Abstract
Aqueous glycerol is a proposed green extractant for anthocyanins and polyphenols as an alternative to conventional solvents. The aim of this study was to investigate the potential use of aqueous glycerol to extract anthocyanins from Syzygium cumini fruit pulp with high yields. The ultrasound-assisted extraction process was also examined to enhance the extraction yield. The application of ultrasound-assisted extraction along with glycerol as a modifier remarkably enhanced the extraction of anthocyanins compared to the conventional extraction. Aqueous glycerol (70%) was screened against conventional solvents (ethanol, methanol and water), where glycerol showed promising outcomes. The optimised ultrasonication time and extraction temperature (25 °C for 5 min) were selected based on our previous study of NADESs. The results showed that glycerol gave the highest amount of anthocyanin content (201.05 mg C3G/100 g fresh weight) compared to conventional solvents for retrieving anthocyanins from S. cumini fruit pulp. Besides the anthocyanin content, the extract yield, phenolic content, and antioxidant activities using DPPH and FRAP were also determined. Glycerol showed a higher phenolic recovery (0.9 mg GAE/g dry sample), resulting in higher antioxidant activity (DPPH activity-73.35% inhibition). Therefore, the application of UAE with aqueous glycerol provides accessibility and enhanced anthocyanin extraction efficiency, thus fulfilling the green and sustainable approach to anthocyanin extraction.
Similar content being viewed by others
1 Introduction
There is an increasing interest in using natural food colourants to prepare jams, cakes, juices and other food items. Food products with vibrant colours have an edge on the sensory scale, which plays a critical role in the product’s palatability [25]. However, the food industry has been utilising synthetic food colours for a long time due to their stability, efficiency and cost-effectiveness [3]. However, the safety of synthetic food colours has been questioned in recent years. The research for better and safer alternatives has led to the recovery of plant bioactive compounds, a great source of food colours [18]. These plant bioactive pigments include chlorophylls, carotenoids, betacyanins and flavonoids. Anthocyanins, a class of flavonoids, are considered to have a wide colour range and are currently being explored and validated as a promising alternative to synthetic colours [12].
Syzygium cumini (S. cumini) is one of the underutilised fruits of the Myrtaceae family cultivated in subtropical and tropical regions [33]. Previous studies indicate that the pulp of S. cumini fruit is a rich source of major anthocyanins named malvidin, cyanidin, petunidin, delphinidin, and peonidin [48, 52, 56]. The fruit is also a rich source of antioxidant compounds where the anthocyanins may act similarly [6]. Despite its commercial use in juice processing, there is limited research data on anthocyanin extraction from S. cumini [47, 54]. However, it is a trending research topic [17], D. [29], N. [30, 46].
Applying a suitable extraction technique is a crucial factor to consider for enhancing the recovery of bioactive compounds [50]. Conventional extraction techniques have lately been associated with many disadvantages, such as longer extraction times, lower stability, thus higher degradation, and use and consumption of organic solvents [44]. The use of solvents such as methanol for the extraction processes and the extracts are not preferred in food products due to their toxicity, flammability and environmental pollution [15]. These solvents and techniques, therefore, don’t align with the concept of “green extraction processes” [26].
Glycerol has been acknowledged as a promising alternative for being a green and high-performing extraction solvent [1]. As glycerol is a by-product of the biodiesel industry, it is readily available due to the high production of biodiesel [35]. It has been identified as one of the green solvents attributed to its biodegradability, natural origin, and safety [55]. Glycerol is also considered a cheaper alternative to conventional solvents. It also lowers the cost of the overall extraction process as the number of unit operations is reduced, for there is no requirement to separate the solvent from the extract [37, 45]. The solvent seems highly attractive because it has a high polarity and boiling point [40]. Glycerol, being highly polar, has the potential for targeted recovery of anthocyanins, where there is minimal chance of other components being extracted. Water/glycerol systems have been considered effective in anthocyanin recovery due to a lowered dielectric constant, aiding in increased pigment diffusion with the solvent [34, 39]. Compared to other organic solvents, such as ethanol and methanol, glycerol is a non-toxic solvent with considerable extraction efficiency [23].
Ultrasonication has been utilised to improve anthocyanin extraction processes in various fruits and waste [14]. Ultrasonication effectively accelerates the chemical reactions through cavitation that causes a structural change internally into the food matrices [13, 57]. Ultrasonic pre-treatments can accelerate extraction, reduce processing times and save energy [21]. On an industrial scale, glycerol can prove to be a safer option due to its lower vapour pressure, and in combination with ultrasonication, it may help in energy-efficient methods [53]. The previous experiments also showed that using ultrasonication with green solvents caused the internal structural changes of food matrices, thus increasing the recovery of anthocyanins and phenols [43]. Yet the research is limited to fully assessing the potential for efficient recovery of anthocyanins utilising energy efficient, time-saving and cost reducing technique and solvent combinations, specifically from the fruit pulp of S. cumini.
The present study uses ultrasound-assisted extraction to enhance recovery and screen conventional solvents (water, ethanol and methanol) against aqueous glycerol. A comparative analysis was conducted of the extracts for various parameters such as antioxidant activity, total anthocyanin and phenolic content and total yield. The main objective is to find an alternative green solvent to extract anthocyanins from the S. cumini fruit pulp.
2 Materials and methods
2.1 Experimentation material
The fruit of S. cumini was collected from a local vendor in the Patiala region of Punjab, India. The extraction solvents glycerol, ethanol and methanol were purchased from the Loba Chemie Pvt. Ltd. company. Distilled water for the extraction was collected from the distillation apparatus setup in the lab. The pulp of S. cumini fruit was separated from seeds, dried and powdered proportionately before use. The anthocyanin content and other assays were determined spectrophotometrically using a UV–Vis spectrophotometer (Shimadzu Scientific Instruments, Japan).
2.2 Ultrasound-assisted anthocyanin extraction using glycerol and comparative analysis
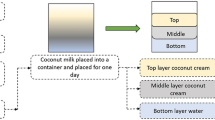
The whole process has been summarized in the figure. S. cumini dried-powdered pulp was weighed (2 g) and placed in a conical flask (solid-solvent ratio, 1: 20). An amount of solvent (40 ml) was added in accordance. The viscosity of the solvent is a critical factor that directly affects the extraction process. The high viscosity of solvents can hinder the diffusion process, leading to lower recovery of anthocyanin or phenolic compounds [16]. Multiple studies have reported that glycerol is a viscous solvent, so 70% aqueous glycerol was used. Higher concentrations would not extract higher yields of anthocyanins [32, 34, 37, 39]. The extraction temperature plays a critical role in the recovery of anthocyanins. Anthocyanins are temperature-sensitive compounds; thus, a lower temperature yields higher anthocyanins, which may further differ based on solvents or extraction techniques [26, 41]. The temperature (25 °C) for the anthocyanin extraction from the sample was kept constant for all the solvents. The pre-treatment of ultrasonication was given for 5 min, and then extraction was performed for 2 hours on an incubator shaker at the mentioned temperature. A comparative study was designed to investigate the influence of green solvent (glycerol) over conventional solvents (water, ethanol, methanol) in the anthocyanin recovery from the fruit pulp of S. cumini. The extracts were then collected and filtered, where filtrate was used to perform further assay procedures.
2.3 Evaluating extract yield
To calculate the extract yield of the sample, the extracts were dried in a hot-air oven to free the extract from the solvent. The total yield (TY) was calculated using the dried crude extract using the formula given in Eq. 1. And expressed as a percentage (%). All measurements were conducted in triplicates.
2.4 Determining total phenolic content (TPC)
The total phenolic content was determined using the Folin–Ciocalteau method Swer et al. (2018) adopted with slight modifications. To 0.2 ml diluted sample, 2.5 ml of Folin–Ciocalteau reagent was added and incubated for 5 min at room temperature. Then 2 ml sodium carbonate (7.5 g/100 ml) was added, mixed and incubated at room temperature for 2 h. After incubation, the mixture was shaken, and the absorbance was measured at 754 nm against distilled water as blank in UV–Vis spectrophotometer.
The results were calculated based on the calibration curve of gallic acid, and the results were expressed as Gallic acid equivalents (mg GAE/100 g).
2.5 Total anthocyanin content (TAC)
The total anthocyanin content of the S. cumini anthocyanins was determined spectrophotometrically using the method given by Giusti & Wrolstad [19] with slight modifications. The aqueous aliquots of test samples were separately diluted with potassium chloride buffer (pH 4.5) and sodium acetate buffer (pH 1.0) and allowed to equilibrate for 1 h at room temperature. The absorbance was measured simultaneously at 530 and 700 nm against a blank (distilled water). The total anthocyanin content expressed as cyanidin-3-glucoside was calculated using the following equation:
where, A is the absorbance which is finalised after finding the difference in absorbances at 530 and 700 nm in different buffers, M.W. is the molecular weight of cyanidin-3-glucosides (449.2 g/mol), DF is the dilution factor, ε is the molar absorptivity of cyanidin-3-glucoside (26,900 L/cm mol), and 1 is the cuvette path length (in cm).
2.6 DPPH antioxidant assay
DPPH radical scavenging assay was performed following the method of Brand-Williams et al. [11]. An aliquot of the extract solution was diluted 1:4 times before estimation to obtain a reading within the spectrophotometer’s linear range. Briefly, 0.1 ml of the diluted sample was treated with 3.9 ml of 0.1 mm methanolic DPPH solution and allowed to stand for 30 min in the dark at 37 °C. The absorbance was recorded at 517 nm immediately against methanol as blank. Per cent inhibition of the DPPH radical by the sample was calculated using the following equation:
where, Asample = absorbance of DPPH on reaction with the sample extract, Ablank = absorbance of DPPH with methanol as blank instead of sample.
2.7 FRAP antioxidant assay
The ferric reducing antioxidant power (FRAP) assay was performed according to the method given by [9] with minor modifications. The sample extract was diluted 1:100 times with distilled water to obtain a reading within the linear range of the spectrophotometer at 593 nm. Briefly, 0.1 ml of the diluted sample was added to 3 ml of FRAP reagent consisting of acetate buffer (300 mM, pH 3.6), 2,4,6-Tripyridyl-s-Triazine-TPTZ (0.031 mg in 10 ml 40 mM HCl) and ferric chloride (20 mM) in the ratio of 10:1:1. After 4 min the absorbance was recorded at 593 nm against FRAP as blank. The results of absorbance values directly indicated the sample’s antioxidant activity.
All the experiments were conducted with three replications and the results reported are mean values recorded (Fig. 1).
3 Results and discussion
3.1 Total extraction yield
The pulp of S. cumini fruit was subjected to four different solvent treatments with varying extraction yields. The yields of extracts using the different solvents are indicated in Fig. 2. Among the solvents used, the maximum extraction yield of 31.9% was obtained for glycerol. The results show that the extract yield is influenced by the solvent adopted for the extraction. The water/glycerol mixture, under similar conditions applied for other solvents, was able to solubilise a higher amount of pigment, leading to a higher extraction yield. This may be attributed to two main factors: firstly, the viscosity of the glycerol solution was lowered with the addition of water, facilitating the solvent penetration into the solid particles of the sample; secondly, the higher polarity of the extraction system assisted in efficient solute–solvent complex transfer into the extract [5]. Moreover, the higher extraction yield could also be associated with the multiple hydrogen bonding networks formed by glycerol and water, which facilitate the extraction of anthocyanins. As glycerol is a highly polar solvent and anthocyanins are also a group of polar flavonoids, their extraction must be prominent with the solvent [58]. Another critical factor in increased yield may be the higher solvent-to-solid ratio, which increases the mass transfer ratio, thus resulting in a higher diffusion rate and maximum extraction yield [28]. A similar study by Özkan et al. [41] reported a 10–15% higher extraction yield with aqueous glycerol compared to ethanol and butanediol from gülfatma flowers. Glycerol is considered to be highly beneficial in maintaining the stability of anthocyanins, which may be due to the multi-hydroxyl group structure of the solvent, thus retaining the higher amount of anthocyanins [20]. Glycerol, via hydrogen bonds, is known to form a cage-like structure in interaction with water, contributing to higher anthocyanin recovery [16]. The extraction yield also depends on extraction conditions, where ultrasonication pre-treatment plays an important role. It is an effective way to accelerate the diffusional process, thus contributing to higher and more efficient recovery of anthocyanins [10]. According to a study by Jovanović et al. [24], who extracted anthocyanins from bilberry fruits using ultrasound with NADES solvent, report a positive influence of sonication for a shorter time, yielding higher anthocyanins.
3.2 Total anthocyanin content
Figure 3 presents the results of anthocyanin content in the extracts obtained with different solvents from the pulp of S. cumini fruit. Anthocyanin content in the pulp of S. cumini fruit varied from 87.94 mg C3G/100 g (ethanol) to 201.05 mg C3G/100 g (glycerol). In the case of solvents, the highest anthocyanin concentrations were noted for water (188.69 mg C3G/100 g) and glycerol (201.05 mg C3G/100 g) at 25 °C. In the presented experiment, glycerol has been identified as the best extraction system for anthocyanins. According to Kowalska et al. [32], water-glycerol extraction systems increased the content of anthocyanins compared to pure water in black chokeberry and elderberry extracts. In the given studies, the authors demonstrated extraction efficiency. The results show that a 70% aqueous glycerol mixture provided a very satisfactory extraction yield in anthocyanins higher than water, ethanol, and methanol. A similar pattern was observed in a study by Soares et al. [51], where the anthocyanin content yield (49.8 mg/g) for aqueous glycerol solution along with ultrasonication was 28.7% higher than water. In agreement with our results, a study of anthocyanin extraction from frozen blueberry honeysuckle by Kaniewska et al. (2013) indicated that water/glycerol extraction systems prove to be better solvents than water. The better efficiency of water-alcohol extraction solvent may result from the affinity of anthocyanins to polar solvents. Glycerol enhances the solubility of more polar flavonoids, resulting in higher recovery [1]. It is also suggested that the intermediate concentration of the solvent, which is around 70% (v/v), is the best condition for the higher recovery of anthocyanins [36].
3.3 Total phenolic content
Figure 4 shows the results for total phenolic content, which was approximately equal for water as a solvent compared to glycerol. The TPC was recorded with the maximum value of 0.998 mg GAE/g dry sample (water), while glycerol led to an equally efficient phenolic extraction of 0.9 mg GAE/g dry sample. Similar to water and methanol, the aqueous glycerol solvent system’s polarity helped to influence the solvent’s affinity towards extracted compounds, thus facilitating the anthocyanin extraction at a suitable ratio [8]. A similar study by [1] explored the efficacy of aqueous glycerol for polyphenolic extraction from rice bran, where they concluded that increasing glycerol volume increased the yield of total phenols. The difference in the polyphenolic extraction efficiency may be due to the varied functionality of glycerol with different plant materials. In a study by [2], acidified glycerol treatments were given to waste orange peels where the yield of total polyphenols achieved was approximately 30% higher than the conventional extraction techniques in a significantly shorter processing time. The glycerol’s viscosity may have slowed the diffusion of phenolics in water/glycerol mixtures compared to pure solvents [42].
3.4 Antioxidant activities (DPPH and FRAP)
The results of the DPPH assay have shown that the higher anthocyanin content is proportionate to the antioxidant capacity, but this is not a general principle [49]. To ascertain the stated fact, the anthocyanin extracts of each solvent were assayed with two antioxidant tests: antiradical activity (DPPH) and reducing power (FRAP).
As can be seen, the experimental values of antiradical activity in terms of the DPPH assay of S. cumini pulp extract are presented in Fig. 5. The DPPH free radical scavenging activity of glycerol (73.35% inhibition) was significantly higher than the conventional solvents. The radical scavenging activity for the conventional solvents was similar (water–70.74, ethanol–70.47 and methanol–70.62% inhibition). The water-glycerol extract displayed a higher antiradical activity, which is proportionate to the higher phenolic and anthocyanin content. These findings concurred with our previous study with NADES (in the communication), where the antiradical activity was proportionate to anthocyanin content. This outcome strongly demonstrated that the antioxidant activity of the extracts varies with the type of solvent used given the same conditions. Anis & Ahmed [4] observed similar results for antiradical activities of phenols and flavonoids from Rumex hastatus extracted with water-glycerol extraction systems, where the highest antiradical activity (90.62%) was obtained with the higher phenolic content. Increasing solvent polarity promotes higher antioxidant extraction [22]
As stated earlier, despite the consolidated concept that higher phenolic content is accompanied by proportionally higher antioxidant capacity, several other investigations highlighted that a correlation between phenolic content and antioxidant activity is not always significant [38]. The differences in anti-radical activity and reducing power may reflect differences in the total amount of polyphenols and interactions amongst them, which may affect the antioxidant activity of the extracts. It has been reported that anthocyanins account for a major part of antioxidant capacity, followed by other phenolic compounds [59]. The results (Fig. 6) showed a proportionate decrease in the reducing power of the extracts between the solvents as opposed to their phenolic content. The water-glycerol extract showed a lower reducing power when compared to the conventional solvents, where the antiradical activity was higher for the same. There is enough significance in the interactions between antioxidant constituents of the extracts, where the higher reducing and antiradical activities may be displayed by the polar fractions.
The differences between the antiradical activity and reducing power may be attributed to the synergism/antagonism mechanism reflecting the differences in interactions amongst the polyphenols, thus affecting the antioxidant activity of extracts [27, 31, 42]. Similar results were observed for antiradical activity and reducing power by Philippi et al. [42] while extracting polyphenols from eggplant peel using aqueous glycerol aided with ultrasonic treatments. The reducing power was quite lower compared to antiradical activity, where the difference between the results for glycerol and ethanol was insignificant. A significant difference between the results of DPPH and FRAP was observed for the phenolic extraction of lotus seedpod using aqueous glycerol, where antiradical was higher for ultrasound with glycerol but reducing power was higher for water bath incubation with glycerol [7].
Summarising the results of the presented study, the efficient extraction of anthocyanins can be alternatively carried out by glycerol as a solvent. It is clear from the results that a higher yield of anthocyanins was obtained compared to the conventional solvents.
4 Conclusion
Anthocyanins, being vacuolar pigments, may not be completely extracted with the conventional techniques of solvent extraction, where the sample may not be able to completely disperse into the substrate, thus making them unavailable for extraction. Using a green extraction process as a pre-treatment, such as ultrasonication, may help reduce the utilisation of organic solvents. Green solvents such as glycerol are natural substances without toxicity, making them a suitable candidate for separating bioactive compounds from food matrixes. This study has validated the efficiency of recovering phenolic compounds, that is, anthocyanins, from S. cumini fruit pulp. For a potential industrial application, such a procedure would be desirable as the production costs would be significantly lowered, and the extracts would not require further processing. They could be directly incorporated into food products. Pre-treatment techniques, such as ultrasound, would lower the time taken, thus increasing the efficiency of the whole process. This investigation may be regarded as the first step in applying an integrative process development approach that provides alternatives for recovering anthocyanins from the pulp of S. cumini fruit. However, the scalability of the extraction methods needs to be explored for commercial success of this method.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Abbreviations
- UAE:
-
Ultrasound-assisted extraction
- NADESs:
-
Natural deep eutectic solvents
- C3G:
-
Cyanidin-3-glucoside
- TAC:
-
Total anthocyanin content
- TPC:
-
Total phenolic content
- DPPH:
-
2, 2-Diphenyl-1-picrylhydrazyl
- FRAP:
-
Ferric reducing antioxidant power
- TPTZ:
-
2,4,6-Tri-pyridyl-s-triazine
- TY:
-
Total yield
References
Aalim H, Belwal T, Jiang L, Huang H, Meng X, Luo Z (2019) Extraction optimization, antidiabetic and antiglycation potentials of aqueous glycerol extract from rice (Oryza sativa L.) bran. LWT 103:147–154. https://doi.org/10.1016/J.LWT.2019.01.006
Abdoun R, Grigorakis S, Kellil A, Loupassaki S, Makris DP (2022) Process optimization and stability of waste orange peel polyphenols in extracts obtained with organosolv thermal treatment using glycerol-based solvents. ChemEngineering 6(3):35. https://doi.org/10.3390/chemengineering6030035
Albuquerque BR, Pinela J, Pereira C, Calhelha RC, Oliveira I, Heleno S, Oliveira MBPP, Barros L (2023) Optimization and comparison of heat- and ultrasound-assisted extraction methods for anthocyanin recovery from sicana odorifera fruit epicarp. Biomass Conversion and Biorefinery. https://doi.org/10.1007/s13399-023-05097-6
Anis N, Ahmed D (2022) Modelling and optimization of polyphenol and antioxidant extraction from rumex hastatus by green glycerol-water solvent according to response surface methodology. Heliyon 8(12):e11992. https://doi.org/10.1016/J.HELIYON.2022.E11992
Apostolakis A, Grigorakis S, Makris DP (2014) Optimisation and comparative kinetics study of polyphenol extraction from olive leaves (Olea europaea) using heated water/glycerol mixtures. Sep Purif Technol 128:89–95. https://doi.org/10.1016/J.SEPPUR.2014.03.010
Aqil F, Gupta A, Munagala R, Jeyabalan J, Kausar H, Sharma RJ, Singh IP, Gupta RC (2012) Antioxidant and antiproliferative activities of anthocyanin/ellagitannin–enriched extracts from Syzygium cumini L. (Jamun, the Indian Blackberry). Nutr Cancer 64(3):428–438. https://doi.org/10.1080/01635581.2012.657766
Bao N, Wang D, Fu X, Xie H, Gao G, Luo Z (2021) Green extraction of phenolic compounds from lotus seedpod (receptaculum nelumbinis) assisted by ultrasound coupled with glycerol. Foods 10(2):239. https://doi.org/10.3390/foods10020239
Belwal T, Huang H, Li L, Duan Z, Zhang X, Aalim H, Luo Z (2019) Optimization model for ultrasonic-assisted and scale-up extraction of anthocyanins from pyrus communis ‘starkrimson’ fruit peel. Food Chem 297:124993. https://doi.org/10.1016/J.FOODCHEM.2019.124993
Benzie IFF, Strain JJ (1996) The ferric reducing ability of plasma (frap) as a measure of “antioxidant power”: the frap assay. Anal Biochem 239(1):70–76. https://doi.org/10.1006/abio.1996.0292
Brahmi F, Blando F, Sellami R, Mehdi S, De Bellis L, Negro C, Haddadi-Guemghar H, Madani K, Makhlouf-Boulekbache L (2022) Optimization of the conditions for ultrasound-assisted extraction of phenolic compounds from opuntia ficus-indica [L.] Mill. flowers and comparison with conventional procedures. Ind Crops Prod 184:114977. https://doi.org/10.1016/j.indcrop.2022.114977
Brand-Williams W, Cuvelier ME, Berset C (1995) Use of a free radical method to evaluate antioxidant activity. LWT—Food Science and Technology 28(1):25–30. https://doi.org/10.1016/S0023-6438(95)80008-5
Câmara JS, Locatelli M, Pereira JAM, Oliveira H, Arlorio M, Fernandes I, Perestrelo R, Freitas V, Bordiga M (2022) Behind the scenes of anthocyanins—from the health benefits to potential applications in food. Pharmaceutical and Cosmetic Fields Nutrients 14(23):5133. https://doi.org/10.3390/nu14235133
Das P, Nayak PK, Kesavan krishnan R (2022) Ultrasound assisted extraction of food colorants: principle, mechanism, extraction technique and applications: a review on recent progress. Food Chemistry Advances 1:100144. https://doi.org/10.1016/J.FOCHA.2022.100144
Demirdöven A, Özdoğan K, Erdoğan-Tokatli K (2015) Extraction of anthocyanins from red cabbage by ultrasonic and conventional methods: optimization and evaluation. J Food Biochem 39(5):491–500. https://doi.org/10.1111/JFBC.12153
dos Santos FN, de Souza EJD, Jéssica Siebeneichler T, Buchveitz Pires J, Hüttner Kringel D, Dillenburg Meinhart A, Renato Guerra Dias A, da Rosa ZE (2022) Multivariate analysis as tool for optimization of anthocyanins extraction from jambolan (Syzygium cumini L.). Food Anal Methods 15(9):2524–2536. https://doi.org/10.1007/S12161-022-02313-3/TABLES/5
Fu X, Du Y, Zou L, Liu X, He Y, Xu Y, Li L, Luo Z (2022) Acidified glycerol as a one-step efficient green extraction and preservation strategy for anthocyanin from blueberry pomace: new insights into extraction and stability protection mechanism with molecular dynamic simulation. Food Chem 390:133226. https://doi.org/10.1016/J.FOODCHEM.2022.133226
Gaibor FM, Rodríguez D, García MA, Casariego A (2024) Influence of a natural colorant powder from Syzygium Cumini L. (Skeels) on sensory and physicochemical properties during storage of a heat-treated flavored fermented milk. Bionatura 9(1):1–10. https://doi.org/10.21931/RB/2024.09.01.59
Ghosh S, Sarkar T, Chakraborty R, Shariati MA, Simal-Gandara J (2024) Nature’s palette: an emerging frontier for coloring dairy products. Crit Rev Food Sci Nutr 64(6):1508–1552. https://doi.org/10.1080/10408398.2022.2117785
Giusti MM, Wrolstad RE (2001). Characterization and Measurement of Anthocyanins by UV-Visible Spectroscopy. Current Protocols in Food Analytical Chemistry, 00(1), F1.2.1-F1.2.13. https://doi.org/10.1002/0471142913.FAF0102S00
Guo N, Ping-Kou Jiang YW, Wang LT, Niu LJ, Liu ZM, Fu YJ (2019) Natural deep eutectic solvents couple with integrative extraction technique as an effective approach for mulberry anthocyanin extraction. Food Chem 296:78–85. https://doi.org/10.1016/J.FOODCHEM.2019.05.196
Hilali S, Wils L, Chevalley A, Clément-Larosière B, Boudesocque-Delaye L (2022) Glycerol-based NaDES as green solvents for ultrasound-assisted extraction of phycocyanin from arthrospira platensis—RSM optimization and ANN modelling. Biomass Conversion and Biorefinery 12(S1):157–170. https://doi.org/10.1007/s13399-021-02263-6
Iqbal S, Bhanger MI, Anwar F (2005) Antioxidant properties and components of some commercially available varieties of rice bran in Pakistan. Food Chem 93(2):265–272. https://doi.org/10.1016/J.FOODCHEM.2004.09.024
Jabłonowska M, Ciganović P, Jablan J, Marguí E, Tomczyk M, Zovko Končić M (2021) Silybum marianum glycerol extraction for the preparation of high-value anti-ageing extracts. Ind Crops Prod 168:113613. https://doi.org/10.1016/J.INDCROP.2021.113613
Jovanović MS, Krgović N, Živković J, Stević T, Zdunić G, Bigović D, Šavikin K (2022) Ultrasound-assisted natural deep eutectic solvents extraction of bilberry anthocyanins: optimization, bioactivities, and storage stability. Plants 11(20):2680. https://doi.org/10.3390/PLANTS11202680
Jurić S, Jurić M, Król-Kilińska Ż, Vlahoviček-Kahlina K, Vinceković M, Dragović-Uzelac V, Donsì F (2022) Sources, stability, encapsulation and application of natural pigments in foods. Food Rev Intl 38(8):1735–1790. https://doi.org/10.1080/87559129.2020.1837862
Karakashov B, Grigorakis S, Loupassaki S, Makris DP (2015) Optimisation of polyphenol extraction from hypericum perforatum (St. John’s Wort) using aqueous glycerol and response surface methodology. Journal of Applied Research on Medicinal and Aromatic Plants 2(1):1–8. https://doi.org/10.1016/j.jarmap.2014.11.002
Karvela E, Makris DP, Karathanos VT (2012) Implementation of response surface methodology to assess the antiradical behaviour in mixtures of ascorbic acid and α-tocopherol with grape (Vitis vinifera) stem extracts. Food Chem 132(1):351–359. https://doi.org/10.1016/J.FOODCHEM.2011.10.091
Katsampa P, Valsamedou E, Grigorakis S, Makris DP (2015) A green ultrasound-assisted extraction process for the recovery of antioxidant polyphenols and pigments from onion solid wastes using box-behnken experimental design and kinetics. Ind Crops Prod 77:535–543. https://doi.org/10.1016/J.INDCROP.2015.09.039
Kaur D, Yousuf B, Qadri OS (2024) Syzygium cumini anthocyanins: recent advances in biological activities, extraction, stability, characterisation and utilisation in food systems. Food Production, Processing and Nutrition 6(1):34. https://doi.org/10.1186/s43014-023-00177-6
Kaur N, Aggarwal P, Kumar V, Kaur S (2022) Ultrasound–Assisted Extraction of Phytochemicals from Java Plum (Syzygium cumini L.) Pomace: Process Optimization, Phytochemical Characterization Using HPLC, FTIR, SEM and Mineral Profiling. Waste and Biomass Valorization. https://doi.org/10.1007/s12649-022-01915-6
Khiari Z, Makris DP, Kefalas P (2009) An investigation on the recovery of antioxidant phenolics from onion solid wastes employing water/ethanol-based solvent systems. Food Bioprocess Technol 2(4):337–343. https://doi.org/10.1007/S11947-007-0044-8/FIGURES/2
Kowalska G, Wyrostek J, Kowalski R, Pankiewicz U (2021) Evaluation of glycerol usage for the extraction of anthocyanins from black chokeberry and elderberry fruits. Journal of Applied Research on Medicinal and Aromatic Plants 22:100296. https://doi.org/10.1016/j.jarmap.2021.100296
Kumar S, Sharma S, Kumar V, Sharma A, Kaur R, Saini R (2023) Jamun (Syzygium cumini (L.) skeels): the conventional underutilized multifunctional plant-an exotic gleam into its food and functional significance. Ind Crops Prod 191:115873. https://doi.org/10.1016/j.indcrop.2022.115873
Kyriakidou K, Mourtzinos I, Biliaderis C, Makris D (2016) Optimization of a green extraction/inclusion complex formation process to recover antioxidant polyphenols from oak acorn husks (quercus robur) using aqueous 2-hydroxypropyl-β-cyclodextrin/glycerol mixtures. Environments 3(1):3. https://doi.org/10.3390/environments3010003
Li Y, Fabiano-Tixier AS, Tomao V, Cravotto G, Chemat F (2013) Green ultrasound-assisted extraction of carotenoids based on the bio-refinery concept using sunflower oil as an alternative solvent. Ultrason Sonochem 20(1):12–18. https://doi.org/10.1016/j.ultsonch.2012.07.005
Madalão MCM, Lima EMF, Benincá DB, Saraiva SH, de Carvalho RV, Silva PI (2021) Extraction of bioactive compounds from juçara pulp (Euterpe edulis M.) is affected by ultrasonic power and temperature. Ciência e Agrotecnologia 45:e024820. https://doi.org/10.1590/1413-7054202145024820
Makris DP, Lalas S (2020) Glycerol and glycerol-based deep eutectic mixtures as emerging green solvents for polyphenol extraction: the evidence so far. Molecules 25(24):5842. https://doi.org/10.3390/molecules25245842
Moharram HA, Youssef MM (2014) Methods for determining the antioxidant activity: a review. Alexandria Journal of Food Science and Technology 11(1):31–42. https://doi.org/10.12816/0025348
Mourtzinos I, Anastasopoulou E, Petrou A, Grigorakis S, Makris D, Biliaderis CG (2016) Optimization of a green extraction method for the recovery of polyphenols from olive leaf using cyclodextrins and glycerin as co-solvents. J Food Sci Technol 53(11):3939–3947. https://doi.org/10.1007/s13197-016-2381-y
Mourtzinos I, Prodromidis P, Grigorakis S, Makris DP, Biliaderis CG, Moschakis T (2018) Natural food colorants derived from onion wastes: application in a yoghurt product. Electrophoresis 39(15):1975–1983. https://doi.org/10.1002/elps.201800073
Özkan A, Zannou O, Pashazadeh H, Koca I (2023) Application of biosolvents for the extraction of anthocyanins from gülfatma flowers (Alcea apterocarpa (Fenzl) Boiss): optimization and stability approaches. Biomass Conversion and Biorefinery 1:1–17. https://doi.org/10.1007/S13399-022-03730-4/FIGURES/6
Philippi K, Tsamandouras N, Grigorakis S, Makris DP (2016) Ultrasound-assisted green extraction of eggplant peel (Solanum melongena) polyphenols using aqueous mixtures of glycerol and ethanol: optimisation and kinetics. Environmental Processes 3(2):369–386. https://doi.org/10.1007/S40710-016-0140-8/TABLES/7
Rahaman A, Zeng XA, Kumari A, Rafiq M, Siddeeg A, Manzoor MF, Baloch Z, Ahmed Z (2019) Influence of ultrasound-assisted osmotic dehydration on texture, bioactive compounds and metabolites analysis of plum. Ultrason Sonochem 58:104643. https://doi.org/10.1016/J.ULTSONCH.2019.104643
Rodríguez-Mena A, Ochoa-Martínez LA, González-Herrera SM, Rutiaga-Quiñones OM, González-Laredo RF, Olmedilla-Alonso B (2023) Natural pigments of plant origin: classification, extraction and application in foods. Food Chem 398:133908. https://doi.org/10.1016/j.foodchem.2022.133908
Ruesgas-Ramón M, Figueroa-Espinoza MC, Durand E (2017) Application of Deep Eutectic Solvents (DES) for phenolic compounds extraction: overview, challenges, and opportunities. J Agric Food Chem 65(18):3591–3601. https://doi.org/10.1021/acs.jafc.7b01054
Safriani N, Husna NE, Tamamy MM (2024) Application of ultrasound-assisted extraction of anthocyanin from syzygium cumini fruit. IOP Conference Series: Earth and Environmental Science 1290(1):012048. https://doi.org/10.1088/1755-1315/1290/1/012048
Sahu PP, Behera L, Nayak S, Samal KC (2020) Health benefits of jamun (Syzygium cumini) an underutilised fruit: a ray in nanotechnology field. Journal of Pharmacognosy and Phytochemistry 9(5):74–80
Sharma M, Dash KK (2022) Microwave and ultrasound assisted extraction of phytocompounds from black jamun pulp: Kinetic and thermodynamics characteristics. Innov Food Sci Emerg Technol 75:102913. https://doi.org/10.1016/j.ifset.2021.102913
Shehata E, Grigorakis S, Loupassaki S, Makris DP (2015) Extraction optimisation using water/glycerol for the efficient recovery of polyphenolic antioxidants from two artemisia species. Sep Purif Technol 149:462–469. https://doi.org/10.1016/j.seppur.2015.06.017
Shirsath SR, Sonawane SH, Gogate PR (2012) Intensification of extraction of natural products using ultrasonic irradiations—a review of current status. Chem Eng Process 53:10–23. https://doi.org/10.1016/j.cep.2012.01.003
Soares BP, Ferreira AM, Justi M, Rodrigues LGG, Oliveira JV, Pinho SP, Coutinho JAP (2023) Juçara fruit (Euterpe Edulis Martius) valorization combining emergent extraction technologies and aqueous solutions of alkanediols. Molecules 28(4):1607. https://doi.org/10.3390/MOLECULES28041607/S1
Swami SB, Kalse SB (2020) Bioactive compounds in jamun (Syzygium cumini L.). The Pharma Innovation Journal 9(11):161–167 (http://www.thepharmajournal.com)
Vieira V, Calhelha RC, Barros L, Coutinho JAP, Ferreira ICFR, Ferreira O (2020) Insights on the extraction performance of alkanediols and glycerol: using Juglans regia L. leaves as a source of bioactive compounds. Molecules 25(11):2497. https://doi.org/10.3390/molecules25112497
Wadibhasme U, Athmaselvi KA, Hemke J, Kalkote S (2020) Development and evaluation of Jamun (Syzygium cumini) fortified “Instant drink mix” by utilizing the spray drying technique. The Pharma Innovation Journal 9(4):103–110
Wolfson A, Dlugy C, Shotland Y (2007) Glycerol as a green solvent for high product yields and selectivities. Environ Chem Lett 5(2):67–71. https://doi.org/10.1007/S10311-006-0080-Z/TABLES/2
Xue H, Shen L, Wang X, Liu C, Liu C, Liu H, Zheng X (2019) Isolation and purification of anthocyanin from blueberry using macroporous resin combined sephadex LH-20 techniques. Food Science and Technology Research 25(1):29–38. https://doi.org/10.3136/fstr.25.29
Yusoff IM, Mat Taher Z, Rahmat Z, Chua LS (2022) A review of ultrasound-assisted extraction for plant bioactive compounds: phenolics, flavonoids, thymols, saponins and proteins. Food Res Int 157:111268. https://doi.org/10.1016/J.FOODRES.2022.111268
Zannou O, Koca I (2022) Greener extraction of anthocyanins and antioxidant activity from blackberry (Rubus spp) using natural deep eutectic solvents. LWT 158:113184. https://doi.org/10.1016/j.lwt.2022.113184
Zhao X, Yuan Z (2021) Anthocyanins from pomegranate anthocyanins from pomegranate. Chem Biodivers 18(10):e2100399. https://doi.org/10.1002/cbdv.202100399
Funding
The authors would like to thank Dean of Research and Development Cell, Thapar Institute of Engineering and Technology, Patiala for providing funding under SEED Grant (TU/DORSP/57) to undertake this study. Thapar Institute of Engineering and Technology, TU/DORSP/57, Ovais Shafiq Qadri.
Author information
Authors and Affiliations
Contributions
Darshanjot Kaur: Writing and experimentation. Ovais Shafiq Qadri: Conceptualization, review, and correspondence.
Corresponding author
Ethics declarations
Conflict of interest statement
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kaur, D., Qadri, O.S. Green extraction of anthocyanins from Syzygium cumini fruit pulp using aqueous glycerol through ultrasound-assisted extraction. J.Umm Al-Qura Univ. Appll. Sci. (2024). https://doi.org/10.1007/s43994-024-00152-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43994-024-00152-y