to 43 °C. During the heat stress condition, the electrolytic leakage of MdSUMO2 RNAi plants was much lower than the GL-3, indicating the relative stability of plasma membranes of MdSUMO2 RNAi leaves (Fig. 3A). The malondialdehyde (MDA) content acts as a marker of lipid peroxidation (Del Rio et al. 2005). We found that high temperature treatment enhanced the MDA concentration of the GL-3 and MdSUMO2 RNAi plants, while the MDA content of the MdSUMO2 RNAi plants was lower than GL-3 (Fig. 3B). This result showed the mild damage to MdSUMO2 RNAi plants caused by high temperature than GL-3 plants. Abiotic stress usually leads to ROS burst in plants (Niu et al. 2022). Our results of DAB staining showed that heat stress caused the accumulation of H2O2 content in apple plants. As compared to the GL-3 plants, the leaves of the MdSUMO2 RNAi plants were lightly stained with brown coloration (Fig. 3C). Meanwhile, we determine the H2O2 contents of GL-3 and transgenic plants under control and heat treatment. The results were in agreement with the DAB staining (Fig. 3D). However, in case of NBT staining, no significant differences were observed between the GL-3 and MdSUMO2 RNAi plants (data not shown). Furthermore, we detected the activity of catalase (CAT), which is responsible for H2O2 scavenging. The results showed that the CAT activity was significantly increased in MdSUMO2 RNAi plants than GL-3 after heat stress (Fig. 3E). Altogether, these findings indicated that knock-down of the MdSUMO2s in apple enhanced the thermotolerance by reducing the accumulation of H2O2.
The activated catalase contributes the thermotolerance of MdSUMO2 RNAi transgenic apple plants than GL-3. A, The electrolytic leakage of leaves under high temperature stress. B, Malondialdehyde (MDA) concentration in GL-3 and transgenic apples treated with or without high temperature. C, DAB staining of MdSUMO2 RNAi transgenic apple plants and GL-3 under control and heat treatment. D, H2O2 contents of GL-3 and transgenic plants under control and heat treatment. E, Catalase (CAT) activity in MdSUMO2 RNAi transgenic apple and GL-3 under control and heat treatment. Error bars indicate SD, n = 3. Asterisks indicate significant differences between MdSUMO2 RNAi transgenic apple plants and GL-3 plants in each group (control and heat treatment). Student’s t test was performed and statistically significant differences were indicated by *P ≤ 0.05, **P ≤ 0.01
Heat caused the accumulation of MdDREB2A in MdSUMO2 RNAi transgenic apple plants
Under heat stress condition, the transcription factor DREB2A is regarded as a positive regulator (Sakuma et al. 2006). SUMOylation of DREB2A facilitates its protein stability and improve Arabidopsis thermotolerance (Wang et al. 2020). Nevertheless, SUMOylation of apple MdDREB2A mediates its ubiquitination and following degradation under drought stress condition (Li et al., 2022a). Here, when exposed to heat stress, we found that apple MdDREB2A was quickly activated, and that MdSUMO2 RNAi transgenic apple plants accumulated more of it than GL-3 (Fig. 4A). As SUMOylation can affect the protein stability, so we examined the SUMOylation level of MdDREB2A protein in both GL-3 and MdSUMO2 RNAi transgenic plants under control and heat stress conditions. The Fig. 4B shows that 45 °C heat stress treatment significantly increased the level of SUMO conjugates to MdDREB2A in GL-3 plants, which was much lower than in MdSUMO2 RNAi transgenic plants. Because of the tight crosstalk between SUMOylation and ubiquitination, we further investigated the ubiquitination level of MdDREB2A in response to heat stress condition. The results showed a synergistic effect of SUMOylation and ubiquitination on MdDREB2A, as the ubiquitination level of MdDREB2A increased lower in MdSUMO2 RNAi transgenic plants than GL-3 in response to heat stress (Fig. 4B). To investigate the mechanism of SUMO-mediated DREB protein degradation further, we treated plants with MG132, a 26S proteasome pathway inhibitor. The western bolt result demonstrated that MdDREB2A accumulated more in MdSUMO2 RNAi transgenic apple plants than the wild type GL-3 under heat stress treatment significantly. MG132 was applied to check the stability of MdDREB2A was whether associated with ubiquitination, and the result showed that the MdDREB2A protein degradation was inhibited both in GL-3 and MdSUMO2 RNAi transgenic lines. However, MdDREB2A still accumulated more than GL-3 (Fig. 4C). The analysis of the stability of the MdDREB2A protein under heat stress revealed that the 26S proteasome pathway was involved in the SUMOylation-mediated degradation of MdDREB2A.
The transcription factor MdDREB2A was accumulated in MdSUMO2 RNAi transgenic apple plants than GL-3. A, The proteins of MdDREB2A in MdSUMO2 RNAi transgenic plants were induced more than GL-3 under heat stress. B, SUMOylation and ubiquitination of MdDREB2A in GL-3 and MdSUMO2 RNAi transgenic plants in response to heat stress treatment. * indicates SUMOylated substrates; ★indicates ubiquitinated substrates. C, The stability of MdDREB2A was affected by 26S proteasome mediated degradation in response to heat stress condition. MG132 was applied as the inhibitor of 26S proteasome mediated ubiquitination
Silencing of MdSUMO2 in apple upregulated the down-stream genes of MdDREB2A under heat stress
We next explored whether MdDREB2A accumulation affected the down-stream genes expression in response to heat stress. Six homologous genes of Arabidopsis which were proved to be down-stream of DREB2A were selected to detect mRNA expression levels (Sakuma et al. 2006). Except MdHSP26.5, other five genes were found the conserved DRE motif on their promoters (Fig. 5A). There is only one DRE motif on the promoters of MdTLP1 and MdCYP18.1, two motifs on MdHSP18.2 and MdHSFA3, and three motifs on MdHSP70. We further used quantitative RT-PCR to confirm the expression patterns of these heat stress responsive genes. As Fig. 5B showed, MdHSFA, MdHSP70, MdCYP18-1 and MdTLP1 were found the similar expression level under control condition, but induced significantly more in MdSUMO2 RNAi transgenic plants than GL-3. The expression levels of MdHSP18.2 and MdHSP26.5 were lower in MdSUMO2 RNAi transgenic plants under control condition, but they were induced clearly higher in MdSUMO2 RNAi transgenic plants in response to heat stress condition (Fig. 5B). These results suggest that MdSUMOs are involved in heat stress response by inducing heat-responsive genes expression which acted as the down-stream genes of MdDREB2A.
The transcription factor MdDREB2A activates the expressions of down-stream genes related to heat response. A, A scheme of the down-stream genes. The coding region is shown in indigo and beige indicates the promoter and 5 ‘UTR. The binding sites of MdDREB2A are shown with pink. B-G, Expression of MdDREB2A downstream genes in MdSUMO2 RNAi transgenic apple plants and GL-3 plants under control and heat treatment. Error bars indicate SD, n = 3. Asterisks indicate significant differences between MdSUMO2 RNAi transgenic apple plants and GL-3 plants in each group (control and heat treatment). Student’s t test was performed and statistically significant differences were indicated by *P ≤ 0.05, **P ≤ 0.01
Discussion
Global warming is making heat waves hotter, longer, and more common. Plants have evolved multiple characteristics to adapt the heat stress condition since they are sessile organisms (Walters et al. 2022; Zhu 2016). At the organellar level, leaf properties such as leaf color and leaf angle are changed when plants exposure to heat stress, following with the stomatal closure, inhibited photosynthesis and carbon assimilation (Blum 1986; Slattery and Ort 2019). Heat stress during flowering, gametogenesis and loral meristem stages especially results in a huge loss of crop yield and quality (Jagadish et al. 2021; Torres et al. 2013). Severe heat stress can even lead to the death of plant. At the molecular level, the heat stress caused the damage of cellular phospholipid membranes can be sensed by a series of integral membrane proteins rapidly (Scharf et al. 2012). Following, the calcium signaling, MAPK activation, ROS, NO, phospholipid signals, protein SUMOylation, and proteasomal degradation initiate physiological and biochemical changes (Hasanuzzaman et al. 2013; Zhu 2016). In our study, we identified that all the SUMOs in apple genome were up-regulated under heat stress condition (Fig. 1), implying the potential role of SUMO in response to heat stress. Furthermore, we found that the MdSUMO2 RNAi transgenic apple plants exhibited enhanced thermotolerance than the wild type—GL-3 (Fig. 2A-B). The one-month-old MdSUMO2 RNAi transgenic apple plants showed high survival rate under heat stress condition, and the 3-month-old plants sustained green leaves as compared to the scorched leaves of GL-3 after exposing to heat condition (Fig. 2C). Additionally, the 8-month-old MdSUMO2 RNAi plants displayed much less injured new shoots than GL-3 in greenhouse condition. Since the cellular phospholipid membranes usually act as an important senser to heat stress, so we investigated the permeability of membranes (Huo et al. 2020; Zhang et al. 2021). The results showed that MdSUMO2 RNAi transgenic apple plants retained the integrity of the membranes as compared to the GL-3 plants upon heat stress exposure. In a coincide, the MDA content was found much lower in MdSUMO2 RNAi plants after high temperature treatment. ROS scavenging including H2O2 and O2 −, are usually regarded as an effective way for plants to adapt heat stress condition (Hasanuzzaman et al. 2013; Naing and Kim 2021). Our result showed lower H2O2 content in MdSUMO2 RNAi transgenic apple plants than GL-3, but no significant changes in O2 − (Fig. 3). Moreover, the catalase activity was found higher in MdSUMO2 RNAi transgenic apple plants. So, we speculated the activated catalase was responsible for the lower accumulation of H2O2 content in MdSUMO2 RNAi plants.
SUMOylation is a kind of post-translation modification, which widely and rapidly participates in plant heat stress response (Castro et al. 2012; Miller and Vierstra 2011; Morrell and Sadanandom 2019). Among various substrates of SUMO, the HSFA2 plays a positive role in heat stress condition. SUMOylation of HSFA2 mediated by SUMO1 exhibits an inactive state in the cell nucleus and deSUMOylation of HSFA2 leads to an active form of this protein when expose to heat stress (Cohen-Peer et al. 2010). SUMOylation also facilitates the importation of chloroplast proteins during heat stress and further regulates the nonphotochemical quenching level of plants (Zheng et al. 2022). Additionally, chromatin-associated SUMOylation regulates the balance between plant development and heat stress responses by controlling the transcriptional switch (Han et al. 2021). Here, we found that MdDREB2A was accumulated more rapidly in MdSUMO2 RNAi transgenic apple plants as compared to GL-3 under heat stress condition (Fig. 4A). Additionally, DREB2A has been proved to be a positive regulator in plant heat stress response, including Arabidopsis (Sakuma et al. 2006; Wang et al. 2020), maize (Qin et al. 2007), and soybean (Mizoi et al. 2013). Since the DNA-binding capability of DREB2A to the down-stream genes in apple and Arabidopsis are the same in regulating drought stress (Li et al. 2019; Li et al., 2022a), we speculate that the accumulated MdDREB2A in MdSUMO2 RNAi transgenic apple plants was responsible for their strengthened thermotolerance. Previously it was found that SUMOylation of the MdDREB2A mediated its degradation via the 26S protease pathway through MdRNF4 in response to drought stress condition (Li et al., 2022a). So, we are curious about the role of SUMOylation of MdDREB2A in regulating the stability of MdDREB2A in response to heat stress. We got the similar results for MdDREB2A under drought stress condition, MdDREB2A was both less SUMOylated and ubiquitinated in the MdSUMO2 RNAi transgenic apple plants than GL-3 under heat stress condition (Fig. 4B). So, we supposed that SUMOylation of MdDREB2A regulated the drought and heat stress response of apple in the same manner, which was different from Arabidopsis under heat condition (Wang et al. 2020). Previous research has suggested that the AtDREB2A protein contains a 30-amino-acid NRD (negative regulatory domain) domain after translation of the AP2 domain. This domain interacts with BPMs (BTB/POZ AND MATH DOMAIN proteins) and DRIP1/2 (DREB2A-INTERACTING PROTEINS 1 and 2) to degrade AtDREB2A (Morimoto et al. 2017; Qin et al. 2008). Under drought or high-temperature stress, the expression of DRIP1/2 and BPMs is reduced, which prevents the hydrolysis of AtDREB2A, thereby promoting its regulation of downstream genes and enhancing plant stress resistance. However, apple MdDREB2A is structurally dissimilar to AtDREB2A except for the AP2 domain, suggesting that there may be inconsistencies in the response of MdDREB2A to stress compared to Arabidopsis. Previously, we discovered that the SUMOylation of apple MdDREB2A could mediate the ubiquitination of MdDREB2A via 26S protease pathway under drought stress condition (Li et al. 2022). In this study, we found that the similar regulation manner of apple MdDREB2A in response to heat stress as drought stress—the accumulation of MdDREB2A in response to heat stress is also controlled by SUMOylation mediated degradation. As a transcription factor, the DREB2A regulates the expression of the down-stream genes by binding to the DRE motif on their promoters (Li et al. 2019; Sakuma et al. 2006). According to the previous study, we identified the homologous genes of Arabidopsis in apple (Sakuma et al. 2006), including MdHSFA3, MdHSP26.5, MdHSP18.2, MdHSP70, MdCYP18-1 and MdTLP1. All these genes had DRE motif on their promoters, except MdHSP26.5. Furthermore, the qRT-PCR results showed that these genes were induced more in MdSUMO2 RNAi transgenic apple plants as compared to the GL-3 under heat stress condition, implying their contribution in the thermotolerance of the MdSUMO2 RNAi plants.
In conclusion, our results demonstrated that interfering small ubiquitin modifiers (SUMO) improves the thermotolerance of apple by facilitating the activity of MdDREB2A. The exposure of MdSUMO2 RNAi transgenic apple plants to heat stress led to much lighter damage of cellular phospholipid membranes and lower MDA content than GL-3. Moreover, the catalase activity was found to be responsible for ROS scavenging in MdSUMO2 RNAi plants. In addition, the accumulated protein of MdDREB2A caused up-regulated heat-responsive genes contributed to the thermotolerance of MdSUMO2 RNAi plant, too. These findings will provide the candidate genes for future apple breeding in confronting the extreme climate change.
Materials and methods
Plant material and growth conditions
The experimental material used for gene cloning was ‘Golden Delicious’ (Malus × domestica Borkh. cv. Golden Delicious), which grown in Northwest A&F University, Yang ling, Shaanxi (34°20′N, 108°24′E). Leaves of ‘Golden Delicious’ was used for RNA extraction, RT-PCR, and gene cloning. For apple transformation, the wild type GL-3, a progeny of 'Royal Gala' (Malus × domestica), was subcultured on Murashige and Skoog (MS) medium (4.43 g/L MS salts, 30 g/L sucrose, 0.2 mg/L 6-BA, 0.2 mg/L IAA and 7.5 g/L agar, pH 5.8) under long-day condition (14 h light [cool white, ~ 100 umolm−2 s−1, T5 LED batten]: 10 h dark) for 4 weeks at 25 °C (Xie et al. 2018). MdSUMO2 RNAi plants were produced as described in a previous study (Li et al., 2022a). The transgenic plants and GL-3 were rooted, transplanted into substrate (Pindstrup, Denmark), and grown in a growth chamber at Northwest A&F University (16 h light: 8 h dark, 25 °C, ~ 55% relative humanity).
RNA extraction and qRT-PCR analysis
RNA extraction and quantitative reverse transcription PCR (qRT-PCR) analysis were performed as described previously (Xie et al. 2018).
The extracted RNA was detected by agarose gel electrophoresis and the concentration was determined by UV spectrophotometer (Thermo Nanodrop 2000). For qRT-PCR analysis, approximately 1 ug of RNA was reverse transcribed with oligo-dT to first-strand cDNA using a RevertAid First Strand cDNA Synthesis Kit (Thermo Scientific, K1622, USA). qRT-PCR was performed on the CFX96 real-time system (Bio-Rad, USA), using a ChamQ Universal SYBR qPCR Master Mix (Vazyme, C601, China). Primers used in this study are list in Table 1.
Thermotolerance assays
Thermotolerance stress was performed as described previously (Guan. et al. 2013). For analyzing the expression patterns of MdSUMO2s under heat stress, one-month-old GL-3 plants were put into a growth chamber with 45 °C for 120 min. For analyzing the phenotype of one-month-old apple plants, GL-3 and MdSUMO2 RNAi transgenic apple plants with a consistent growth state were exposed to 45 °C for 8 h and allowed to grow for an additional 14 d. The leaves collected at 120 min were used for gene expression and protein analysis. The 3-month-old GL-3 and MdSUMO2 RNAi transgenic apple plants were exposed to 40 °C for 7 d in outside environment. In the summer, the 8-month-old GL-3 and MdSUMO2 RNAi transgenic apple plants grew in greenhouse suffered from high temperature at noon when the temperature reached to 50 °C, and the treatment lasted from 11:00 to13:00 for one month.
Determination of H2O2 content, CAT activity, MDA content and ion leakage
H2O2 content was performed using a Hydrogen Peroxide Assay Kit (Comin, H2O2-1-Y, China).
CAT activity and MDA content were measured as described previously (Niu et al. 2022). For CAT activity and MDA content measurement, approximately 0.1 g leaf was used to extract. The extraction buffer includes 100 mM phosphate buffer [pH = 7.0], 1 mM ethylenediaminetetraacetic acid [EDTA], 0.1% Triton-X-100, and 1% polyvinyl pyrrolidone [PVP].
The electrolytic leakage assay was performed as previously described (Niu et al. 2022). Briefly, the leaves were collected from one-month-old GL-3 and MdSUMO2 RNAi transgenic apple plants. The leaves were cut into leaf discs and placed in a glass tube. Then the discs were soaked in deionized water for 12 h and the tubes were then placed into the high-temperature aqua bath cycle instrument (Thermo Fisher PC200-A40) with the following heat temperature regime: successive 4 °C increases each hour from 23 °C to 35 °C, then hold at 35 °C for 1 h; then successive 4 °C increases at 1-h intervals, then hold at 39 °C for 1 h; then successive 4 °C increases at 1-h intervals, then hold at 43 °C for 1 h. Finally, the discs in tubes were boiled at 100 °C for 30 min and the conductivity was determined using a benchtop conductivity meter (Thermo Scientific, STARA2120, USA). Experiments were performed at least three times.
In vivo SUMOylation and ubiquitination analysis
The SUMOylation and ubiquitination analysis were performed as previously described (Li et al., 2022b). In brief, the samples of analysis were collected at 0 h or 0.5 h or 1 h or 2 h under heat stress. Then the total proteins of the samples were extracted by protein extraction buffer (50 mM Tris–HCl, 150 mM NaCl, 2 mM EDTA, 1% Np-40, 10% Glycerin, 1 mM PMSF, 5 mM DTT, pH 7.5). Finally, the proteins were immunoprecipitated with anti-SUMO (ab5316, Abcam) or anti-Ubiquitin (P4D1, Cell Signaling Technology®) antibodies.
Statistical analysis
For statistical analysis, the Student’s t test was used by using Prism 8.0 software (GraphPad Software, USA). Differences were identified significant if P < 0.05 or 0.01.
Availability of data and material
All data generated or analyzed during this study are included in this published article.
Abbreviations
- CAT:
-
Catalase
- DAB:
-
3,3'-Diaminobenzidine tetrahydrochloride
- DRE:
-
Dehydration responsive element
- DTT:
-
Dithiothreitol
- EDTA:
-
Ethylene diamine tetraacetic acid
- MDA:
-
Malondialdehyde
- NBT:
-
Nitro-blue tetrazolium
- PMSF:
-
Phenylmethylsulfonyl fluoride
- PVP:
-
Polyvinyl pyrrolidone
- qRT-PCR:
-
Quantitative reverse transcription PCR
References
Blum A (1986) The effect of heat stress on wheat leaf and ear photosynthesis. J Exp Bot 37:111–118. https://doi.org/10.1093/jxb/37.1.111
Castro PH, Tavares RM, Bejarano ER, Azevedo H (2012) SUMO, a heavyweight player in plant abiotic stress responses. Cell Mol Life Sci 69:3269–3283. https://doi.org/10.1007/s00018-012-1094-2
Chang HM, Yeh ETH (2020) SUMO: from bench to bedside. Physiol Rev 100:1599–1619. https://doi.org/10.1152/physrev.00025.2019
Cohen-Peer R, Schuster S, Meiri D, Breiman A, Avni A (2010) Sumoylation of Arabidopsis heat shock factor A2 (HSFA2) modifies its activity during acquired thermotholerance. Plant Mol Biol 74:33–45. https://doi.org/10.1007/s11103-010-9652-1
Dai A (2013) Increasing drought under global warming in observations and models. Nat Clim Chang 3:52–58. https://doi.org/10.1038/nclimate1633
Del Rio D, Stewart AJ, Pellegrini N (2005) A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr Metab Cardiovasc Dis 15:316–328. https://doi.org/10.1016/j.numecd.2005.05.003
Dong Q, Duan D, Zheng W, Huang D, Wang Q, Li X, Mao K, Ma F (2021) MdVQ37 overexpression reduces basal thermotolerance in transgenic apple by affecting transcription factor activity and salicylic acid homeostasis. Hortic Res 8:220. https://doi.org/10.1038/s41438-021-00655-3
Fang H, Dong Y, Yue X, Chen X, He N, Hu J, Jiang S, Xu H, Wang Y, Su M et al (2019) MdCOL4 interaction mediates crosstalk between UV-B and high temperature to control fruit coloration in apple. Plant Cell Physiol 60:1055–1066. https://doi.org/10.1093/pcp/pcz023
Franco AC, Ball E, LÜttge U (1992) Differential effects of drought and light levels on accumulation of citric and malic acids during CAM in Clusia. Plant Cell Environ 15:821–829. https://doi.org/10.1111/j.1365-3040.1992.tb02149.x
Geng X, Fu YH, Hao F, Zhou X, Zhang X, Yin G, Vitasse Y, Piao S, Niu K, De Boeck HJ, Menzel A, Peñuelas J (2020) Climate warming increases spring phenological differences among temperate trees. Glob Change Biology 26:5979–5987. https://doi.org/10.1111/gcb.15301
Giorno F, Guerriero G, Baric S, Mariani C (2012) Heat shock transcriptional factors in Malus domestica: identification, classification and expression analysis. BMC Genomics 13:639. https://doi.org/10.1186/1471-2164-13-639
Guan Q, Wen C, Zeng H, Zhu J (2013) A KH Domain-Containing Putative RNA-Binding Protein Is Critical for Heat Stress-Responsive Gene Regulation and Thermotolerance in Arabidopsis. Molecular Plant. 6(2):386–95. https://doi.org/10.1093/mp/sss119
Guerra D, Crosatti C, Khoshro HH, Mastrangelo AM, Mica E, Mazzucotelli E (2015) Post-transcriptional and post-translational regulations of drought and heat response in plants: a spider’s web of mechanisms. Front Plant Sci 6:57. https://doi.org/10.3389/fpls.2015.00057
Han D, Chen C, Xia S, Liu J, Shu J, Nguyen V, Lai J, Cui Y, Yang C (2021) Chromatin-associated SUMOylation controls the transcriptional switch between plant development and heat stress responses. Plant Commun 2:100091. https://doi.org/10.1016/j.xplc.2020.100091
Hasanuzzaman M, Nahar K, Alam MM, Roychowdhury R, Fujita M (2013) Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int J Mol Sci 14:9643–9684. https://doi.org/10.3390/ijms14059643
Hay RT (2005) SUMO: a history of modification. Mol Cell 18:1–12. https://doi.org/10.1016/j.molcel.2005.03.012
Huang LJ, Cheng GX, Khan A, Wei AM, Yu QH, Yang SB, Luo DX, Gong ZH (2019) CaHSP16.4, a small heat shock protein gene in pepper, is involved in heat and drought tolerance. Protoplasma 256:39–51. https://doi.org/10.1007/s00709-018-1280-7
Huo L, Sun X, Guo Z, Jia X, Che R, Sun Y, Zhu Y, Wang P, Gong X, Ma F (2020) MdATG18a overexpression improves basal thermotolerance in transgenic apple by decreasing damage to chloroplasts. Hortic Res 7:21. https://doi.org/10.1038/s41438-020-0243-2
Huo L, Guo Z, Wang P, Sun X, Xu K, Ma F (2021) MdHARBI1, a MdATG8i-interacting protein, plays a positive role in plant thermotolerance. Plant Sci 306:110850. https://doi.org/10.1016/j.plantsci.2021.110850
Jagadish SVK, Way DA, Sharkey TD (2021) Plant heat stress: Concepts directing future research. Plant, Cell Environ 44:1992–2005. https://doi.org/10.1111/pce.14050
Kumar A, Nayak AK, Das BS, Panigrahi N, Dasgupta P, Mohanty S, Kumar U, Panneerselvam P, Pathak H (2019) Effects of water deficit stress on agronomic and physiological responses of rice and greenhouse gas emission from rice soil under elevated atmospheric CO2. Sci Total Environ 650:2032–2050. https://doi.org/10.1016/j.scitotenv.2018.09.332
Li X, Xie Y, Lu L, Yan M, Fang N, Xu J, Wang L, Yan Y, Zhao T, van Nocker S, Ma F, Liang D, Guan Q (2019) Contribution of methylation regulation of MpDREB2A promoter to drought resistance of Mauls prunifolia. Plant Soil 441:15–32. https://doi.org/10.1007/s11104-019-04149-z
Li X, Zhou S, Liu Z, Lu L, Dang H, Li H, Chu B, Chen P, Ma Z, Zhao S et al (2022) Fine-tuning of SUMOylation modulates drought tolerance of apple. Plant Biotechnol J 20:903–919. https://doi.org/10.1111/pbi.13772
Lobell DB, Bänziger M, Magorokosho C, Vivek B (2011a) Nonlinear heat effects on African maize as evidenced by historical yield trials. Nat Clim Chang 1:42–45. https://doi.org/10.1038/nclimate1043
Lobell DB, Schlenker W, Costa-Roberts J (2011b) Climate trends and global crop production since 1980. Science 333:616–620. https://doi.org/10.1126/science.1204531
Miller MJ, Vierstra RD (2011) Mass spectrometric identification of SUMO substrates provides insights into heat stress-induced SUMOylation in plants. Plant Signal Behav 6:130–133. https://doi.org/10.4161/psb.6.1.14256
Miller MJ, Barrett-Wilt GA, Hua Z, Vierstra RD (2010) Proteomic analyses identify a diverse array of nuclear processes affected by small ubiquitin-like modifier conjugation in Arabidopsis. Proc Natl Acad Sci 107:16512–16517. https://doi.org/10.1073/pnas.1004181107
Mizoi J, Ohori T, Moriwaki T, Kidokoro S, Todaka D, Maruyama K, Kusakabe K, Osakabe Y, Shinozaki K, Yamaguchi-Shinozaki K (2013) GmDREB2A;2, a canonical DEHYDRATION-RESPONSIVE ELEMENT-BINDING PROTEIN2-type transcription factor in soybean, is posttranslationally regulated and mediates dehydration-responsive element-dependent gene expression. Plant Physiol 161:346–361. https://doi.org/10.1104/pp.112.204875
Morimoto K, Ohama N, Kidokoro S, Mizoi J, Takahashi F, Todaka D, Mogami J, Sato H, Qin F, Kim JSFukao Y, Fujiwara M, Shinozaki K, Yamaguchi-Shinozaki K (2017) BPM-CUL3 E3 ligase modulates thermotolerance by facilitating negative regulatory domain-mediated degradation of DREB2A in Arabidopsis. Proc Natl Acad Sci 114(40):E8528–E8536. https://doi.org/10.1073/pnas.1704189114
Morrell R, Sadanandom A (2019) Dealing with stress: a review of plant SUMO proteases. Front Plant Sci 10:1122. https://doi.org/10.3389/fpls.2019.01122
Naing AH, Kim CK (2021) Abiotic stress-induced anthocyanins in plants: Their role in tolerance to abiotic stresses. Physiol Plant 172:1711–1723. https://doi.org/10.1111/ppl.13373
Niu C, Jiang L, Cao F, Liu C, Guo J, Zhang Z, Yue Q, Hou N, Liu Z, Li X, Tahir MM, He J, Li Z, Li C, Ma F, Guan Q (2022) Methylation of a MITE insertion in the MdRFNR1–1 promoter is positively associated with its allelic expression in apple in response to drought stress. Plant Cell 34(10):3983–4006. https://doi.org/10.1093/plcell/koac220
Qin F, Kakimoto M, Sakuma Y, Maruyama K, Osakabe Y, Tran LS, Shinozaki K, Yamaguchi-Shinozaki K (2007) Regulation and functional analysis of ZmDREB2A in response to drought and heat stresses in Zea mays L. Plant J 50:54–69. https://doi.org/10.1111/j.1365-313x.2007.03034.x
Qin F, Sakuma Y, Tran L-SP, Maruyama K, Kidokoro S, Fujita Y, Fujita M, Umezawa T, Sawano Y, Miyazono K, Tanokura M, Shinozaki K, Yamaguchi-Shinozaki K (2008) Arabidopsis DREB2A-Interacting Proteins Function as RING E3 Ligases and Negatively Regulate Plant Drought Stress-Responsive Gene Expression. Plant Cell 20:1693–1707. https://doi.org/10.1105/tpc.107.057380
Rytz TC, Miller MJ, McLoughlin F, Augustine RC, Marshall RS, Juan YT, Charng YY, Scalf M, Smith LM, Vierstra RD (2018) SUMOylome profiling reveals a diverse array of nuclear targets modified by the SUMO ligase SIZ1 during heat stress. Plant Cell 30:1077–1099. https://doi.org/10.1105/tpc.17.00993
Sakuma Y, Maruyama K, Qin F, Osakabe Y, Shinozaki K, Yamaguchi-Shinozaki K (2006) Dual function of an Arabidopsis transcription factor DREB2A in water-stress-responsive and heat-stress-responsive gene expression. Proc Natl Acad Sci 103:18822–18827. https://doi.org/10.1073/pnas.0605639103
Scharf KD, Berberich T, Ebersberger I, Nover L (2012) The plant heat stress transcription factor (HSF) family: Structure, function and evolution. Biochimica Biophysica Acta 1819:104–119. https://doi.org/10.1016/j.bbagrm.2011.10.002
Slattery RA, Ort DR (2019) Carbon assimilation in crops at high temperatures. Plant, Cell Environ 42:2750–2758. https://doi.org/10.1111/pce.13572
Smith P, Gregory PJ (2013) Climate change and sustainable food production. Proc Nutr Soc 72:21–28. https://doi.org/10.1017/s0029665112002832
Tempe D, Piechaczyk M, Bossis G (2008) SUMO under stress. Biochem Soc Trans 36:874–878. https://doi.org/10.1042/bst0360874
Török Z, Crul T, Maresca B, Schütz GJ, Viana F, Dindia L, Piotto S, Brameshuber M, Balogh G, Péter M, Porta A, Trapani A, Gombos I, Glatz A, Gungor B, Peksel B, Vigh L Jr, Csoboz B, Horváth I, Vijayan MM, Hooper PL, Harwood JL, Vigh L (2014) Plasma membranes as heat stress sensors: from lipid-controlled molecular switches to therapeutic applications. Biochimica Biophysica Acta 1838:1594–1618. https://doi.org/10.1016/j.bbamem.2013.12.015
Torres CA, Sepulveda A, Gonzalez-Talice J, Yuri JA, Razmilic I (2013) Fruit water relations and osmoregulation on apples (Malus domestica Borkh.) with different sun exposures and sun-injury levels on the tree. Sci Hortic 161:143–152. https://doi.org/10.1016/j.scienta.2013.06.035
Torres CA, Sepúlveda G, Kahlaoui B (2017) Phytohormone Interaction Modulating Fruit Responses to Photooxidative and Heat Stress on Apple (Malus domestica Borkh.). Front Plant Sci 8:2129. https://doi.org/10.3389/fpls.2017.02129
Walters J, Zavalnitskaya J, Isaacs R, Szendrei Z (2022) Heat of the moment: extreme heat poses a risk to bee-plant interactions and crop yields. Curr Opin Insect Sci 52:100927. https://doi.org/10.1016/j.cois.2022.100927
Wang F, Liu Y, Shi Y, Han D, Wu Y, Ye W, Yang H, Li G, Cui F, Wan S et al (2020) SUMOylation stabilizes the transcription factor DREB2A to improve plant thermotolerance. Plant Physiol 183(1):41–50. https://doi.org/10.1104/pp.20.00080
Wheeler T, von Braun J (2013) Climate change impacts on global food security. Science 341:508–513. https://doi.org/10.1126/science.1239402
White MA, Diffenbaugh NS, Jones GV, Pal JS, Giorgi F (2006) Extreme heat reduces and shifts United States premium wine production in the 21st century. Proc Nutr Soc 103:11217–11222. https://doi.org/10.1073/pnas.0603230103
Xie Y, Chen P, Yan Y, Bao C, Li X, Wang L, Shen X, Li H, Liu X, Niu C, Zhu C, Fang N, Shao Y, Zhao T, Yu J, Zhu J, Xu L, van Nocker S, Ma F, Guan Q (2018) An atypical R2R3 MYB transcription factor increases cold hardiness by CBF-dependent and CBF-independent pathways in apple. New Phytol 218:201–218. https://doi.org/10.1111/nph.14952
Zhang C, An N, Jia P, Zhang W, Liang J, Zhou H, Zhang D, Ma J, Zhao C, Han M, Ren X, Xing L (2022) MdNup62 interactions with MdHSFs involved in flowering and heat-stress tolerance in apple. BMC Plant Biol 22:317. https://doi.org/10.1186/s12870-022-03698-3
Zhang X, Gong X, Li D, Yue H, Qin Y, Liu Z, Li M, Ma F (2021) Genome-Wide Identification of PRP Genes in Apple Genome and the Role of MdPRP6 in Response to Heat Stress. Int J Mol Sci 22(11):5942. https://doi.org/10.3390/ijms22115942
Zheng X, Wang C, Lin WY, Lin C, Han D, Xie Q, Lai J, Yang C (2022) Importation of chloroplast proteins under heat stress is facilitated by their SUMO conjugations. New Phytol 235:173–187. https://doi.org/10.1111/nph.18121
Zhu JK (2016) Abiotic Stress Signaling and Responses in Plants. Cell 167:313–324. https://doi.org/10.1016/j.cell.2016.08.029
Acknowledgements
We thank Dr. Zhihong Zhang from Shenyang Agricultural University for providing tissue-cultured GL-3 plants.
We also thank Mrs. Wenjing Wang for assistance with the sample collection process.
Funding
This work was supported by the National Key Research and Development Project (2022YFD1602107), the Key S&T Special Projects of Shaanxi Province, China (2020zdzx03-01–02) and the Natural Science Foundation of Shaanxi Province, China (2022JQ-179).
Author information
Authors and Affiliations
Contributions
X.L., Q.G., F.M. and C.N. conceived the study. Z.L., N.B., J.G., S.Z., B.C. and Z.M. performed experiments. Z.L. and N.B. analyzed the data. X.L., Z.L. M.M. and Abid Khan wrote the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All authors consent to participate.
Consent for publication
All authors consent for publication.
Competing interests
Q.G. is a member of the Editorial Board and was not involved in the journal's review of, or decisions related to, this manuscript.
Additional information
Handling Editor: Dr. Shuhua Yang
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, Z., Bian, N., Guo, J. et al. Interfering small ubiquitin modifiers (SUMO) improves the thermotolerance of apple by facilitating the activity of MdDREB2A. Stress Biology 3, 10 (2023). https://doi.org/10.1007/s44154-023-00089-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44154-023-00089-y