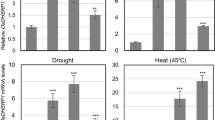
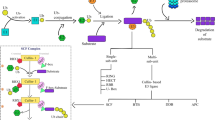
Abstract
Abiotic stresses significantly reduce the grain yield and productivity of cereal crops, especially rice, and this may affect food security in the future. Different abiotic stress adaptation pathways have been investigated and depicted in plants. Among these, the ubiquitin-proteasome system (UPS) has been studied as a key mechanism to understand the protein regulation pathways that enhance the adaptation and survival of plants under various environmental stresses such as drought, salinity, cold, and toxic metalloid exposure. RING E3 ligases constitute a highly diverse and important enzyme group that acts within the 26S UPS, and it also plays a crucial role as a central regulator of plant physiological and cellular processes. This review aimed to highlight recent findings and discoveries regarding the different stress-induced RING E3 ligase genes of major cereal crops and their functions via ubiquitination pathways under different environmental stress conditions. Such genes regulate different physiological responses including protein stabilization, cell membrane integrity, regulation of stomatal opening, and the maintenance of meristematic cells, and they also regulate reactive oxygen species and heavy metal levels via ubiquitination in plants. Hence, the ubiquitination process is considered a potential target for the development of abiotic stress-tolerant crops, and it might be used as an excellent mechanism for stress-tolerant crop improvement programs.
Similar content being viewed by others
References
Al-Salhi M, Ghannam MM, Al-Ayed MS, El-Kameesy SU, Roshdy S. 2004. Effect of gamma-irradiation on biophysical and morphological properties of corn. Nahrung 48: 95–98
Bae H, Kim SK, Cho SK, Kang BG, Kim WT. 2011. Overexpression of OsRDCP1, a rice RING domain-containing E3 ubiquitin ligase, increased tolerance to drought stress in rice (Oryza sativa L.). Plant Sci. 180: 775–82
Bray EA, Bailey-Serres J, Weretilnyk E. 2000. Responses to abiotic stresses, in Biochemistry and Molecular Biology of Plants, eds, BB Buchanan, W Gruissem, RL Jones. Rockville, American Society of Plant Physiologists, pp 1158–1249
Chasapis CT, Spyroulias GA. 2009. RING finger E(3) ubiquitin ligases: structure and drug discovery. Curr. Pharm. Des. 15: 3716–3731
Conde A, Chaves MM, Geros H. 2011. Membrane transport, sensing and signaling in plant adaptation to environmental stress. Plant Cell Physiol. 52: 1583–1602
Deshaies RJ, Joazeiro CA. 2009. RING domain E3 ubiquitin ligases. Annu. Rev. Biochem. 78: 399–434
Dong CH, Agarwal M, Zhang Y, Xie Q, Zhu JK. 2006. The negative regulator of plant cold responses, HOS1, is a RING E3 ligase that mediates the ubiquitination and degradation of ICE1. Proc. Natl. Acad. Sci. USA, 103: 8281–8286
Fang H, Meng Q, Xu J, Tang H, Tang S, Zhang H, Huang J. 2015. Knock down of stress inducible OsSRFP1 encoding an E3 ubiquitin ligase with transcriptional activation activity confers abiotic stress tolerance through enhancing antioxidant protection in rice. Plant Mol. Biol. 87: 441–58
Flick K, Kaiser P. 2012. Protein degradation and the stress response. Semin. Cell Develop. Biol. 23: 515–22
Freemont PS, Hanson IM, Trowsdale JA. 1991. Novel cysteinerich sequence motif. Cell 64: 483–484
Gao T, Wu Y, Zhang Y. 2011. OsSDIR1 overexpression greatly improves drought tolerance in transgenic rice. Plant Mol. Biol. 76: 145–56
Goldstein G, Scheid M, Hammerling U, Boyse EA, Schlesinger DH, Niall HD. 1975. Isolation of a polypeptide that has lymphocyte-differentiating properties and is probably represented universally in living cells. Proc. Nat. Acad. Sci. USA 73: 11–15
Grattan SR, Zeng L, Shannon MC, Roberts SR. 2002. Rice is more sensitive to salinity than previously thought. Calif. Agric. 56: 189–195
Guerra D, Mastrangelo AM, Lopez-Torrejon G, Marzin S, Schweizer P, Stanca AM, Del Pozo JC, Cattivelli L, Mazzucoteli E. 2012. Identification of a protein network interacting with TdRF1, a wheat RING ubiquitin ligase with a protective role against cellular dehydration. Plant Physiol. 158: 777–789
Hwang SG, Chapagain S, Han AR, Park, YC, Park, HM, Kim, YH, Jang, CS. 2017. Molecular characterization of rice arsenic-induced RING Finger E3 ligase 2 (OsAIR2) and its heterogeneous overexpression in Arabidopsis thalaiana. Physiol. Plarumant. 161: 372–384
Hwang SG, Kim JJ, Lim SD, Park YC, Moon JC, Jang CS. 2016b. Molecular dissection of Oryza sativa salt-induced RING finger protein 1 (OsSIRP1): possible involvement in the sensitivity response to salinity stress. Physiol. Plant. 158: 168–179
Hwang SG, Park HM, Han AR, Jang CS. 2016a. Molecular characterization of Oryza sativa arsenic-induced RING E3 ligase 1 (OsAIR1): Expression patterns, localization, functional interaction, and heterogeneous overexpression. J. Plant Physiol. 191: 140–148
Jung CG, Lim SD, Hwang S, Jang CS. 2012. Molecular characterization and concerted evolution of two genes encoding RING-C2 type proteins in rice. Gene 505: 9–18
Koiwai H, Tagiri A, Katoh S, Kathoh E, Ichikawa H, Minami E, Nishizawa Y. 2007. Ring–H2 type ubiquitin ligase EL5 is involved in root development through the maintenance of cell viability in rice. Plant J. 51: 92–104
Kosarev P, Mayer KF, Hardtke CS. 2002. Evaluation and classification of RING-finger domains encoded by the Arabidopsis genome. Genome Biol. 3: Research0016
Kovacs E, Keresztes A. 2002. Effect of gamma and UV-B/C radiation on plant cells. Micron 33: 199–210
Li Z, Hu Q, Zhou M, Vandenbrink J, Li D, Menchyk N, Reighard S, Norris A, Liu H, Sun D, et al. 2013. Heterologous expression of OsSIZ1, rice SUMO E3 ligase, enhances broad abiotic stress tolerance in transgenic creeping bentgrass. Plant Biotech. J. 11: 432–45
Lim SD, Cho HY, Park YC, Ham DJ, Lee JK, Jang CS. 2013. The rice RING finger E3 ligase, OsHCI1, drives nuclear export of multiple substrate proteins and its heterogeneous overexpression enhances acquired thermotolerance. J. Exp. Bot. 60: 2899–914
Lim SD, Hwang JG, Han AR, Park YC, Lee C, Ok YS, Jang CS. 2014a. Positive regulation of rice RING E3 ligase OsHIR1 in arsenic and cadmium uptakes. Plant Mol. Biol. 85: 365–379
Lim SD, Jung CG, Park YC, Lee SC, Lim CW, Kim DS, Jang CS. 2015. Molecular Dissection of a rice microtubule-associated RING finger Protein and its potential role in salt tolerance in Arabidopsis. Mol. Biol. 89: 365–384
Lim SD, Lee C, Jang CS. 2014b. The rice RING E3 ligase, OsCTR1, inhibits trafficking to the chloroplasts of OsCP12 and OsRP1, and its overexpression confers drought tolerance in Arabidopsis. Plant Cell Environ. 37: 1097–113
Lim SD, Yim WC, Moon JC, Kim DS, Lee BM, Jang CS. 2010. A gene family encoding RING finger proteins in rice: their expansion, expression diversity, and co-expressed genes. Plant Mol. Biol. 72: 369–380
Liu C, Hsu Y, Cheng Y, Yen HC, Wu YP, Wang CS, Lai CC. 2012. Proteomic analysis of salt-responsive ubiquitin-related proteins in rice roots. Rapid Commun. Mass Spectrom. 26: 1649–60
Liu H, Zhang H, Yang Y, Li G, Yang Y, Wang X, Basnayake BM, Li D, Song F. 2008. Functional analysis reveals pleiotropic effects of rice RING-H2 finger protein gene OsBIRF1 on regulation of growth and defense responses against abiotic and biotic stresses. Plant Mol. Biol. 68: 17–30
Liu J, Xia Z, Wang M, Yang T, Wu J. 2013. Overexpression of maize E3 ubiquitin ligase gene enhances drought tolerance through regulating stomatal aperture and antioxidant system in transgenic tobacco. Plant Physiol. Biochem. 73: 114–120
Magadum S, Benerjee U, Murugan P, Gangapur D, Ravikesavan R. 2013, Gene duplication as a major force in evolution J. Genet. 92: 155–161
Ning Y, Jantasuriyarat C, Zhao Q, Zhang H, Chen S, Liu J, Liu L, Tang S, Park CH, Wang X, et al. 2011. The SINA E3 Ligase OsDIS1 negatively regulates drought response in rice. Plant Physiol. 157: 242–55
Park GG, Park JJ, Yoon J, Yu SN, Na G. 2010. A RING finger E3 ligase gene, Oryza sativa Delayed Seed Germination 1 (OsDSG1), controls seed germination and stress responses in rice. Plant Mol. Biol. 74: 467–78
Park JJ, Yi J, Yoon J, Cho LH, Ping J, Jeong HJ, Cho SK, Kim WT, An G. 2011. OsPUB15, an E3 ubiquitin ligase, functions to reduce cellular oxidative stress during seedling establishment. Plant J. 65: 194–205
Park YC, Kim JJ, Kim DS, Jang CS. 2015. Rice RING E3 ligase may negatively regulate gamma-ray response to mediate the degradation of photosynthesis-related proteins. Planta 241: 1119–1129
Peters JM, Franke WW, Kleinschmidt JA. 1994. Distinct 19S and 20S subcomplexes of the 26S proteasome and their distribution in the nucleus and cytoplasm. J. Biol. Chem. 269: 7709–7718
Pickart C.M. Back to the future with ubiquitin. 2004. Cell 116: 181–190
Shahbaz M, Ashraf M. 2013. Improving salinity tolerance in cereals. Crit. Rev. Plant Sci. 32: 237–249
Shahid M, Pourrut B, Dumat C, Nadeem M, Aslam M, Pinelli E. 2014. Heavy-metal-induced reactive oxygen species: phytotoxicity and physiocochemical changes in plants. Rev. Environ. Contam. Toxicol. 232: 1–44
Stone SL, Hauksdottir H, Troy A, Troy A, Herschlrb J, Kraft E, Callis J. 2005. Functional analysis of the RING-type ubiquitin ligase family of Arabidopsis. Plant Physiol. 137: 13–30
Takatsuji H. 1998. Zinc-finger transcription factors in plants. Cell Mol. Life Sci. 54: 582–596
Wahid A, Gelani S, Ashraf M, Fooladb MR. 2007. Heat tolerance in plants: an overview. Environ. Exp. Bot. 61: 199–223
Wery J, Silim S, Knights EJ, Malhotra RS, Cousin R. 1994. Screening techniques and sources and tolerance to extremes of moisture and air temperature in cool season food legumes. Euphytica 73: 73–83
Wi SG, Chung BY, Kim JH, Baek MH, Yang DH, Lee JW, Kim JS. 2005. Unstructural changes of cell organells in Arabidopsis stems after gamma irradiation. J. Plant Biol. 48: 195–200
Xia Z, Liu Q, Wu J, Ding J. 2012. ZmRFP1, the putative ortholog of SDIR1, encodes a RING-H2E3 ubiquitin ligase and responds to drought stress in an ABA-dependent manner in maize. Gene 495: 146–153
Yim WC, Lee B-M, Jang CS. 2009. Expression diversity and evolutionary dynamics of rice duplicate genes. Mol. Genet. Genomics 281: 483–493
Zeng S, Fou P, Ziao F, Li Y. 2014. Overexpressing a novel RING–H2 finger protein gene, OsRHP1, enhances drought and salt tolerance in rice (Oryza sativa L.). J. Plant Biol. 57: 357–365
Zhang G, Zhang M, Zhao Z, Ren Y, LI Q, Wang W. 2017. Wheat TaPUB1 modulates plant drought stress resistance by improving antioxidant capability. Sci. Rep. 7: 7549
Zhang M, Zhang GQ, Kang HH, Zhou SM, Wang W. 2017. TaPUB1, a putative E3 Ligase Gene from Wheat, Enhances Salt Stress Tolerance in Transgenic Nicotiana benthamiana. Plant Cell Physiol. 58: 1673–1688
Zhu JK. 2016. Abiotic stress signaling and response in plants. Cell 167: 313–324
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chapagain, S., Park, Y.C. & Jang, C.S. Functional diversity of RING E3 ligases of major cereal crops in response to abiotic stresses. J. Crop Sci. Biotechnol. 20, 351–357 (2017). https://doi.org/10.1007/s12892-017-0104-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12892-017-0104-0