Abstract
In order to achieve global carbon neutrality in the middle of the 21st century, efficient utilization of fossil fuels is highly desired in diverse energy utilization sectors such as industry, transportation, building as well as life science. In the energy utilization infrastructure, about 75% of the fossil fuel consumption is used to provide and maintain heat, leading to more than 60% waste heat of the input energy discharging to the environment. Types of low-grade waste heat recovery technologies are developed to increase the energy efficiency. However, due to the spatial and temporal mismatch between the need and supply of the thermal energy, much of the waste thermal energy is difficult to be recovered. Thermal energy storage (TES) technologies in the forms of sensible, latent and thermochemical heat storage are developed for relieving the mismatched energy supply and demand. Diverse TES systems are developed in recent years with the superior features of large density, long-term, durable and low-cost. These technologies are vital in efficient utilization of low-grade waste heat and expected for building a low or zero carbon emission society. This paper reviews the thermal storage technologies for low carbon power generation, low carbon transportation, low carbon building as well as low carbon life science, in addition, carbon capture, utilization, and storage are also considered for carbon emission reduction. The conclusion and perspective are raised after discussing the specific technologies. This study is expected to provide a reference for the TES technologies in achieving zero-carbon future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
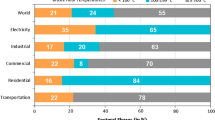
Due to the intense carbon emission since industrial revolution, the carbon dioxide concentration in the atmosphere rapidly increases from 280 to 420 ppm within a short period of ~ 300 years. Due to the high transmittance in the solar wavelength and low absorbance in the far infrared wavelength range, the greenhouse effect occurs and contributes to a large increment in global temperature. The global temperature increment leads to severe consequences such as glacier melting, sea level rise and climate anomaly. In order to avoid the consequences that threaten the human survival, it is necessary to reduce the emission of greenhouse gases (GHG) in all sectors of the world. To ensure the global temperature rise less than 2 °C, carbon net zero emission should be achieved at the second half of the twenty-first century [213]. In order to make the global temperature rise less than 1.5 °C, carbon zero emissions must be achieved at around 2050 [37]. According to the global GHG emissions by sector in 2016, the global CO2 emissions are about 49.4 billion tons, of which the GHG emissions from industrial energy consumption account for the major part, including iron & steel, chemical & petrochemical, food & tobacco, non-ferrous metals and other manufacturing industries. The large portion of CO2 emission from industrial process is due to the heavy dependence on thermal energy from fossil fuel combustion. The second largest CO2 emission is from building sector, of which residential emissions account for 10.9% and commercial building emissions account for 6.6%. Among them, domestic heating, cooling and hot water supply of residential buildings account for 70% of the energy consumption of buildings [10]. The third largest CO2 emission is from the transportation sector, which mainly includes road transportation, aviation transportation and shipping transportation, accounting for 11.9%, 1.9% and 1.7% respectively. Although electric driven transportation is boosted in recent years, the combustion of fossil fuels is still the major measure for transportation energy supply.
From the above analysis one can find that no matter in industry, building, or transportation sectors, the carbon emissions are mainly from the combustion of fossil fuel for providing thermal energy. Due to the massive demand on thermal energy in the aforementioned energy utilization sectors, the dependence on fossil fuels could not be replaced in a short period of time. However, the utilization of thermal energy would lead to severe heat loss inevitably. The heat loss appears in the forms of exhaust gas from the chimney of thermal power plant, convection and radiation heat loss from the surface of equipment and thermal pipelines, as well as the exhaust gas from gas turbine and internal combustion engines. It is reported that more than 60% of the energy consumption is lost as low-grade thermal energy. The efficient utilization of the low-grade waste thermal energy could significantly reduce the utilization of fossil fuels and contribute to the development of a carbon neutrality. Diverse technologies for recycling the low-grade waste thermal energy are developed including organic rankine cycle power generation system [214], heat pumps (HP) [275], solid-state power converters (thermoelectric [73], thermoelectrochemical cells [43], thermo-osmotic systems [255], thermally regenerative cells [120] and so on. The utilization of the above heat recovery technologies could increase the overall efficiency of fossil fuel combustion to a certain extent. However, the thermal energy could not be recycled by these technologies in some occasions. This could be attributed to three reasons. First, the low-grade waste thermal energy is typically discontinuous. The discontinuous thermal energy supply leads to adverse operating condition for the organic rankine cycle system,Second, the location mismatch between supply and demand of thermal energy. The low-grade waste thermal energy is typically generated in industrial zone where is rich of thermal energy. However, the demand on low-grade thermal energy typically happens in urban zones. The location mismatch induces heat loss and cost-efficiency problems for waste energy recovery,Third, the timing mismatch between the supply and demand of thermal energy. For example, the low-grade waste thermal energy is typically generated in the industrial process during daytime, while the demand on waste thermal energy is typically at night for domestic heating purposes. These three reasons severely limit the wide application of waste thermal energy recovery technologies and hinder the overall conversion efficiency of the fossil fuel chemical energy. A technology ensures the highly efficient utilization of the low-grade waste thermal energy is highly desired.
TES is considered to be a technology that can solve the problems of heat source discontinuity as well as location and timing mismatch between heat source and heat load for efficiently recover the thermal energy from both industrial and renewable resources [14, 96]. In the past decades, the research on TES is boosting [170, 260] but the commercialization shows a slow pace. This is mainly attributed to the issues raised from the cost, technical maturity as well as policy guidance [16, 154]. The high-grade thermal energy is typically recycled in the industry with good payback period and economic benefits. As a contrary, the recycle on low-grade thermal energy is less attractive. However, with the urgent goal for achieving carbon neutrality globally, diverse policies (e.g. carbon emission tax and peak-valley price on electricity) have been made for charging excess carbon emission. These policies induce more worthwhile efforts on recycling the low-grade thermal energy.
On the other hand, the development on renewable sources (e.g. solar and wind energy) is equally important as compared to waste energy recovering for contributing to a low-carbon emission society. However, the solar and wind energy face the same discontinuity issues as the waste energy. Storage units are essential for the efficient utilization of renewable energy. There are diverse commercial storage technologies including [173], such as compressed air energy storage [299, 300], flywheel energy storage [49], pumped hydro energy storage [202], battery energy storage [28], hydrogen storage [36], TES [194] and the novelty carnot battery [164]. The energy storage efficiency, density, cost and other parameters of common energy storage methods are shown in Table 1. From the viewpoints of storage scale, capacity and cost, TES system with the scale of hundreds of MWh, capacity up to several months and cost of energy [123] as low as 0.1 €/kWh is attractive among the storage technologies.
TES has shown great potential in the applications of renewable sources development as well as low-grade energy recovery, which are the two important contents in achieving carbon neutrality. In addition, the IPCC (Intergovernmental Panel on Climate Change) special report on global warming of 1.5 °C states that CCUS (Carbon Capture Utilization and Storage) technology can effectively improve the global climate change, and it is clearly pointed out that CCUS technology is significant to achieve zero carbon emissions by 2050. The combination of CCUS and TES will be the most effective and direct means of carbon emission reduction, with great emission reduction potential, wide application scenarios and high potential benefits. Although there have been many reviews on TES technology, most of them focus on technical aspects such as material improvement, equipment design and system optimization. None of the published review article is from the viewpoint of TES contribution in achieving a carbon neutrality. This article reviews the TES technology in achieving zero-carbon power generation, zero-carbon transportation, zero-carbon building, zero-carbon life science and zero-carbon society, as shown in Fig. 1. The aforementioned energy consumption sectors require different temperature range thermal storage materials and systems. The sensible, latent and thermochemical thermal storage materials and systems for diverse temperature ranges are also compared for clarifying the pros and cons. This paper firstly reviews the classification of TES and the mechanism of energy storage (Section 2). According to the type of thermal storage, the applications of using sensible heat storage (SHS) for developing a zero-carbon future is summarized in Section 3, followed by using latent heat storage (LHS) in Section 4 and thermochemical heat storage (TCHS) in Section 5. The future requirements on TES technology for achieving carbon neutrality is discussed in Section 6. Finally, the conclusions and perspectives are provided in Section 7. This review is beneficial for subsequent researchers and engineers to design a more efficient and more reasonable TES system suitable for developing a zero-carbon future.
2 Overview of TES technologies
TES is a heat storage technology that collects, stores and releases heat with relatively large capacity. This feature allows the feasible integration of TES with diverse energy systems such as solar energy, wind energy, geothermal energy and industrial waste heat. With the difference in storage mechanism, TES can be classified as SHS, LHS and TCHS.
2.1 SHS
The heat storage capacity of SHS is related to the mass, specific heat capacity and temperature change of the heat storage medium. During the entire heat storage process, the temperature of the storage medium would increase or decrease to store or release heat. Therefore, the amount of heat stored could be expressed as:
According to formula (1), the stored thermal energy is proportional to the mass, specific capacity and temperature variation of the thermal storage material. Considering the specific capacity, cost and temperature range, solid and liquid are generally chosen as the heat storage medium. Solid materials (temperature changes over 100 °C) such as non-metallic sand, gravel [125], concrete [131], soil bedrock [99, 100] and high-temperature metal materials are typically employed as high-temperature thermal storage medium, while water [84], aquifer, various heat transfer oils [233] and high-temperature molten salts [23], can typically employed for different temperature range thermal storage in liquid materials. In the heat storage process, SHS materials typically have good thermal reliability and chemical stability after repeated thermal cycles. At the same time, the SHS materials are also limited by the relatively larger size of the container. The following Table 2 shows the advantage and disadvantage applications of some representative SHS materials, which have been widely used in commercial and domestic applications.
2.2 LHS
LHS has become a hot topic of research in recent years. In the initial stage of heat storage, the same as the SHS, as the temperature of the heat storage material increases, the heat absorbed gradually increases, but the difference is that when the temperature reaches the phase transition point, the heat storage material continues to absorb heat without further increment of temperature during phase transition process. After the phase transition is complete, the temperature continues to increase as SHS. Therefore, the amount of heat stored could be expressed as:
Although there also exists sensible heat during the whole thermal storage process, the whole heat storage process is dominated by the latent heat value \(\Delta H\) since the heat of fusion is generally several times to the sensible heat. The heat storage medium is called phase change material (PCM), and the PCM is divided into solid–solid, solid–liquid, solid–gas and liquid–gas according to the type of phase transition. Among them, the solid–solid phase transition mainly refers to the change of the internal lattice structure of the molecule, and the latent heat of phase transition is generally much lower than that of the solid–liquid phase transition process. For solid–gas and liquid–gas phase transition, the volume change before and after the phase transition is severe, thus leading to few practical applications in industry. The solid–liquid phase transition has the characteristics of large value of latent heat, small volume change during phase change, and wide range of phase transition temperature. The types of PCMs are shown in Fig. 2 including organic PCMs [244, 308], inorganic PCMs and eutectic PCMs. The organic PCMs can be classified as paraffins, sugar alcohols, fatty acids and esters. The inorganic PCMs [137] can be classified as crystalline hydration salts, molten salts, metals and alloys. The eutectic PCMs [156, 158, 160, 235, 296], can be synthesized between organic-organic, organic–inorganic, and inorganic-inorganic PCMs. Although solid–liquid PCMs have been developed for many years, have shown features of high heat storage density, and have already been put into commercial applications, there are still some problems in the process of energy storage [104, 105, 176]. Table 3 shows the advantages and disadvantages of using all kinds of PCM in practical applications. The problems mainly include low thermal conductivity, poor cycling performance, ease for leakage and corrosion and so on. In response to this problem, many measures have been taken to improve the overall performance of the PCM, which are well discussed in the review articles [216, 264, 286].
2.3 TCHS
TCHS can be further divided into chemical reaction heat storage, ad/absorption heat storage [294]. For chemical reaction energy storage, it mainly utilizes chemical bond formation and bond breaking in forward/reverse reactions of chemical reactions to achieve thermal storage or release. The adsorption heat storage can be defined as the adsorbent in the condensed state, through physical or chemical adsorption to fix and capture the adsorbate. The formula for heat storage in a chemical reaction is as follows:
According to the chemical heat storage formula (3), this TES technology relies on the chemical reaction. The process of combining the two separately stored reactants A and B through chemical bonds to form a single product is accompanied by heat release. When the thermal energy is stored, the product AB absorbs heat and decomposes to products A and B through the decomposition reaction. The products could be kept separately for long-term storage. Common chemical reaction energy storage methods such as carbonate decomposition [19, 178], metal hydride hydrogen production [225, 246], ammonia synthesis and methane reforming [71, 72] have shown large heat storage capacity and long heat storage duration. The TCHS system typically shows a 2–5 times larger specific storage capacity as compared to LHS and 8–10 times larger to SHS [307]. However, such systems require higher activation energy before heat storage. For adsorption heat storage, the capture and adsorption of adsorbate by solid or liquid matrix materials, such as zeolite and silica gel systems [106, 130]. Liquid absorption such as solution of Lithium salt [57], sodium hydroxide [279], etc., which also can be employed for TES. In practical applications, Table 4 provides a more detailed comparison and analysis of TCHS.
Among the three TES methods, SHS has the highest maturity, stable cycle, low cost, wide source availability. Besides, the system is simple and easy to be implemented. The disadvantage is that it is greatly affected by environmental factors. Consideration on thermal insulation measures need to be taken. Only short-term heat storage can be performed, and the amount of heat storage is affected by the volume of the system. LHS has gradually begun to be used due to its advantages of high heat storage density, wide phase transition temperature range, constant phase transition temperature, and large heat storage per unit volume. The disadvantage is that the cycle stability is poor, especially the inorganic PCMs have supercooling and phase separation phenomena. The energy storage density per unit volume of TCHS is about 8 to 10 times that of SHS, and more than twice that of LHS. It also has the advantages of long heat storage duration and less heat loss. However, larger reactor equipment and storage tanks are required for thermal storage for thermochemical reactions. Compared with the other two heat storage methods, TCHS is seldom used commercially due to its complex operation, high reaction cost constraints, and poor cycling performance [191, 227]. In Table 5, we summarize and compare the advantages and disadvantages of three different TES in more detail, such as energy storage density, efficiency, cost and technology maturity.
3 SHS in achieving carbon neutrality
This section reviews the SHS technology in the practical applications for achieving carbon neutrality. The employment of using SHS for zero-carbon power generation, zero-carbon building, zero-carbon heating and zero-carbon transportation is reviewed. Issues with material selection, system integration and durability are discussed.
3.1 SHS in zero-carbon power generation
SHS is typically employed in the concentration solar (photothermal) power (CSP) which target for a great reduction in carbon emission during the power generation process. The International Energy Agency (IEA) has set a target to generate about 630 GW of electricity by 2050 using CSP technology [97]. As shown in Fig. 3, the main idea of a CSP system is to collect the solar energy from the arrays of heliostat for photothermal conversion. The heated receiver with a temperature of over 600 °C could be employed for power generation via either Rankine cycle or Brayton cycle. The CSP system typically operates either in a direct or indirect mode. SHS systems are vital in both the direct steam power generation (DSPG) and the indirect steam power generation (ISPG). The SHS system with storage capability is able to bring part of the photothermal energy from daytime to the night to achieve continuous power generation which is important for pursuing high energy efficiency, better load matching as well as long lifespan of the power generation system. Other than the concentration solar energy, the SHS could also be employed in industrial waste recovery power generation system, for storing and balancing the fluctuating waste heat source. In these systems, the selected SHS material is mainly high-temperature molten salt, liquid metal, heat transfer oil, concrete, rock, solid particles, steam, etc., which have the characteristics of high thermal conductivity, high boiling point, low melting point, high heat capacity, high thermal stability, low cost, and low corrosiveness to metal containers [203].
Schematic diagram of a concentration solar power system, with permissions requested from [272]
3.1.1 DSPG
In a DSPG system, the SHS material serves both as a heat storage medium as well as a heat transfer fluid (HTF). In such systems, the tower like solar concentration system is typically employed and the receiver temperature could be greater than 500 °C. The heat transfer oil cannot be employed for its relatively low decomposition temperature (< 400 °C). Under this circumstance, liquid phase SHS (molten nitrate salts [272], fluidized particles [65, 129], and liquid metal [61]) are typically employed.
The dual functions of the SHS as well as HTF material are favorable for reducing the system complexity and the construction cost. The first molten salt based DSPG system was introduced in 1984 [71, 72]. With a system configuration as shown in Fig. 4a [67, 254], the thermal energy collected from the tower-type concentration system is transferred to the molten salt. Molten salts as both HTF and SHS material, which the feed temperature is 290 °C and the molten salt is heated to over 565 °C after heat transfer with the high temperature receiver. The heated molten salt flows to the steam generator to power the Rankine cycle via a TES tank. Figure 4b shows the two-tank molten salt TES system of the Andasol 350 MW CSP plant in Spain, which contains ~ 28,500 metric tons Solar Salt for 7.5 h storage [67]. The heat storage process using high-temperature fluidized particles as HTF is shown in Fig. 5. The solar radiation is collected and the heat is exchanged with the fluidized particles through the wall of the vertically placed tubular absorber. Silicon carbide is typically employed as the fluidized particles with the features of high-dense particle suspension and unstable fluidization to improve heat transfer in the fluidized bed [228], which is favorable in obtaining an increased system efficiency. In addition to silicon carbide as fluid particles, Minerva Díaz-Heras et al. [64] also tested sand, carbo Accucast ID50 and SiC particles for CSP. The results show that the main attraction of SiC and carbo is their very high absorptivity, around 0.9 or more than 0.9, which ensures a higher ability to absorb direct solar radiation, thereby increasing the temperature and efficiency of the system. While sand is an abundant material, 4–5 times cheaper than SiC and carbo, it can be transported/fluidized at lower pumping costs. Other than the molten salts and fluidized particles, low melting point liquid metal material has become an attractive heat transfer medium widely used in CSP systems due to its unique properties such as low melting point, high thermal conductivity, high latent heat, non-flammability, and non-toxicity [61]. In recent years, Salyan proposed [229] a method of adding liquid metal Ga plug-in to the solar energy storage system to improve the heat storage capacity. Ga's high thermal conductivity, low specific heat, and its liquidus temperature facilitate Ga's use as a thermal energy carrier in the TES device, and a maximum power output of 0.64 kW was obtained during the curing cycle. In addition to liquid SHS material, steam accumulator is typically employed for waste heat recovery from industry. The steam accumulator is a heat storage device that directly stores high-temperature steam at higher pressure and releases lower pressure steam when needed. In January 2016, only two commercial tower power plants using steam accumulator thermal energy storage were in operation: PS10 and PS20, located in Spain, became the first two commercial solar towers in the world [201]. The first generation of CSP columns used saturated steam technology (Fig. 6a). The PS10 storage system provides 20 MWh of storage capacity, during plant operation steam is generated in the receiver and sent to a turbine where it expands to generate mechanical work and electricity, excess steam is stored in a steam accumulator for later use. The second generation CSP direct steam tower adopts superheated steam technology (Fig. 6b). Utilizing a second receiver to reheat the steam produced by the first receiver to reach a higher temperature, the high-temperature steam produced can reach a temperature of 540 °C and a pressure of 130 bar, compared with its predecessor PS20, the power cycle's efficiency increased by 30% [95]. Although steam can be directly used as TES for CSP system, additional high-pressure energy storage tanks and anti-corrosion treatment of impurities in water are required, which increases additional costs.
Fluidized particles based DSPG, with permissions requested from [35]
At present, the commonly used SHS materials are mainly molten salts and metal oxides. As far as future DSPG technology is concerned, with the development of photothermal technology, low-cost, stable, high-efficiency, and environmentally friendly sensible heat materials need to be developed, which will have a great impact on improving power plant efficiency and reducing construction and operating costs. The steam generator is also a key component of solar steam generation. Exploring new steam generators is of great significance to improve the efficiency of power generation and the reliability of power plants.
3.1.2 ISPG
In the ISPG system, the SHS material only serves as the TES medium. Heat transfer oil and antifreeze fluids are typically employed as the HTF [95].
As compared to the DSPG, the ISPG system shows two distinct features. The first feature is that the operation temperature is relatively lower (< 400 °C) than the DSPG, thus parabolic trough solar concentrators could meet the requirements for power generation [182]. Second, since the TES and HTF fluids transfer heat in an indirect way, the system is more complex than the DSPG system due to the existence of extra heat exchangers, valves and pumps as shown in Fig. 7a. The figure shows the 1 MWe CSP system in Yanqing, Beijing [162], which includes solar field, indirect heat storage double tanks, steam generation system and power generation module. The double-tank heat storage is mainly that the molten salt can store additional heat energy, and directly exchange heat with the HTF heat transfer oil to generate steam for power generation. The temperature of the low-temperature heat storage tank is 292 °C, and the temperature of the high-temperature heat storage tank is 386 °C. When the solar radiation heat is insufficient or encountered in cloudy and rainy days, it is necessary to use a high temperature heat storage tank to preheat the heat transfer oil to provide extra heat into the system. Herrmann et al., [112] proposed a trough solar double-tank heat storage and collector field. The high-temperature heat transfer oil absorbs heat through the trough-type collector, and the low-temperature molten salt is heated to 385 °C. During the power generation process, the high temperature molten salt and the heat transfer oil releases heat to the HTF. The temperature of the molten salt drops to 300 °C and flows into the low temperature heat storage tank. At the same time, the HTF exchanges heat to generate steam for power generation. Solid SHS material could also be employed in the ISPG since the SHS only plays a role as TES medium. Compared with the double-tank liquid SHS heat storage system, solid heat storage medium becomes more attractive due to its lower investment, lower maintenance costs, wider range of sources such as sand, gravel, concrete, etc. Using concrete block as heat storage medium, as it is easy to handle, available all over the world, and with no environmentally critical components. Laing et al., [148] designed and tested a ISPG system as shown in Fig. 7b. The concrete storage module consists of tube register and storage concrete. The heat transfer medium is oil, and the entire storage module is insulated with mineral wool. To improve the heat transfer effect, the charging and discharging process uses anisotropic flow to ensure a heat transfer temperature difference of 100 K. However, concrete as a thermal storage medium is affected by working temperature. When the temperature exceeds 150 °C, there will be a loss of free water and other components in concrete [150]. When the temperature continues to rise to 500 °C, the mass of cement continues to decrease, and after several cycles, the compressive strength of concrete will drop to 30–50% of its initial value, reaching the maximum usable strength of concrete. As a result, the applicable temperature of using concrete as a thermal storage medium should not exceed 500 °C.
In the ISPG system, SHS material is used as the heat storage medium in the CSP system. Compared with the DSPG system, although the construction cost of solar thermal power generation is higher, it can effectively solve the problems of insufficient sunlight and intermittent power generation. Due to the complexity of the ISPG system, the synergy between the various components is more important. Optimizing system design and integration can achieve more efficient solar power generation, in addition, the use of artificial intelligence and Internet of Things technology for intelligent monitoring and maintenance of ISPG systems can also improve system stability.
3.1.3 Redundant power to power
At present, the energy directly used by human beings is mainly electric power. The redundant power generated by renewable energy needs to be stored during the low peak period of power consumption. At present, battery storage power stations have been used for energy storage. However, it will face disadvantages such as high investment and maintenance costs, low safety, and serious self-discharge of storage power stations. SHS, as a mature heat storage technology, can store redundant electricity in low peak periods as heat energy. During the peak period of electricity consumption, heat energy is converted into electricity and put into use.
In the process of converting electricity into heat, Joule heat is used, and the high-temperature heat generated (the highest temperature exceeds 700 °C) is mainly stored through solid heat storage media and high-temperature molten salt materials. As shown in Fig. 8a, Siemens Gamesa Renewable Energy has begun operation of its electric TES system [307], which contains about 1,000 tons of volcanic rock as the energy storage medium. The electrical energy is converted into hot air by electric resistance heaters and blowers, heating the rocks to 750 °C. When demand peaks, the stored energy is re-energized using a steam turbine. Operational results show that the plant can store up to 130 MWh of thermal energy for about a week and keep it constant throughout the charging cycle. Thermo-mechanical energy storage technology that uses thermoelectricity as the main output energy source and stores electrical energy as thermal energy is called Carnot batteries. As shown in the Fig. 8b, the electric-thermal-electric system is made up of three main components [39], the power block, the Carnot battery and the NuScale nuclear reactor. The role of the Carnot battery is to ensure continuous superheating of the steam and to store excess power from the grid. For this purpose, it consists of an electric heater, two TES tanks, superheater and circulating pump. The storage medium is a mixture of molten salts, similar to the CSP heat storage medium, this mixture consists of 60wt% NaNO3-40wt% KNO3. The cold tank and hot tank temperature are specified in this study at 267 °C and 590 °C, respectively, to keep the storage medium at acceptable operating conditions.
The use of solid and molten salt sensible heat materials as the heat storage part of the Carnot battery is economical and environmentally friendly compared to other types of power storage methods.
Although the efficiency of the existing Carnot battery technology for power generation is about 40%, using redundant low-cost electricity and abandoning the burning of coal can greatly reduce carbon dioxide emissions. In the future, the electric heating part can be replaced by a heat pump. Driving electricity still comes from renewable energy surplus electricity. In addition, the hot molten salt tank will be changed to "hot molten salt tank and high temperature radiator", and the cold molten salt tank will be changed to "cold molten salt tank and low temperature heat source". Compared with resistance heaters, the heat pump charging efficiency can reach 120%, so that the overall efficiency of the system can reach 50%.
3.2 SHS in zero-carbon building
The main energy requirement in building includes electricity, thermal and cooling. The heating load could be great in buildings especially in winter. The heating load in buildings comes from domestic hot water and space heating. To achieve a zero-carbon or low-carbon building, the heating load should be supplied from renewable energy or industrial waste heat. Similar to the scenario in power generation sector, the application of both the solar energy and industrial waste heat faces the problems of discontinuity and fluctuation. The application of SHS in zero-carbon buildings is mainly divided into passive type and active type heat storage in building.
3.2.1 Passive type heat storage in buildings
Passive type heat storage in buildings typically employs different SHS on the façade of the buildings. The materials with high thermal capacity will have a positive impact on building thermal comfort and reduction in building energy consumption [223]. These SHS materials could shift the solar energy from peak to off-peak periods [287] and contributes for stabilizing indoor temperature fluctuations [13, 290]. To reduce the indoor temperature fluctuations, DM et al., [198] evaluated the effect of four different thermal mass levels on reducing the indoor maximum daytime temperature in different environmental climates. A formula was proposed in their study for predicting the maximum daily indoor temperature of buildings at high equatorial elevations. Karlsson et al., [135] investigated the addition of high thermal capacity SHS in the building walls to reduce heating energy consumption and propose the relationship between heating energy requirement and solar input. Although the passive type building thermal management could not provide thermal or cooling energy to the building, the size and thermophysical properties of SHS could be tuned to retrofit buildings for thermal insulation and heat dissipation. Al-Sanea et al., [1] investigated the effect of adding high thermal inertia concrete materials at internal and external wall positions on buildings. The results showed that installing a concrete layer on the inner side of the wall had a better effect on indoor thermal comfort than installing it on the outer layer. However, different conclusions have been reached in other studies, Bloomfield and Fisk et al., [31, 242] reported that additional thermal inertia in weighty buildings during daily intermittent heating does not provide any substantial energy savings. The achievement of positive results depends on many factors. Climatic conditions, acceptable indoor comfort demand, and high thermal mass designs may introduce conflicting requirements for winter heating and summer cooling.
3.2.2 Active type heat storage for buildings
Active type TES is the use of external energy sources such as solar energy and off-peak electricity for domestic heating to improve the thermal comfort inside the building. Domestic heating most commonly uses hot water as the SHS material to store solar heat. The temperature in the collector is generally less than 100 °C and the temperature difference between hot water and heat exchanger is between 5–10 °C. In addition to hot water heating, underground water, sand, and soil are used as heat storage medium for large buildings' thermal active energy storage with heat pump (HP) and so on. In addition to the application of renewable energy sources, off-peak electricity can also be used for space heating in residential buildings with better economic performance. Off-peak electricity is used to heat high heat capacity SHS materials on building walls.
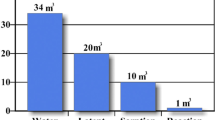
Heat storage with water has attracted widespread attention, the configurations of hot water tanks can be divided into three categories as shown in Fig. 9. They are direct water tank with heat insulation, shell-and-tube heat exchangers, and the third is the double-pipe heat exchanger. Since SHS has the characteristics of large heat loss due to the influence of external temperature. To solve the problem, underground heat storage can be selected for large-scale building heat storage including underground water, sand and soil. This technology is mature for large-scale heat storage [74, 284, 285]. Xu et al [284] proposed to use underground soil as a heat storage medium for agricultural greenhouse environmental insulation to replace the auxiliary electric heating system. As shown in Fig. 10a, water is used as a heat transfer medium and the soil as the storage material. The heat is released only through the heat radiation and heat convection of the heat exchanger when the temperature of greenhouse is low. The results show the maximum ambient temperature could be increased by 13 °C. Large-scale application of underground water as heat storage media such as [103, 215] is shown in Fig. 10b with similar system configuration but different storage medium. Meister et al., [179] also propose a full-scale experimental solar thermal system with a 36 m3 underground tank, which not only provides space heating but also domestic hot water. The results showed that the solar system can provide 15 GJ of space heat load including 86% of domestic hot water load (total load = 13 GJ). In addition to soil and water, there also exist other types of SHS media such as rock bed. Zhao et al. [304], using the TRNSYS model to simulate and establish the air heating system using pebble bed as the heat storage medium. It was demonstrated the system could be able to meet 32.8% of the heating demand in winter and 84.6% of the energy demand in the non-heating season. In some parts of Europe, the use of underground water, soil, rocks, etc. to store heat accounts for about 9–10% of the European energy supply, while the direct use of solar energy for heating only accounts for 3.2% [88]. The use of SHS materials, with its reliable heat storage capacity and low cost, has a great application market and prospects in the direction of large-scale cross-seasonal energy storage in the future.
SHS for heating water in three categories
The use of off-peak electric energy for heat storage and heating is also one of the recent popular space heating methods [124, 252]. As shown in Fig. 11a, the electric energy during off-peak periods is used to convert the electric energy into thermal energy to heat the firebricks material for heat energy storage [307]. During application, ambient air could be injected into the heat storage system to extract heat and for heating water to achieve space heating, the distribution of the hot and cold flows is regulated by adjusting the opening of the dampers to determine the final output temperature in the mixer to ensure that the mixing temperature is stable around the set point (Tset). The initial temperature is set to 1000 °C and the air inflow temperature is set to 60 °C. It is found that the mixer output temperature is effectively stabilised at each Tset, such as Tset = 100 °C or 200 °C in Fig. 11b. The use of this redundant electric heat storage heating can also save energy and reduce emissions, but it is necessary to consider the heating temperature control equipment to meet the heat load demand [307].
3.2.3 Cold storage for buildings
As a commonly used as energy storage medium, SHS can not only store or release thermal energy in heating, but also shows good performance in cold storage applications. The SHS system has been applied to chilled water storage (CWS) to provide cooling for buildings [20]. The use of chilled water as SHS is mainly used as a coolant in large refrigeration units or for air conditioning and cooling of buildings, as shown in the Fig. 12 [38], during the charging period of the CWS, chilled water at 5–6 °C flows from the chiller to the cooling load of the building and the heat storage tank of the CWS respectively. After heat exchange the chilled water temperature rises to 11–12 °C and the higher temperature water at the top of the CWS flows through the chiller again. In the discharging, chilled water at 5–6 °C from the CWS flows to the cooling coil of the buildings and then back to the CWS again at low flow rates when dealing with very low cooling load. When the cooling load demand is greater, a single CWS cannot provide sufficient cooling, so a CWS uses fresh water as storage medium which is considered environmentally friendly. Thu et al., [263] experimentally studied the temperature variation of chilled water in mechanical vapor compression chiller. The results showed that the cooling capacity increased by 40–45% and Coefficient of Performance (COP) increased by 37–40% at chilled water outlet temperature of 17 °C with a storage configuration. Other studies have designed different CWS systems to meet the optimal cold storage requirements. Boonnasa et al. [38], proposed a method for the optimal capacity and operation strategy of CWS systems under different electricity prices. According to the experimental results, the CWS system consists of two continuously operating chillers and a TES system with a volume of 5175 m3 showed the optimal performance, which reduced the cooling energy consumption of the chillers and the peak demand by more than 2 times and 31.2% respectively. As for the application of CWS in building interiors, it is mainly used to remove excess heat. Vadiee et al., [267] proposed the combination of chilled water technology and underground heat storage in a closed greenhouse system design for meeting daily heating and cooling demand as well as peak loads in a closed greenhouse. From economic point of view, the system shows a payback period of 7–8 years. Andrepont et al., [15] also have successfully carried out CWS technology and the system is considered to be one of the largest CWS systems in the world for distinct cooling, with a storage capacity of 432.6 MWh and a peak cooling rate of 87.9 MW.
Schematic diagram of building cooling system with a CWS, with permissions requested from [38]
In summary, under the condition that the temperature requirement is less than 100 °C and the heat storage volume is not limited, water is a highly stable sensible heat material with high specific heat capacity and cost close to zero. Not only can it be applied to domestic heating and cooling, but it can also be applied to large-scale cross-seasonal energy storage of buildings and other fields.
3.3 SHS in zero-carbon transportation
To reduce energy consumption and carbon emissions in the transport sector. Electric vehicles (EV) are more efficient, faster and produce lower emissions than conventional internal combustion engine vehicles, but the discharge performance of their core lithium batteries is severely affected by temperature. As the most common SHS materials, air and coolant have important impact on improving the charging and discharging performance of battery packs and increasing the cruising range of vehicles.
3.3.1 Air-based SHS materials
Using air as a heat storage material relies on the characteristics of air has strong fluidity, low price, wide source, and high heat transfer intensity. Air can be used not only as a heat storage material but also as HTF, as shown in Fig. 13a, Hong et al., [298] adopted a parallel air-cooling system for the prismatic battery pack. The air was pumped into the battery thermal management system (BTMS), and then dispersed into each cooling channel through the divergence plenum, and collected by the convergence plenum at the end of the cooling channel after heat exchange with the battery pack then flow out later and consider introducing secondary vents at different locations, the maximum temperature of the battery pack is reduced by 5 K or more and the maximum temperature difference is reduced by 60%. Saw et al., [237] used forced convection air in a cylindrical battery pack, as shown in Fig. 13b. The air for forced heat exchange adopts a parallel air flow group, but the air flow group will generate axial flow around the battery due to the structure during the transportation process. The maximum cell pack temperature is 63 °C when the mass flow rate is 5 g/s, and the temperature decreases to 33.2 °C at a mass flow rate of 75 g/s. According to the temperature uniformity test, the battery pack indicates a uniformity temperature change of about 6 °C at a mass flow rate of 5 g/s, which decreases to about 1.5 °C at 75 g/s, as the air flow speed increases, the cooling performance of the battery group is improved. Using air as a HTF material for EV BTMS can not only dissipate heat at high temperature but also heat the battery pack at low temperature. Ji et al. [126], proposed to use electric heaters and fans to externally heat the battery pack. As shown in the Fig. 13c, in a closed space, the air is heated by electric heating, and the air is sent into the battery pack through the fan to increase the temperature of the battery pack. After the battery pack releases heat, it is heated again by an electric heating device. Figure 13d shows that the use of air heat storage materials not only for low-temperature heat release but also high-temperature heat dissipation of the battery pack has the characteristics of simple configuration and low maintenance cost, and does not require separate storage of coolant, occupying less space for the battery pack. Develop an efficient thermal management system, optimize the design of the heat storage system, including the size, shape, and structure of the device, to ensure that the temperature of the heat storage material will not be too high or too low, thereby ensuring the safety and stability of EVs.
a Secondary ventilation air based BTMS, with permissions requested from [298]. b Forced air heat exchange diagram of cylindrical battery, with permissions requested from [237]. c Schematic view of air low temperature heating system, with permissions requested from [126]. d Air based BTMS, with permissions requested from [207]
3.3.2 Liquid-based SHS materials
Compared with air-based BTMS, liquid-based has the characteristics of high thermal conductivity, higher heat storage and release capacity, higher specific heat capacity and faster heat dissipation to the battery under extreme conditions. Among them, water and ethylene glycol as the most mainstream liquid-based materials. For regular shaped square battery packs, Panchal et al., [204] compares the temperature and velocity distribution inside a micro-channel cooling plate placed on a prismatic lithium-ion battery cell using experimental and numerical technologies. The study was performed on water cooling methods at discharge rates of 1C and 2C and at different operating temperatures of 5 °C, 15 °C and 25 °C. Coolant can be used not only as heat release and heat storage medium of battery pack but also as HTF, the results show that the temperature of the microchannel fluid increases with the increase of the discharge rate, and it is experimentally also found that the heat generation at the two poles of the battery is higher than that in the middle of the battery. For the other type cylindrical battery packs, Rao et al., [221] proposed a liquid-based BTMS system based on variable contact surface designed for cylindrical lithium battery packs, as shown in Fig. 14a, by changing the size of the contact surface between the aluminum block and the battery pack. The aluminum block is used as a SHS material, which can absorb the heat of the battery pack. The microchannel in the middle of the aluminum block is filled with cold water as a HTF to take away the heat. The results show that the system with variable contact surface is better than the system with constant contact surface considering the system weight and the pump power consumption. When the inlet velocity is 0.05 m/s, the temperature difference decreases by 6%, 14% and 28% with increasing variable contact surface, while the system weight decreases by 20%, 29% and 47%, of these three slopes, the variable contact surface of 3 mm is the best design of the system. Considering the safety between the battery and coolant, Basu et al., [3] designed a new liquid-based BTMS method for 18,650 battery packs in Fig. 14b, which ensures safe operation under the condition of ensuring heat transfer and economic conditions. The heat generated by the battery is transferred by the SHS medium aluminum material and exchanges heat with the fluid. The simulation result shows at high discharge rate (2.7C) and low coolant flow rate (0.01 m/s), the maximum temperature rise is kept within 7 K. In addition to the heat conduction, the aluminum material can also be used as a separator to prevent liquid leakage and short circuit. However, the traditional water and ethylene glycol used as coolants have shortcomings such as low thermal conductivity. Therefore, a new type of coolant liquid metal material was proposed for use in liquid-based BTMS systems [292]. The results show that under the same flow rate, the liquid metal as a coolant enables lower battery pack temperatures and more uniform temperatures. Liquid-based coolant can not only reduce the overall temperature of the battery module and improve the temperature uniformity of the module [274], but also can alleviate thermal diffusion in the event of a collision or thermal runaway [127].
In addition to heat exchange with the battery pack, for the entire EV heating, ventilation and air conditioning (HVAC) system, not only the battery pack needs temperature control, but also the cabin needs thermal comfort adjustment, Harden et al. [101] designed a coolant as a SHS medium, and integrated the SHS tank into the coolant circuit for low-temperature heating of the EV in winter, as seen in Fig. 14c. There is an air/coolant heat exchanger in the system that transfers heat from the coolant side to the air side. In low temperature environments, grid energy can be used to heat the heat storage medium to the required temperature before departure. The heat storage medium can then partially or completely offset heating needs without the use of a power battery. At the same time, the system also has a positive temperature coefficient (PTC) heater. If the heat storage tank is not stored before departure, the coolant can be heated by the PTC and flow into the heat exchanger to heat the air. Simulation results show that an 80 kg, 80 °C coolant tank can meet all the heating requirements of a 36 km, 1 h and 9 min city driving cycle. Furthermore, annual analysis shows that a 30 kg heat storage tank can reduce the average annual consumption of the battery by up to 20Wh/km or 12%. Likewise, Lajunen et al., [151] performed a similar study by modeling the effect of TES units on a medium-sized passenger EV. Simulation results show that, under very cold conditions (-30 °C), installing a large storage capacity coolant-based TES tank (150 L) and a higher initial storage temperature (80 °C) increases the driving distance by about 25%. However, due to the large volume of SHS, the mass and volume of the vehicle will be too large, which will increase energy consumption compared with high heat storage density materials such as PCM and TCHS.
3.4 SHS in zero-carbon society
The greenhouse effect and global climate change have attracted worldwide attention. With the development of human society, the emission of GHGs has increased greatly, especially the contribution of CO2 to the greenhouse effect is greater than that of other GHGs. According to the IEA report, in the past decade, global CO2 emissions have stabilized at more than 30 Gt/year. 40% of CO2 comes from power plants, 23% from transport, and 22% from steel mills and other industries. We have also gradually realized that only by improving the production process and rationally using resources, we cannot effectively reduce CO2 emissions in society. Climate change caused by large amounts of carbon dioxide can be mitigated in a short period of time through carbon capture and storage (CCS). Applying SHS to CCS can effectively improve energy efficiency and reduce carbon emissions.
CCS, which capture CO2 from fuel combustion or industrial processes, transports it by ship or pipeline, and stores it underground, in depleted oil and gas fields and deep saline formations. At present, due to high energy consumption and high cost, CCS technology is mainly concentrated in power plants [232], using extra steam heat provided by power plants to improve carbon capture efficiency, for iron and steel plants with low steam production, there will also be a large amount of waste heat resources such as rolling mills and steel billets, Zhang et al., [298] developed a method to capture CO2 in the flue gas of the heating furnace by using the sensible heat of the continuous casting slab, and optimized the system parameters. The carbon dioxide absorbed in the solvent is separated by using the sensible heat resource of the continuous casting slab, which improves the regeneration efficiency of the solvent and reduces the extra energy consumption, the results show that the purity of CO2 captured is 98.4%, the annual CO2 capture capacity is about 70 000 tons, and the regeneration energy consumption is 3.67 MJ/kg CO2. The most important aspect of the CO2 capture process is the energy requirement. In a typical thermal adsorption–desorption system, energy is required for material (sorbent and flue gas) transport, sorbent heating (sensible heat), and CO2 desorption [132]. For example, Sjostrom [250] suggested a fluidized bed process for CO2 capture using an indirect sensible heat exchange scheme. In this process, water circulates between two solid–liquid heat exchangers as a heat transfer medium for sensible heat exchange, one solid–liquid heat exchanger is used for the cold solids flow of the adsorption bed and the other is used for the hot flow of the desorption bed. Therefore, SHS technology is of great significance for CCS systems in terms of improving the regeneration rate of solvents, reducing energy consumption, reducing carbon emissions, and utilizing waste heat.
4 LHS in achieving carbon neutrality
This section reviews the practical applications of LHS technologies in achieving carbon neutrality. The LHS materials can be applied to different fields of thermal storage applications due to its features of the wide range of phase change temperatures and large latent heat value. The selection of PCMs, the material thermophysical property improvement and the practical application of the LHS system towards zero carbon development is reviewed.
4.1 LHS in zero-carbon power generation
In the future, a zero-carbon power generation based on solar power plants show superior features for less carbon emission. The utilization of solar energy for power generation could be classified as CSP system as well as the photovoltaic (PV) system. LHS play important roles in these two systems to address the intermittency and fluctuation problems of solar energy and to improve the continuity of power generation. This section will mainly discuss the application of LHS in the field of CSP and PV plants.
4.1.1 Concentrate solar power systems (CSP)
As shown in Section 3.1, CSP system collects solar energy through a reaction mirror field which collect much heat, and then converting the collected heat into steam to drive a turbine to produce electricity. When there is too much light to produce too much heat or when it is too rainy to provide enough heat, the TES integrated in the CSP system can serve to store and release the heat. Considering the existing CSP temperature range (200–600 °C), high temperature PCMs are selected for use in TES systems [52, 197]. High temperature PCMs usually include inorganic PCM such as molten salts, metals and alloys, multi-component molten salt eutectic PCMs, etc. According to the literature [116], for molten salt or eutectic salt the phase transition temperature is in the range of 500 K-1300 K, among these single-component salts such as nitrate, chloride salt, carbon salts and fluoride salts, the latent heat of chloride salts is large, and the cost is relatively low (0.3 $/kg), so chloride salt has high attractiveness [139, 283]. Utilizing the characteristics of multi-component eutectic salts, eutectic salts with a large phase transition temperature range can be prepared. Chloride salt which can be prepared the ternary chloride salt eutectic PCMs as 59.98 wt.% MgCl2-20.42 wt.% KCl-19.60 wt.% NaCl, using TGA/DSC to start the detection and heating from 120 °C to 450 °C, the melting point temperature is 381.47 °C, the average latent heat is 198.55 kJ/kg. Preparing the 34.81 wt.% NaCl-32.28 wt.% KCl-32.91 wt.% LiCl ternary chloride salt, the phase transition temperature is 351.36 °C, and the phase transition latent heat is 131.96 kJ/kg [94], it can be used as a high temperature heat storage material in the CSP system. In addition to the chloride salts used above, there are also nitrate-based PCMs used in CSP systems. In early 2008, Laing et al., [149] proposed that NaNO3 was used as a PCM, the melting temperature was 306 °C and the latent heat value was 175 kJ/kg, which build and designed a laboratory pipeline with a concentrating heat field of 5 KW, the experiments result also has been used to fabricate a 700 kWh PCM storage. In addition to utilizing single component PCM, a cascade PCMs system approach can be taken depending on the temperature. Prieto et al., [212] designed a PCM with four different temperatures of chloride salt and nitrate compound, as shown in Fig. 15, the heat storage and discharging process is carried out by the HTF flowing through different heat storage tanks. The heat discharging process is to start the HTF from the low temperature storage tank and finally flow through the high temperature storage tank, and vice versa. Simulation results show that the TES heat storage efficiency of this cascade system throughout the year is 90.8%, and the power generation is 3% different from that of the traditional two-tank molten salt type, which is technically feasible, but the subsequent need to account for economic costs and other issues.
Cascade system PCM charging and discharging process, with permissions requested from [212]
In addition to the cascade systems mentioned above, Galione et al., [85] proposed the heat storage concept of multilayer phase change materials (MLPCM), As shown in Fig. 16a, the upper layer is PCM with higher phase transition temperature, the bottom layer is PCM with lower phase transition temperature. Compared with the single solid heat storage tank of the same volume, the MLPCM heat storage tank has better heat storage efficiency and heat storage capacity. Elfeky et al., [75] proposed a transient Concentric-Dispersion model to apply MLPCM heat storage to high-temperature CSP generation. As shown in Fig. 16b, it is analyzed and verified that the three-stage PCM has faster heat storage and release performance than the single-stage PCM under similar working conditions, and the overall system the energy and exergy efficiencies vary between 60.6–75.76% and 41.5–75.18%, respectively. Although molten salt-based PCMs are widely used in CSP systems, they suffer from low thermal conductivity, high phase change volume expansion, phase separation, and high supercooling.
Compared with molten salt-based PCM, metal and alloy PCMs [64] with lower vapour pressure have more and more advantages in improving thermal conductivity, reducing phase separation, and reducing volume change before and after phase transition, which can be used in the field of high-temperature power generation. Common alloy materials such as aluminum-based materials, zinc-based materials and magnesium-based heat storage materials. In particular, silicon-aluminum alloys have a large heat of solution and are widely used. Khare et al., [142] proposed a multi-objective optimization method to select appropriate metal-based alloys for high temperature steam generation power generation systems, the results show that for Al, Mg, Zn and Si materials, 88wt% Al-12wt% Si and 60wt% Al-34wt% Mg-6wt% Zn are the most suitable materials for steam generation in the temperature range of 400–750 °C, the heat transfer performance is generally better than that of inorganic molten salt, and it is relatively friendly to the environment. Adinberg et al., [8] developed and tested a recirculating heat transfer storage system using metallic PCM, which can be applied to generate superheated steam in the temperature range of 350–400 °C. When the metal component is 70wt% Zn-30wt% Sn, the melting temperature of 370 °C is the best metal alloy material for high temperature steam power generation. Integration of the developed thermal storage system into a 12 MW solar CHP. The storage system will also allow the solar thermal system to operate uninterrupted on a daily basis when the normal solar radiation is not sufficient. Although this metal alloy is much more expensive than inorganic salts, due to its excellent chemical stability and high thermal conductivity, there is still research value.
Existing latent heat materials such as nitrates and chlorides have been commercially used in CSP systems, but due to the limitation of heat storage density and thermal conductivity, it is necessary to develop new latent heat materials with high heat storage density in the future, and in order to prevent PCMs from Leakage and cracking also need to optimize the wrapping and fixing methods of heat storage materials. Also optimizing the system design of latent heat materials has an important impact on improving heat storage performance and service life.
4.1.2 PV-Thermal
Solar PV is a photoelectric conversion method that converts photon energy into electrical energy to generate electricity, and its core component is a solar cell. Although many emerging solar cells have been proposed, the power generation efficiency is still low due to the influence of temperature. According to the literature [217], when the temperature is increased by 1 °C, the photoelectric conversion efficiency decreases by 0.3%-0.65%, and the good working temperature range is -40 °C-85°C [136]. It is essential to perform thermal management in PV cells and their components using PCMs can significantly improve the photoelectric conversion efficiency of PV cells [171, 174].
PV/PCM has become a new type of temperature management module, compared with traditional methods such as spraying cooling water, the use of PCMs to cooling PV can not only control the temperature of PV, but also collect the heat. Hasan et al., [11] studied the application of PCM in PV power generation, using PCM to absorb 41% of the heat in PV modules to heating water, increasing the photoelectric conversion efficiency by 1.3%. Kibria et al., [144] studied the addition of three PCMs with different phase transition temperatures in PV panels by numerical simulation method, which effectively confirmed that PCM can improve the thermal performance of PV cells by 5% as an effective means to suppress the temperature rise of PV cells. Compared with conventional photovoltaic electric/thermal (PV/T), it is capable of heat collection and heating water in addition to heat dissipation, Browne et al., [175] proposed a novel PV/T/PCM system that can delay heat generation from the collector by adding PCM to the PV/T system, where the heat is first stored in the collector. The PCM selected a eutectic mixture of fatty acid and palmitic acid with a phase transition temperature of 17.7 °C. As shown in Fig. 17, by setting 4 different PV/T collector systems, compared with the system without PCM, the water temperature was increased by 5.5 °C at 6:00 am, and it was confirmed that PCM could improve the PV temperature to increase the output energy of the system. Islam et al., [185] utilized a PV/T/PCM system to provide 33% thermal storage potential compared to conventional photovoltaic hot water systems (PV/T/W) with extended thermal availability 75–100%, heat production increased by about 9%, and the cooling effect of the PV/T/PCM is also better than that of the conventional PV/T/W system. In areas rich in solar energy resources, the combination of collectors and photovoltaic solar cells can reduce heat load demand for space heating and supply domestic hot water, and is of great significance for reducing the temperature of solar panels and increasing power generation.
PV/T/PCM composite power generation and heating system, with permissions requested from [175]
4.2 LHS in zero-carbon building
In areas with harsh environments, LHS has been widely used in construction fields such as space heating and domestic hot water, which alleviates peak power consumption and energy waste to a certain extent. The energy consumption of the building part accounts for 30% of the main energy consumption [181]. To reduce energy consumption and improve thermal comfort of buildings, review [245] pointed out that organic PCMs have good applications in heating/cooling buildings. Storage concepts applied to the building sector have been classified as passive and active systems, in this section we will discuss in detail.
4.2.1 Passive building applications
The thermal control of passive buildings mainly uses the characteristics of PCMs with small temperature changes during the phase change, and directly incorporated PCMs into building structures such as gypsum wallboard, concrete, or porous materials by direct incorporation, immersion, encapsulation and microencapsulation.
Organic PCMs such as paraffin wax, fatty acid and their blends are considered as an efficient PCMs that can be applied in the field of passive building energy conservation. Kenisarin et al., [138] analyzed and summarized the application of PCMs in passive thermal control in the building field, and summed up the cases that have been successfully applied commercially to maintain the temperature fluctuation in the building at 18 °C-25°C, also mentioned the application of three methods to integrate PCMs into building structures, the first is to immerse porous materials into PCMs, second is to prepare micro-encapsulated PCMs into building structures, and the last is introduce macro-encapsulated PCM into building materials. Sharifi et al., [243] using the first method different kinds of paraffin in gypsum board by immersion method, and showed that the use of paraffin in the building material can increase the maintenance time of indoor thermal comfort and reduced the HVAC energy demand by 17% for a full year which 6% of the heating demand and 35% of the cooling demand. Mohammad et al., [239] using the second method prepared shape-stable nano-PCMs with fatty acids and expanded graphite (EG), incorporated nano-PCMs into gypsum wallboards using a 3-layer wallboard design, and conducted diurnal temperature tests with a control group (no nano-PCMs), as shown in the Fig. 18a. Studies have shown that the indoor temperature fluctuation (18.5–26.5 °C) of using nano-PCMs in wall panels is smaller than that without nano-PCMs (13–32 °C), and it can also delay the time to reach the peak temperature. Under the requirement of indoor thermal comfort, numerical simulation studies show that the incorporation of nano-PCMs into wall panels reduces energy consumption by 79%. Park et al., [206] impregnated a series of PCMs into expanded perlite and vermiculite to prepare EP/EV-gypsum boards in Fig. 18b, and replaced the traditional gypsum boards of Korean standard residential buildings, and carried out circulating water bath tests under Korean climate conditions. The study showed that, the composite PCMs obtained by configuring n-octadecane and n-heptadecane at a mass ratio of 7:3 showed the greatest energy-saving effect when cooled in the thermal comfort range, and the phase transition temperature was 23–24 °C. Although the use of micro-encapsulated PCMs can prevent the leakage and improve thermal conductivity, it still faces problems such as high cost and cumbersome process in practical application. In response to this, the use of a macro-encapsulation PCM is proposed. Ramakrishnan et al., [219] used a numerical simulation method to simulate the addition of macro-encapsulated Bio-PCM™ mat-like PCMs with fatty acids as the main component to the walls and ceilings of a single-storey residential building in Melbourne in summer. Under heat wave conditions, the period of severe discomfort is reduced by 65% reducing indoor thermal stress. The application of PCM to actual construction projects not only needs to consider the requirements of its reliability on the thermal comfort of the indoor environment, but also needs to consider the economic cost. Taking a Chinese city as an example, Mi et al., [183] studied five cities in different geographical locations, and simulated the filling of PCM with a phase transition temperature of 27 °C and a latent heat value of 230 kJ/kg. The results show that the economic benefits of filling PCM are more prominent only in areas with relatively harsh environments, such as Shenyang and Zhengzhou in cold winter and Changsha in hot summer. The application of LHS in passive building applications should improve the thermal conductivity of PCM in building walls in the future to meet the requirements of packaging PCM processing convenience and wall safety.
4.2.2 Active building applications
An attractive solution using LHS in building active systems is the application of new or retrofitted buildings, such as the realization of renewable energy for HVAC space heating and cooling, domestic water heating, the improvement of the performance of current installations or the possible application of peak shifting strategies.
Residential, commercial, and industrial buildings typically require hot water around 60 °C, while bathing, laundry, and cleaning operations in the domestic sector typically require hot water around 50 °C [62]. As a representative of organic PCMs, paraffin wax is a very commonly used thermal storage material for solar hot water. Khalifa et al., [141] installed solar collector tubes in paraffin PCM. When there is no sunlight, the paraffin-filled collector tubes can continue to heat water by using paraffin as a heat source, as shown in Fig. 19a, the paraffin is filled under the copper tube, which can utilize the radiant heat of the sun as much as possible, which the experimental results show that the temperature of the heat collector tube filled with PCM its thermal storage efficiency is between 45 and 54%. Mahfuz et al., [172] filled paraffin into a shell-and-tube hot water heat storage device for heat storage to improve the thermal energy utilization rate of the system, as shown in Fig. 19b, when the mass flow rate of HTF increased from 0.033 to 0.167 kg/min, the system energy efficiency increased from 63.88% to 77.41%. Single-stage PCMs have low efficiency for medium and low temperature waste heat utilization. Fan et al. [291] proposed a PCM cascade system to increase the waste heat recovery rate from 15.8% to 63.4% using a composite system of erythritol and paraffin. This is the high efficiency cascade LHS system has great potential to recover medium and low temperature waste heat and accelerate the decarbonization of building space heating in the future. In addition to the use of solar energy for TES, Konyk et al., [146] also proposed a new mobile heat storage vehicle with carrier-filled PCM by using industrial waste heat through quantitative and qualitative regulation in the heat distribution process to reduce heat loss during transportation by virtue of the latent heat properties of the PCMs. If the cost problem can be solved, the mobile heat storage vehicle can solve the mismatch of space waste heat resources in the future.
For the use of solar energy for hot water heat storage, not only organic PCMs can be used for heat storage, hydrated salt inorganic PCMs have large latent heat value of phase change and suitable phase change temperature and are also widely used. Najafian et al., [192] selected PCM filled with sodium acetate trihydrate as the main filling at a phase transition temperature of 50 °C to 70 °C, and added 10% graphite to improve the thermal conductivity of PCM, and used the TRNSYS to simulate the thermal conductivity of PCM. The influence of the amount, the position in the container and the size of the container on the heat release time of the PCM was calculated. In addition, the supercooling energy storage using hydrated salt inorganic materials can be adapted to long-term energy storage research. As shown in the Fig. 20, Fan et al. [55, 76], for the first time studied the use of sodium acetate trihydrate to stabilize supercooling vacuum tubular collectors, water tanks and PCM unit solar combination system performance, with a 22.4 m2 collector area and 5 PCM units of 200 L each, a solar fraction of 71% was calculated for the annual heat supply. The system meets year-round passive domestic water as well as daily heat loads in Denmark. Using the sensible heat of sodium acetate trihydrate and supercooling latent heat energy storage to meet 7 different working modes, the highest solar energy utilization rate can reach 69%.
Sodium acetate trihydrate as PCM for long term heat storage, with permissions requested from [76]
In addition to using solar energy to heat hot water, direct space heating in building interiors using PCM. Weinläder et al., [280] proposed the LHS unit was integrated into the top of the room ceiling using a ventilation system as shown in the Fig. 21a. During the daytime, the ventilation was operated as a pure cycle to direct the warm room air into the PCM, at night the PCM was regenerated using outdoor air. It is able to reduce the maximum room temperature by 2 °C during the daytime in summer, but cooling at night must take into account the inability of cold air to enter the ceiling for PCM regeneration. In addition to ceilings, PCM can be filled on wall facades for cooling, Gracia et al., [59] design the thermal performance of a ventilated façade containing PCM in the channel was experimentally evaluated by adjusting the gates in different inlet directions so that the air in the cavity was below or even lower than the external ambient temperature during the peak load. As a result, the compartment is exposed to less heat gain through this enclosure, thus reducing the energy consumed by the HP during this period. As an important part of the building's free cooling system [108], the TES system stores the cold energy in the environment, as shown in Fig. 21b. The cold storage process is that when the ambient temperature at night is lower than the phase transition temperature of the PCM, the cooling capacity is carried by an electric fan. When the heat of the PCM is taken away, the PCM solidifies. When the cold energy is released during the day, the hot air in the room exchanges heat with the PCM, and the melting process of the PCM reduces the indoor air temperature. Solar energy, as a renewable energy source, has been used for household daily heating and domestic hot water. Compared with sensible heat materials, the use of latent heat materials reduces the volume and increases the heat storage density. It can also be used in new application scenarios and energy systems such as ground source heat pump heating systems and biomass water heaters in the future.
4.2.3 Phase-change cold storage
PCMs have a wide temperature range and can not only be used for high-temperature heat collection and power generation, but also can be used for cold storage at low temperatures. Currently, the commonly used cold storage methods are mainly divided into chilled water cold storage, ice cold storage and eutectic hydrated salt cold storage [104, 105]. For ice storage, the latent heat of phase transition is 334 kJ/kg [122], but due to the low phase transition temperature, the chiller needs to provide a cooling capacity of about -5 °C during the charging process. In addition to that, eutectic hydrated salt has the largest cold storage capacity, especially for inorganic hydrated salt PCMs, the hydrated salt solution formed with excess water forms binary eutectic PCMs, and its eutectic temperature is lower than 0 °C, low temperature cold storage can be carried out. Khan et al., [9] performed an experimental study of a household refrigerator using two different PCMs (i.e., water and eutectic solution with a melting point 0 and -5 °C, respectively) placed behind the evaporator. COP significantly increased through the use of PCM, improving 20–27%. They also found that by increasing the quantity of PCM, COP increased about 6%. Comparing the two PCMs, eutectic solutions was found to increase the COP more than water. Not only that, Abdolmaleki et al., [5] a eutectic mixture of polyethylene glycol with a phase change temperature of -20 °C (PEG200:PEG300 = 30wt%:70wt%) was prepared for application in a freezer chamber. The results showed that the energy consumption of the freezer with the PCM was much lower than that of the normal freezer, with 8.37% energy saving when 1.5 kg of PCM was added and at a melting temperature of -20 °C. Although the hydrated salt solution has a lower phase transition temperature and a higher cold storage density, the hydrated salt solution faces supercooling at low temperature and phase separation during long-term operation, exploring stable and efficient cold storage materials is the mainstream research direction. Wang et al., [277] presented a review summarizing clathrate hydrate, refrigerant clathrate hydrate, carbon dioxide clathrate hydrate, hydrocarbon clathrate hydrate and multicomponent inclusion hydrates, compared with frozen water and ice cold storage, it has higher cold storage density and phase transition temperature, and has better cycle stability and heat transfer characteristics than eutectic salts.
4.3 LHS in zero-carbon transportation
LHS is a widely researched energy storage technology, not only as a cooling material for coolant in traditional internal combustion engine vehicles and for preheating before cold start of vehicles, but also in the face of emerging new energy EVs, which have more impact on their power battery packs whose performance is seriously affected by temperature. Therefore, this section summarizes the application of LHS in zero carbon transportation.
4.3.1 LHS in internal combustion engine vehicles
At present, the global transportation is still based on internal combustion engine vehicles, and which can produce a large amount of GHG emissions and consume a large amount of petroleum fuel. Although a lot of work has been done to optimize vehicle combustion and injection systems [273], in recent years, research into efficient cooling of automotive engines could reduce harmful emissions and improve fuel economy. In early 2008, Vetrovec et al., [271] proposed to add a PCM-rich collector to the cooling system of an vehicle, heat can be collected by the collector and discharged with low heat consumption, reducing the weight of the cooling system, save vehicle fuel consumption. Kim et al., [2] selected a latent heat value of erythritol with a phase transition temperature of 118 °C and a latent heat value of 339.8 kJ/kg as the heat storage material. The result is a reduction in the weight of the vehicle's cooling system and the ability to reduce coolant volume by 30%, and the stored heat can also be used to warm up the engine. In the same way, Gumus et al., [98] applied a TES system in the car to reduce the energy consumption during the cold start of the internal combustion engine vehicle. The TES system mainly used sodium sulfate decahydrate as the PCM, it can increase the engine temperature by 17.4 °C before cold start, the maximum thermal efficiency of the TES system can reach 57.5%, reducing CO and CH compound emissions by 64% and 15%, respectively. In short, PCMs have been studied and applied in automobiles, such as engine preheating [220, 270], improving thermal comfort of automobiles [32], and three-way catalyst preheating [41, 42].
4.3.2 LHS in EVs
The core of EVs is the power battery pack. Lithium-ion batteries have the advantages of high energy density, fast charge and discharge rate, and high cycle life, but they also face thermal runaway phenomenon in the summer or in the winter extreme environment, the discharge voltage is too low and the car cannot start.
Compared with air-based/liquid-based active cooling methods that consume additional electrical energy and other auxiliary energy, PCMs have been widely used in power battery packs as a passive BTMS relying only on their own heat storage characteristics [156, 165, 288], which can improve the performance of the battery and the service life. Hallaj et al., [12] conducted a temperature analysis of the discharge process of the 18,650 battery pack with or without PCM, and found that the battery pack filled with PCM can discharge more stably for a long time. The PCMs used in battery packs are mainly paraffin-based organic PCMs, Stefan et al., [147, 282] designing a fast-charging TES device using paraffin PCM to store heat for fast-changing urban electric buses. The system is designed to keep the cabin temperature around 18 °C and can be fully charged in just a few minutes using the terminal's high-voltage charger. As shown in Fig. 22b. The core TES module consists of a paraffin PCM layer, an electric heater and a cooling tube, and the module is packaged in an incubator. Each module has a storage capacity of 0.76 kWh and a storage density of 30Wh/kg. The charging power is 11 kW, and it can be fully charged in 316 s. Multiple modules can be installed at different locations on the electric bus to meet heat dissipation requirements. Due to their low thermal conductivity, there has been a lot of literature by adding high thermal conductivity materials, such as graphene [93, 177], carbon fiber [230], carbon nanotubes [248] and metal particles and metal foams [117, 301, 303]. In order to improve the heat dissipation performance of the battery pack, there are also some new TES systems combined with PCM heat storage technology. Wu et al., [281] proposed a combination of HP technology and PCMs for BTMS. As shown in Fig. 22a, where the battery pack and the PCM are closely together, one end of the HP combined with the PCM acts as an evaporator, and the other end uses a fan to blow cold air to exchange heat with HP, this BTMS combined with heat pipe technology maintains the battery temperature below 50 °C as much as possible. Other research proposed a BTMS method combining PCM and cooling water plate. Bai et al., [21] used a combination of cooling water plate and PCM for the different locations of local heat generated during the discharge process of the power battery, as shown in Fig. 22c, the maximum temperature of the battery can be reduced by setting the cold water plate at the electrode with higher heat generation, and the PCM is arranged at the rest of the battery to improve the uniformity of the heat generated by the battery.
a PCM combined with HP applied to prismatic battery, with permissions requested from [281]. b Fast-charge TES devices for electric buses using paraffin PCMs, with permissions requested from [282]. c PCM combined with cooling water plate applied to prismatic battery, with permissions requested from [21]
The above heat pipe and cooling water plate BTMS methods combined with PCMs are only suitable for prismatic batteries. For cylindrical batteries, improving the PCM and heat pipe or fluid-based heat transfer structure forms a new BTMS for cylindrical batteries. Jiang et al., [128] surrounded the cylindrical battery with a paraffin/EG composite PCM (CPCM) and wrapped it with an aluminum shell. Through forced air convection heat exchange with the battery pack in Fig. 23a, the temperature difference of the entire component did not exceed 2 °C. Using air baffles can increase the interaction between the cells and the air to improve heat transfer efficiency. Zhao et al., [305] improved the PCM-based temperature control under natural convection conditions by using a technology of combining PCMs with HPs in a cylindrical battery pack, as shown in Fig. 23b. Under the same conditions, the maximum temperature of PCM/HP coupled BTMS can be controlled below 50 °C for a longer time, filling PCM can reduce the temperature difference by about 33.6%, and embedding HP in PCM can further reduce 28.9%, PCM/HP is applied in the maximum temperature difference of BTMS can be controlled below 5 °C for a long time. Filling the PCM in the power battery pack can not only dissipate the high temperature heat generated during the operation of the battery pack, but also some scholars have proposed that the large supercooling characteristic can heat up the battery pack in a low temperature environment in winter, without using additional heat, only the heat released by the discharge of the battery pack absorbed by the PCM can be used. Sun et al., [258] proposed to prepare a ternary eutectic PCM by using a hydrated salt eutectic PCM, which can maintain stability at -20 °C after trigger the PCM to release the heat, raising the temperature around the battery pack to 0 °C, as shown in Fig. 23c. Commercial companies have designed different TES devices for EVs and electric buses [29]. These TES units can be used for EV cabin heating and dehumidification. These TES devices use low-temperature PCMs such as sodium acetate trihydrate and strontium bromide hexahydrate as heat storage materials. Large TES units for buses consist of small TES units with a heat storage capacity of 2.5 kWh, which the small TES unit is a shell-and-tube structure and the HTF is a liquid.
Whether it is heating the EV battery pack in winter or cooling it in summer, it needs to be considered that EVs need a battery management system with a compact size and a high storage density per unit volume. This raises the requirement that compact materials with high heat storage density need to be considered in follow-up studies.
4.4 LHS in zero-carbon life science
LHS can be used not only in zero-carbon power generation, buildings, transportation, but also in textiles, medical treatment and food refrigeration etc. Ultimately reduce energy consumption and reduce greenhouse gas emissions, thereby creating a zero-carbon life science.
4.4.1 Phase-change textiles
When choosing PCM for textile products, phase transition temperature between 28 °C and 35 °C should be selected as far as possible. When the ambient temperature or the surface temperature of the human body rises, the PCM undergoes a solid–liquid phase change to absorb heat, and in the low temperature environment in winter, it can release heat to keep the human body warm. Zhao et al., [306] a novel microcapsules of n-octadecane with natural silk cellulose were synthesized using a self-assembly method in oil/water emulsions. The maximum encapsulation rate and phase change enthalpy of the melting process are 46.65% and 88.23 kJ/kg, respectively, and the onset decomposition temperature of the microcapsules of PCMs was determined to be 178.5 °C. Therefore, the CPCMs prepared in this study exhibited high thermal storage capacity and good thermal stability and could be the best candidates for thermoregulated textiles or fibers as well as biomedical materials. Behrouz et al., [110] prepared a binary eutectic PCM with lauric acid: palmitic acid = 69wt%:31wt% based on two fatty acids according to experimental tests, and the phase transition temperature was 32.17 °C. As shown in Fig. 24, Liu et al. [166], report for the first time a versatile strategy for designed assembly of high enthalpy flexible phase change nonwovens by wet-spinning hybrid graphene-boron nitride fiber and subsequent impregnating paraffins. These materials exhibited an enthalpy value of 206.0 kJ/kg, excellent thermal reliability and anti-leakage capacity, superb thermal cycling ability of 97.6% after 1000 cycles, and ultrahigh water vapor permeability. This novel strategy further expands the advanced functional application of PCMs, and it will have a wide range of applications in the field of human wearable passive thermal management in real-world environments.
Wearable thermal management in face masks during summer and winter, with permissions requested from [166]
4.4.2 Phase-change medical treatment
The fatty acid-based organic PCM with non-toxicity to human body and good biocompatibility is screened out, and a fatty acid eutectic PCM whose phase transition temperature is in line with human body temperature is prepared, which can be used for drug-directed medical treatment. Zhu et al., [309] used lauric acid: stearic acid to prepare a binary fatty acid eutectic PCM with a phase transition temperature of 39 °C in a ratio of 4:1, as a gated material for near-infrared-triggered drug release. When the drug reaches the pathological cells, the drug is released through near-infrared heating. As shown in Fig. 25a, Li et al., [159] by adding 5wt% sodium hydroxide to erythritol, the erythritol supercooling degree can be increased and the phase change heat release temperature can be maintained above 40 °C for a longer period of time more than 7 min, which can be used for skin thermal therapy. In addition, some researchers have prepared encapsulated PCMs to provide thermal protection to surrounding tissues, which is beneficial to the transport of drugs [209]. In addition to fatty acid-based organic PCM, Chen et al., [275] developed a biodegradable silicone-based nano system utilizing CuS nanocrystals as a photothermal agent and PCM (1-tetradecanol) as a stimuli-responsive "gatekeeper" in this system, as shown in Fig. 25b. The system features dual stimuli-responsive drug delivery, which can effectively suppress tumors by stimulating drug release through synergistic chemotherapy and photothermal therapy.
Due to the fact that the temperature of the phase change process is basically constant, PCMs have good performance in the biological field, such as temperature control, drug delivery, tissue repair, etc. The efficient and stable heat storage materials developed now need to consider biocompatibility and safety issues, and optimize the performance of PCMs according to the temperature requirements of different organisms.
4.4.3 Phase-change food refrigeration
In order to maintain the quality of food that is seriously affected by temperature, some food must be stored under refrigerated or frozen conditions. Therefore, avoiding food spoilage, prolonging the shelf life of food and maintaining the cold chain during storage, transfer, delivery and sale are crucial. PCMs are commonly used in heat storage and transport systems in the food industry, such as thermal processing sections, refrigeration and packaging applications.
Cuneyt Erdinc Tas et al., [261] proposed polyethylene-based flexible nanocomposite food packaging films with thermal buffer properties at cold-chain temperatures were fabricated using PCM-impregnated halloysite nanotubes as nanofillers. The result shows that food samples demonstrate the effectiveness of nanocomposite packaging films in keeping food cold for extended periods of time. Saurav Sarkar et al. mentioned in the review [236] that the polyurethane doped with PCMs prevents food spoilage and low-temperature refrigeration, it includes the selection and synthesis of PCMs, the synthesis method and its application in refrigerated food in -10 °C-15°C polyurethane PCMs. Particularly for non-paraffinic, organic PCMs include esters, fatty alcohols, glycols, etc. Despite the different structural components, they all share some common characteristics, such as low supercooling, and instability at high temperature, low thermal conductivity, and flammability. Unlike paraffins, these "green" PCMs are "food-grade," meaning they're safe to eat [297]. Large-scale cold chain transportation based on PCM has also been commercially applied. The University of Birmingham and China CRRC [203] have developed the world's first 40-foot container that uses storage and release of cold energy materials. This container is used for the transportation of fresh fruits and vegetables in the market. PCM can maintain an internal temperature between 5–12 °C for up to 120 h. The technology has recently completed commercial trials, with 35,000 km of road and 1,000 km of rail transport carried out in different climate zones.
4.5 LHS in zero-carbon society
The TES technology and CCS technology are placed in the comprehensive energy system modeling framework [299], which considers the power system and thermal system. For the power system, conventional power generation, renewable energy and CCS are considered. For heating systems, both district heating networks come from industrial-scale cogeneration, high-voltage and TES systems. Cogeneration and high voltage on the district heating network and end-user side play an important role in linking the electricity and heat systems, enabling operational synergies through energy system integration. TES mainly refers to LHS and TCHS, which can increase the saving rate of the entire system from 7.3% to 18.3%. In addition to CCS, the utilization of high latent heat (501–507 kJ/kg), an appropriate phase change temperature range (0–15 °C), low material cost, and high chemical safety and stability CO2 hydrate as a cold storage medium in different types of air-conditioning systems can be used to reduce peak electricity consumption and alleviate energy supply intermittency and the paper [278] reviews the research progress of CO2 hydrate thermodynamics, kinetics and transport phenomena related to cold energy storage on the CO2 hydrate formation. Although CO2 hydrate has been used for cold storage in the laboratory, CO2 hydrate is seriously affected by temperature and pressure [211], which will lead to operational instability and the need to build additional gas storage for replenishment, resulting in further cost increases.
5 TCHS in carbon neutrality
As the TCHS with the highest heat storage density, there are two main types of heat storage: adsorption/absorption heat storage and chemical reaction heat storage. This chapter focuses on the research and application of different kinds of thermal storage methods on the zero-carbon field.
5.1 TCHS in zero-carbon power generation
Compared with the other two heat storage methods, because of its potentially higher energy storage density, TCHS systems emerge as an attractive alternative for the design of next-generation power plants, which are expected to operate at higher temperatures (300–1000 °C). The application of TCHS in the field of solar power generation the most important part is the selection of thermochemical reaction material.
According to Table 4, the solar power generation technology adapted to high temperature reaction conditions utilizes thermochemical reaction TCHS. There have been many large-scale industrial applications, carbonates which can store and release the heat through endothermic calcination and exothermic carbonation processes. It can be used as high-temperature thermochemical storage materials. The most common carbonates material is the calcium carbonate, which is in abundance on earth and widely used in power plants due to its low cost and high operating temperature (> 550 °C). Carbonate TCHS technology was first proposed in 1970s [77], but faced with the problem of CO2 storage, in order to reduce CO2 emissions, a calcium looping (CaL) integrated in solar power plants was proposed. In recent years, Chen et al., [46] proposed a novel CSP-CaL plant system which can eliminate the dependence on carbonation for power generation during sunshine time and free power dispatch can be easily realized, as shown in the Fig. 26a, the system is divided into sunshine and night modes, by driving CaCO3 to calcine to absorb solar energy and convert the energy into CaO and the chemical form of CO2 in sunlight mode, the heat after the reaction is used to generate electricity and preheat the reactants. When it goes into night mode. The energy demand is met by releasing energy through the exothermic reaction of CaO and CO2. The global storage exergy efficiency and power efficiencies can reach 37.60% and 48.04%, respectively, under optimal operation conditions. However, conventional CaCO3 pellets suffer from slow reaction kinetics, poor stability, and low solar absorption. To improve the heat storage capacity and enhance the mechanical, optical, and thermal properties of CaL, novel SiC/Mn co-doped CaO particles were prepared by extrusion-spheronization [155]. When the mass ratio of CaO:SiC:MnO2 was 100:5:5, the co-doped CaO pellets improved the stability of CaL and exhibited excellent heat storage performance compared with pure CaO particles, the heat storage density improved by 41.5%, the light absorption capacity and high thermal conductivity are 17.6 times and 3.2 times that of the original, respectively. In addition, Yuan et al. [295] proposed a composite CaCO3 particle in which binary sulfate and Al-Mn-Fe oxide synergistically improved cycle stability, mechanical strength, and solar energy absorption, the energy storage density of composite CaCO3 particles has only a slight decay rate of 4.91% after 100 cycles, and still has a high energy storage density of 1455 kJ/kg.
a Schematic of the proposed CSP-CaL integration system, with permissions requested from [48]. b Schematic diagram of the CSP plant using MH-TES system, with permissions requested from [180]. c SOEC mode and SOFC mode in RSOFC mode, with permissions requested from [259]. d A schematic diagram of an amino TCHS system, with permissions requested from [153]
Furthermore, Using high-energy–density and high-temperature metal hydrides (MHs) for TCHS is an attractive concept, and a two-reactor MH system uses high and low-temperature metal hydrides. High temperature metal hydride (HTMH) is used as energy storage media, while low temperature metal hydride (LTMH) is used as hydrogen storage media. Those MHs that have been primarily investigated to date for high-temperature energy-storage applications are MgH2, TiH2, and CaH2. Figure 26b illustrates a CSP plant coupled with an MH-TES system to run the steam power plant [180]. This system comprises of three sections: solar concentration section, MH-TES system, and steam power plant based on the Rankine cycle. The TES system shown uses LTMH and HTMH. Excess solar energy during the day is stored in HTMH via HTF. HTMH can absorb a large amount of high-temperature solar energy through the endothermic reaction of releasing hydrogen. LTMH, on the other hand, absorbs the hydrogen released by HTMH through an endothermic reaction. The chemical process can be reversed when solar energy is not available and advanced thermal energy is required to run the power plant. The hydrogen stored in the LTMH can flow from the LTMH reactor (using waste heat desorption) to the HTMH reactor for high-grade heat output. The absorption of hydrogen by HTMH results in the generation of high-temperature heat required by steam generators through an exothermic reaction. The biggest problem of using metal hydrides for heat storage is to solve the problem of hydrogen storage. According to the experimental and modeling results, Jensen et al. [226] designed and manufactured a HTMH for 600–800 °C power generation of about 200 kWh/m3, and a near-room-temperature LTMH to store hydrogen in sunlight until it is needed to generate electricity. Experiments have proved that the prototype can operate reversibly for at least 60 cycles at 635–645 °C and 1 bar hydrogen pressure, and the actual gravimetric energy density is about 800 kJ/kg, it can not only meet the requirements of heat storage but also meet the design requirements of low-temperature hydrogen storage. The use of different types of MH for heat storage in thermochemical reactions at different operating temperatures has been summarized in the review [50].
In addition to integration with solar thermal plants and waste heat recovery, hydrogen-based TES systems can also be integrated with reversible solid oxide fuel cells (RSOFC). The RSOFC device is divided into two modes, the hydrogen storage system is the SOEC mode, the heat storage system is the SOFC mode, and the MH-based TES system can be considered at the same time, as shown in the Fig. 26c [259]. In SOEC mode, the hydrogen released from the RSOFC is absorbed by the MH reactor, and the process releases heat for an exothermic reaction. This heat is used to generate steam, which is fed back to the RSOFC. In the SOFC mode, the MH reactor desorbs H2 in an endothermic reaction by absorbing the waste heat generated by the RSOFC, and combines it with O2 in the RSOFC to generate external power [247]. Giap et al. [90] proposed a novel RSOFC system combined with MH and waste steam. RSOFC works at 1.1 bar and operation temperature is 750 °C MgH2-5 at.% V and LaNi5 are studied as MH materials. The whole adsorption/desorption process takes 8 h, and the system round-trip efficiency of HTMH system and LTMH system is 45.6% and 48.1%, respectively.
In addition to the above-mentioned TCHS materials using carbonate, MH and MH-based RSOFC for zero-carbon power generation, the industrial use of high-temperature, high-pressure and catalytic ammonia synthesis reactions also generate a lot of heat. The schematic diagram of the ammonia TCHS system is shown in Fig. 26d. Lavine et al., [153] conducted an ammonia-based study of the US Department of Energy's solar thermal cost target by integrating an endothermic reactor into a tower receiver and then synthesizing ammonia into steam that can be used to generate supercritical Steam Rankine cycle. The purpose of this study is to promote the use of ammonia TCES systems in CSP. At temperatures above 650 °C, the operating cost of the developed TCES is less than 15$/kWh. Inorganic hydroxides, mainly Ca (OH)2, are non-toxic and inexpensive. Its decomposition reaction is accompanied by a large amount of reaction heat, so it can be applied to medium and high temperature TCES systems. It is feasible to choose Ca (OH)2-CaO-H2O system for power generation which the cyclic stability and reversibility of the system were also tested [241]. In addition, Liu et al., [167] proposed a high-temperature metal oxide redox pair Mn2O3/Mn3O4 and Co3O4/CoO systems for TCES, with O2 involved in the endothermic process, the operating temperature is 1115 K-1179 K, the findings were used in the design of a 10 kW TCES pilot plant that is currently being tested in a concentrated solar furnace.
Although TCHS has a high heat storage density in the field of high-temperature power generation, the cycle stability, reaction kinetics, reversibility, and thermal conductivity of solid–gas TCES materials must be addressed. In the next stage, researchers should reduce the operating cost of the CSP system by improving the economical, reliable and ecologically compatible performance of storage materials and optimizing the reactor process parameters.
5.2 TCHS in zero-carbon building heating
Building heating as a future heating method most likely to be replaced by renewable energy, and TCHS applied to building heating at a charge temperature of less than 140 °C. Compared to TCHS where thermochemical reactions occur, These TCHS methods occurs by means of adsorption/absorption reactions in the form of solid gas or liquid gas, so this section focuses on the application of adsorption/absorption TCHS to building heating.
5.2.1 Absorption TCHS application
Absorption TCHS, a physical or chemical process in which the molecules of a substance penetrate the surface layer of a liquid or solid by combining with the structure of a solid or liquid, resulting in a change in the composition of the structure. Usually occurs in the liquid–gas absorption process, the liquid acts as the absorbents and the gas acts as the absorbates. Absorption TCHS processes are more focused on cooling and air conditioning systems.
Regarding the absorption technologies developed, absorption refrigeration is the most commonly used technology for solar cooling, Ramen Kanti De et al., [60] proposed a solar-assisted lithium bromide-water vapor absorption system as a new type of system for refrigeration. Solar energy provides thermal energy demand for the steam absorption system, and the side of the cold storage can use the electric energy provided by PV panels. The performance evaluation of the single-effect and double-effect steam absorption systems was carried out. The annual COP of the double-effect steam absorption system is 80% higher than that of the single-effect system, and the exergy efficiency is about 16% higher. Similarly, As shown in Fig. 27, Gao et al., [86] also believed that the energy storage density of the double-stage system for the LiBr-H2O solar vapor absorption system is much higher than that of the single-stage system, which are 233 kJ/kg and 93 kJ/kg, respectively. Increasing the evaporation temperature leads to better performance with higher COP and energy storage density. The experimental results show that the energy storage density is 67 kWh/m3 at the absorption temperature between 12 and 15 °C and the heat storage density is 85 kWh/m3 at the absorption temperature between 15 and 18 °C. Another most common solar vapor absorption refrigeration system uses NH3-H2O as the working medium [186], and the dynamic modeling of the system is carried out. The charging and discharging process is changed according to the concentration of ammonia vapor and ammonia solution. As the ambient temperature of the evaporator decreases, the vapor flow rate gradually decreases, and changes in the refrigerant flow rate, weak solution flow rate, and solution mass density will affect the stored density. It involves very low or no power consumption, and for the same cooling capacity, the size of absorption refrigeration units is mostly smaller compared to other units due to the large amount of heat and mass transfer.
Operation principle of the single-stage and double-stage absorption thermal storage, with permissions requested from [86]
Liquid absorption TCHS can be used not only for absorption liquid refrigeration, but a fraction of material can also be used for space heating and domestic hot water, Fumey et al., [83] a liquid absorption method with sodium hydroxide/water as absorbent mass/agent is proposed in which the solution is diluted from 50wt% to 27wt% and the absorber temperature is raised by 35 K. During desorption, the concentration is raised from 25wt% to 53wt% at a temperature difference of 44 K between the desorber and the condenser. In terms of the concentration difference, a theoretical energy density of 435 kWh/m3 is achieved relative to the discharged absorber. This development achieves compact, lossless, long-term thermal storage for space heating and domestic hot water.
5.2.2 Adsorption TCHS application
Adsorption thermochemical reaction heat storage is defined as a surface phenomenon in which gas or liquid molecules attach to the solid surface of an adsorbent. Adsorption is generally based on the property of solid surfaces to reversibly immobilize certain molecules through weak van der Waals bonds. When adsorption is mentioned, it is generally considered solid/gas adsorption, and according to the binding force between the two phases, adsorption is classified into two forms: physisorption and chemisorption [89]. The main interaction force of physisorption is the van der Waals force, which can be defined as a type of adsorption that occurs when most particles come into contact with a solid or liquid surface. Chemisorption is characterized by chemical interactions using covalent forces similar to those that occur in compound formation.
Common physisorption mainly includes a surface adsorption on solid surfaces such as zeolite, metal–organic frameworks (MOF), and silica gel, while chemisorption mainly includes dehydration/water absorption reactions of different types of hydrated salts. According to the IEA [121], about 36% of the world's energy consumption is used in buildings, accounting for about 40% of the total global CO2 emissions. Most of the energy used in buildings is for space heating and domestic hot water. For adsorbents as pure solids, relevant experiments have been carried out in the early years, in 2007, ZAE has produced a 7000 kg open system using zeolite 13X [106], and proposed the direct use of zeolite 13X for heat storage [114], as shown in Fig. 28a, Johannes et al., [130] designed an open system using solar heat storage in building heating, using 40 kg of zeolite, setting the airflow inlet at 20 °C, flow rate of 180 m3/h, specific humidity of dry air, the test results show that the temperature at the outlet of each zeolite container rose by an average of 38 °C in 8 h, and the discharge temperature at 120–180 °C to meet the summer temperature requirements. Another experiment found that an open adsorption reactor loaded with 40 kg of pure zeolite could provide a constant power of 2.25 kW for more than two hours, which corresponds to a material exothermic capacity of 27.5 W/kg [262]. In addition to zeolite, another solid porous adsorbent material that is commonly adapted to open systems is silica gel. Hesham et al., [109] proposed a laboratory study using silica gel as an adsorbent, and the maximum heat storage density was 325.8 MJ/m3 when the filling volume was 0.5 L, as the air humidity increased, the heat storage density increased and the emission temperature also increased. Strong et al., [256] varied the operating parameters (relative humidity, particle size, desorption temperature and flow rate) to optimize the performance of an open batch silica gel/water vapor adsorption energy storage system for an open TCHS system. It was found that for a 50 g silica gel system the desorption temperature was 120 °C, the flow rate was 24 SLPM, the relative humidity of the adsorption inlet was 90%, and the 12–20 mesh particle size. Under these conditions, an energy storage density of 200.7 kWh/m3 was achieved with a maximum temperature rise of 28.5 °C and maximum and average system thermal power of 308 W/kg and 118 W/kg, respectively. The same MOF [111] are also used as common pure solids for thermal storage.
In addition to pure solids as adsorbent, chemisorption heat storage using hydrated salts has been studied and experimentally applied. For example, MgSO4 [268] with a charge/discharge temperature of 150/30 °C and a thermal storage density of 2.8 GJ/m3, K2CO3 [134] with a thermal storage density of 1.3 GJ/m3 and MgCl2 and CuSO4 with a thermal storage density of more than 2.5 GJ/m3 [69]. In the latest research progress, Li et al., [159] evaluated dozens of different types of brine compounds for adsorption-assisted thermal storage matched to domestic hot water, as shown in Fig. 28b, for a pressurized-assisted adsorption system driven by low-grade solar energy to achieve different temperature rises by regulating water vapor pressure. Considering the energy density, temperature rise and thermal economy performance, SrBr2-6H2O, K2CO3-1.5H2O and LiOH-H2O are the more promising hydrates in the system with the best energy efficiency of 82.47%, 55.08% and 63.97%. The temperature enhancements achieved by the above salt-based single-stage and two-stage pressurized systems were 17.6–19.9 °C and 32.2–37.8 °C, respectively, compared to the conventional TCHS cycle. Developing new materials, especially cost-effective or composite adsorption TCHS materials with particularly high performance, is an important direction for future adsorption heat storage.
Composite adsorption materials are composed of at least two constituents, one of which provides structural support (host matrix) and the other is the active substance that performs the sorption process. The porosity of the host matrix should be high in order to retain as much active material as possible. The design of the material requires a balance between pores filled by active substances and free pores to improve the ability of the adsorbent to be adsorbed [82]. If the host matrix pore size is small, it can also provide heat for adsorption, however, the amount of hydrated salt will be reduced. On the other hand, a large pore size can accommodate more salt, but the host matrix is not involved in the adsorption process [240]. Miao et al., [184] prepared a composite adsorption TCHS using EG and MgSO4. EG has good thermal conductivity, high porosity and large specific surface area, and is a very promising support matrix. The composite was applied to an open TCHS system in the temperature range of 20–120 °C. The heat of reaction increased significantly with the increase of salt content, and according to the latent heat values of different composites, only the salt in the composite was involved in the thermal storage reaction, while the matrix was not. The use of EG not only increased the thermal conductivity of the composites by about 84.8%, but also reduced the hydration time by about 1/4. In addition to using EG as a host matrix, Chao et al., [45] developed a novel zeolite 13X/MgCl2 high energy density heat absorption cell based on the composite water absorption work pair of Zeolite 13X for integrated heat and cold storage, as shown in Fig. 28c. Compared with zeolite 13X, the energy density of zeolite 13X/MgCl2 can be increased by 15.1%, which exhibits the unique ability to achieve high power/energy density for heat and cold storage, with average power densities of 279.66 W/kg and 242.95 W/kg, average energy densities as high as 686.86 kJ/kg and 597.13 kJ/kg, which is better than the current SHS or LHS. The proposed zeolite 13X/MgCl2 based adsorption type thermal cell provides a promising way to achieve high density heat and cold storage based on one thermal energy storage device simultaneously.
As seen from previously published articles, most studies on adsorption-based thermochemical energy storage of composites are usually directed to the preparation of material aspects. SrBr2/expanded vermiculite [66] composites prepared by impregnation method. Ideal for achieving space heating in low-energy homes, SrBr2 encapsulated in mesoporous MIL-101(Cr) MOF is capable of achieving a thermal storage density of 233 kWh/m3 [53]. Skrylnyk et al., [251] tested a laboratory-scale prototype of a proposed packed-bed adsorption thermochemical reactor for TCHS from domestic waste. In the experiment, a salt-based composite consisting of silica gel and 43wt% CaCl2 salt was used as the test adsorbent, and the air temperature was set in the range of 17–40 °C. A prototype filled with 245 g of material was evaluated for temperature and energy performance at a water vapor inlet pressure of 12.5 mbar. The output has an average temperature rise of 9–13 °C and inside the packed bed an average specific thermal power of 168–267 W/kg, with a volumetric energy storage density between 1.0 and 1.6 GJ/m3. Also, Zhang et al., [301, 303] used an activated alumina/LiCl composite sorbent to study a 1 kWh lab-scale open sorption prototype for storing low-temperature heat for space heating, and the system efficiency ranging from 84.5% to 96.9%. Over a period of 7.1 h, air at temperature over 30 °C could be supplied, equating to a total volumetric storage density of 191 kWh/m3. In the future, it is necessary to develop a composite adsorption heat storage system, research new low-cost ad/absorption materials, and improve the overall heat storage efficiency of the system.
5.3 TCHS in zero-carbon traffic
At present, the research focus of internal combustion engine vehicles is to use three-way catalysts to convert NO into N2. Preheating the engine is considered an important measure to reduce fuel consumption and reduce environmental pollution. The application of TCHS technology to the engine is an effective way to reduce energy consumption and pollutant emissions during the cold start process of automobiles.
5.3.1 Inorganic oxide adsorption reaction
The engine coolant is preheated by inorganic oxide/water adsorption reaction. Darkwa et al., [133] proposed a model for heat generation by adsorption of calcium oxide and water, and carried out experiments and theoretical calculations. During the experiment, 1/2 mol of calcium oxide and water were sampled for adsorption reaction. The temperature is raised from 20 °C to 220 °C, which is 17% different from the theoretical calculation result, and it can be considered to heat the engine coolant. Callaghant et al., [56] utilized the adsorption and desorption of inorganic oxides and atmospheric water vapor for heat release and storage, as shown in Fig. 29a. The adsorption process of moist air and inorganic oxides by turning on the water pump, by the heat generated heats the coolant of the engine, and has a preheating effect on the cold start of the engine. When the temperature reaches a predetermined temperature, the engine is started and the fuel is burned. The high-temperature exhaust gas produced afterward can meet the three-way catalytic temperature requirements. Some waste heat is also used in the desorption process which can recovers the ambient temperature of the engine and the exhaust gas temperature so that the adsorbate can be reused. Adsorption-based TCHS use solid–gas or liquid–gas adsorption as storage materials. These technologies have high heat storage density, long-term heat storage without heat loss, and miniaturization and compact equipment are likely to be used in EVs. Narayanan et al., [193] designed an adsorption-based thermal battery capable of providing heating and cooling to vehicles, as shown in Fig. 29b. The adsorbent in the design is NaX zeolite, and the refrigerant is water. The results show a heating and cooling capacity of more than 2.5 kWh for a compact thermal battery with a volume of up to 30 L.
5.3.2 Metal hydride
In addition to the use of inorganic oxide adsorption processes, for the use of waste heat in engines can also be carried out dehydrogenation reactions of metal hydrides. Beard et al., [24] designed a device consisting of metal and metal hydride capable of storing hydrogen. By controlling the switch, 2 g of hydrogen is transferred to the metal to instantly form a metal hydride, which is accompanied by a large amount of heat. The heat generated in this process heats the exhaust gas, which in turn raises the temperature of the exhaust gas entering the three-way catalytic converter to the set temperature, reducing the emission of pollutants. The heat storage process uses the temperature of the exhaust gas discharged from the engine to decompose the metal hydride to store heat. Considering that the car will need heating, ventilation and cooling during transportation, Fang et al., [78] developed thermal battery prototypes with two TCHS methods, using catalytic MgH2 and TiV0.62Mn1.5 as HT and LT hydride. The results showed that the cooling COP was 0.384, and the total cooling and heating energy were 13.6 and 35.4Wh, respectively.
5.4 TCHS in zero-carbon life science
TCHS can not only be used for power generation, buildings, transportation, etc., but with its high heat of reaction, it has been used in self-heating food in the early days, which can help heat the instant food or beverage inside. An exothermic reaction is used as a heat source. This product is useful for military operations, during natural disasters, mountain climbers, and any time traditional cooking is not possible. Bohra et al., [34] compared the heat released by the reaction of phosphorus pentoxide with water in the presence of calcium oxide and the heat of adsorption of zeolite. Although phosphorus pentoxide can release a large amount of heat energy when it meets water, due to its corrosion to metals and harm to the human body, zeolite 3A was finally selected as the heat source for self-heating food cans. Environmentally friendly and user-friendly, zeolite 3A can be dehydrated after use so it can be reactivated to make it reusable. In addition to utilizing zeolite or calcium oxide as a self-heating heat source, Picard et al. [208] proposed a self-heating group meal assembly using an exothermic chemical heater containing a metallic magnesium-iron alloy. Lamensdorf et al., [152] improved the Mg-Fe alloy heater through experiments to improve the heat release performance. In short, the use of high reaction heat of TCHS for self-heating food has been widely used in the food field, and it can meet the food heating requirements without consuming additional energy, which provides convenience.
5.5 TCHS in zero-carbon society
CCSU are not only the main technical means for the low-carbon utilization of fossil energy, but also the underlying technical guarantee for achieving the goal of carbon neutrality. Calcium cycle technology uses the carbonation and calcination cycle reaction of CaO to capture CO2, which is one of the promising large-scale CO2 capture technologies. CaL is an attractive reaction that has been extensively studied in CCSU as a means of TCHS [265].
In recent years, [266] simulating direct concentrated radiative heating from solar energy sources at the laboratory scale, TCHS and CCUS were experimentally compared using the integration between CSP and CaL, as seen in Fig. 30a. The results show that under the condition of CaL-TCHS, the heat storage density is in the range of 960–1130 MJ/m3. Correspondingly, the energy storage density under CCSU conditions is about 780–960 MJ/m3. The energy storage density are all higher than the SHS density of solar energy using molten salts in the range of 290–565 °C. Additionally, the CaL option has better long-term storage stability and higher temperature levels compared to molten salt. The CaL-TCHS is able to guarantee the maximum energy storage density, however, the CaL-CCSU scheme takes full advantage of the overall reduction of the carbon footprint of the integrated process, capturing concentrated CO2. To increase the share of renewable energy and reduce CO2 emissions, Claudio Tregambi et al. [265] used concentrated solar energy to capture CO2 from combustion power plants via the CaL, and then reacted CO2 with hydrogen obtained from water electrolysis to generate methane (power-to-gas), as shown in Fig. 30b. Electrolyzed water could be powered by PVs or excess renewable energy. Simulation of the operating conditions of a municipal solid waste incineration plant located in Manfredonia (Italy). Modeling results show that CO2 capture rates can vary from 30 to 85%. The input thermal power of concentrated solar power is between 50 and 175 MWth, and the operation is 12 h. A portion of this energy could be integrated into the power cycle for power generation, boosting the potential of the original combustion unit. In the end, the plant has an overall efficiency of 20–22%, while capturing carbon dioxide, harnessing renewable energy solar and producing methane from carbon dioxide. Although CaL can be used for both TCHS and CCUS, a significant disadvantage is that at the required high temperature and high partial pressure of CO2, the sintering of CaO grains leads to gradual deactivation during cycling. However, researchers have proposed several techniques such as adding inert stabilizers Al2O3 [27], SiO2 [231], Sun, [158], to improve the performance and cycle stability of Ca-based materials or doping CaO with binary metal compounds of Mg and Fe by sol–gel method [265]. Han et al [102] proposed to synthesize CaO-based inert oxide-stabilized composites by chemical vapor deposition process, in order to prevent the deactivation of CaO due to sintering. The inert materials of the stabilizer include Al2O3, SiO2 and TiO2. Al2O3 produced the most stable composite during the experiments. The composite containing only 5 mol.% Al exhibits an excellent CO2 absorption capacity, without any deactivation over 50 cycles and maintains an energy density of 1.50 GJ/t, which corresponds to the theoretical maximum 87% of the total. This high stability is attributed to a unique synthesis strategy in which thermally stable oxide nanoparticles are deposited on the surface of CaO grains and prevent their aggregation and overgrowth.
At present, TCHS in the direction of CCUS is mainly based on the CaL, which can not only capture CO2 in the atmosphere but also store heat. However, after many cycles, the overall carbon capture efficiency decreases due to the effect of sintering, therefore, it is of great significance to develop stable new heat storage materials and design new catalysts for CCUS systems.
6 Future development and challenges
Carbon neutrality is becoming increasingly important as the world grapples with the threat of climate change. Many countries and organizations have set ambitious goals to achieve carbon neutrality within a certain period of time, achieving net zero carbon emissions by 2050. To achieve carbon neutrality, carbon emissions can be reduced as much as possible through measures such as improving energy efficiency, switching to renewable energy sources, and reducing waste. Utilizing the existing TES technology to store and release thermal energy to ensure the thermal stability of time and space can also help achieve carbon neutrality. Nevertheless, TES technology still faces challenges in power generation, building, transportation, life sciences, and society. In addition, to achieve carbon neutrality requires the joint efforts of policy measures, technological innovation, personal and social change and so on.
In view of the comparisons of different types of TES already presented in Tables 2, 3, and 4, the problems that need to be solved and the directions of research for the application of TES technology in different aspects of carbon neutrality in the future are as follows.
-
(1)
Taking water as an example, in order to improve the efficiency of SHS, the thermal stratification of water in the heat storage tank should be strengthened in the future, and the flow rate of water inlet and outlet and the size of the heat storage tank should be strengthened. Measures such as adopting more cost-effective insulation technology and reducing heat loss to the outside world. In the future, new SHS materials will be developed to improve the heat storage density and thermal stability of SHS materials. In terms of system design, it is necessary to consider multiple aspects of heat transfer, storage, and release to optimize the SHS system to improve the energy conversion efficiency and service life of the system.
-
(2)
For LHS technology, the core is PCM. At present, the heat storage density of existing PCMs is limited, and problems such as phase separation, supercooling, and low thermal conductivity are prone to occur in long-term use. Future research can explore new heat storage materials, such as developing thickeners, nucleating agents, and high thermal conductivity agents to improve heat storage density and thermal stability. At present, microencapsulated PCM technology can increase the surface heat transfer area, prevent PCM leakage, and solve problems such as supercooling. Therefore, encapsulating PCM technology is a promising approach to improve the performance of LHS in the future. In addition to the combination of LHS technology with traditional solar energy, it can also be combined with ground source heat pump systems and biomass water heaters in the future to achieve more efficient energy conversion and utilization efficiency.
-
(3)
In future, TCHS should focus on the development of low-cost, long-life, high cycle stability heat storage materials and improvements to the reactor to reduce investment costs, matching and control of chemical reaction rates and heat transfer rates. For the selection of reactant materials, the TCHS materials with optimized environment, wide source range and high catalytic performance should be selected as far as possible. The design of the TCHS system should also take into account the optimization of the entire heat storage system, including heat storage, heat transfer pipes, and control systems, etc.
7 Conclusions
In response to the energy crisis and the emission of GHGs, TES technology has played a very important role in achieving the goal of global carbon neutrality as soon as possible. This article summarizes the application of SHS, LHS and TCHS technologies in different zero-carbon fields in recent years. The heat storage mechanism of different types of heat storage methods is summarized, and the application of heat storage methods in different zero-carbon situations and the future improvement direction in the zero-carbon field are discussed. The details are as follows.
-
(1)
To describe the performance of the TES method, it can be described in terms of heat storage density, investment and operating costs, cycle stability and energy storage efficiency. According to the heat storage density, the maximum heat storage density is TCHS, the heat storage density can reach 1000 MJ/m3, followed by the LHS of 300–500 MJ/m3, and finally the SHS with an average energy storage density of 100 MJ/m3. The cost of SHS is low, while the cost of TCHS is high. Considering the cycle stability and energy storage efficiency, TCHS can be stored for a long time for several years without loss, LHS can store heat across seasons, while SHS heat storage capacity can only be maintained for a few days.
-
(2)
For zero-carbon buildings, mainly refers to domestic hot water, space heating and HVAC, high thermal inertia materials are generally used, and also can be used in the building field with low heat dissipation such as groundwater and underground soil. However, SHS heat storage occupies a large volume and is heavily influenced by temperature. At present, building materials are filled with organic PCMs with suitable phase change temperatures such as paraffin and fatty acids, which can absorb solar radiation heat during the day and improve the thermal comfort of buildings. In addition to sensible and latent heat methods, using TCHS methods currently available are solid adsorption methods, which are stored or released in buildings through hot water pipe networks or solar heat.
-
(3)
For zero-carbon power generation, it can be divided into CSP and PV power generation, while CSP is usually divided into two categories: DSPG and ISPG, direct steam generation usually uses high-temperature molten salt, fluidized particles, etc., to generate steam through heat exchange in the tower collector, while indirect steam generation uses the high heat storage characteristics and heat storage characteristics of molten salt and alloys in PCM. For TCHS, the use of chemical reaction heat storage, the high temperature heat of solar radiation is stored, and the heat is released to generate steam for power generation. PV power generation mainly using PCM to regulate the surface temperature of PV panels during the day to improve photoelectric conversion efficiency.
-
(4)
For zero-carbon transportation, the main discussion of this review is that the current fuel vehicles and EVs are the research objects. For fuel vehicles, the use of PCMs is mainly to preheat the vehicle engine and improve the cooling efficiency of the vehicle cooling system. For EVs, the TES method is mainly to dissipate heat from the battery pack, using air or liquid as sensible heat to dissipate heat, which has low heat dissipation efficiency and uneven temperature. PCMs are used to store heat around the battery pack, it can reduce the maximum temperature and improve the uniformity of the battery, and the use of PCMs in winter can keep the battery warm.
-
(5)
For zero-carbon life science, this paper focuses on the application of heat storage technology in food, textile and medical treatment. Among them, the low-temperature heat storage material hydrated salt solution is relatively mature in low-temperature preservation of food, and the problems of phase separation and supercooling also need to be solved. If the phase transition temperature is not much different from body temperature, it can be used in textiles, and heat storage materials with good biocompatibility can be used in medical treatment for drug delivery. In the future, more efficient and stable heat storage materials need to be developed.
-
(6)
For zero-carbon society, carbon capture can fundamentally reduce the concentration of GHGs, carbon storage and carbon utilization can store CO2 permanently in the ground and oceans, or convert CO2 into valuable chemicals and products, reducing dependence on fossil fuels. Heat storage technology can be used in the adsorption and desorption cycle in carbon capture, absorbing greenhouse gases such as CO2, and releasing heat during the desorption process. Especially in industry, areas with rich waste heat resources such as iron and steel plants and cement plants can capture and store CO2. In addition, the use of CaL in the TCHS process can also capture CO2 into CaCO3. It should be noted that sintering phenomenon is prone to occur during CaL, so the development of carbon capture materials with high cycle life is very important in the future.
8 Nomenclature
Tinitial Initial temperature of heat storage material (°C)
Tmelt Phase transition temperature of heat storage material (°C)
Tfinal Final temperature of heat storage material (°C)
Cp, s Solid specific heat capacity (kJ/kg·K)
Cp, l Liquid specific heat capacity (kJ/kg·K)
ΔH Phase transition enthalpy (kJ/kg)
m Mass of heat storage material (kg)
A/B TCHS reactants
AB TCHS products
Availability of data and materials
Not applicable.
Abbreviations
- TES:
-
Thermal Energy Storage
- IPCC:
-
Intergovernmental Panel on Climate Change
- IEA:
-
International Energy Agency
- CCUS:
-
Carbon Capture Utilization and Storage
- CCS:
-
Carbon Capture and Storage
- SHS:
-
Sensible Heat Storage
- LHS:
-
Latent Heat Storage
- TCHS:
-
Thermochemical Heat Storage
- GHG:
-
Greenhouse Gases
- PCM:
-
Phase Change Material
- COP:
-
Coefficient of Performance
- HVAC:
-
Heating, Ventilation and Air Conditioning
- CPCM:
-
Composite Phase Change Material
- MLPCM:
-
Multilayer Phase Change Materials
- EG:
-
Expanded Graphite
- EV:
-
Electric Vehicle
- BTMS:
-
Battery Thermal Management System
- DSPG:
-
Direct Steam Power Generation
- DSC:
-
Differential Scanning Calorimeter
- ISPG:
-
Indirect Steam Power Generation
- CSP:
-
Concentrate Solar Power
- HTF:
-
Heat Transfer Fluid
- CWS:
-
Chilled Water Storage
- HP:
-
Heat Pump
- CaL:
-
Calcium Looping
- MH:
-
Metal Hydride
- HTMH:
-
High Temperature Metal Hydride
- LTMH:
-
Low Temperature Metal Hydride
- MOF:
-
Metal-Organic Frameworks
- PV:
-
Photovoltaic
- PTC:
-
Positive Temperature Coefficient
- SOEC:
-
Solid Oxide Electrolyzer Cell
- SOFC:
-
Solid Oxide Fuel Cell
References
Sami A. Al-Sanea, Zedan MF, Al-Hussain SN (2012). Effect of thermal mass on performance of insulated building walls and the concept of energy savings potential. Appl Energy 89(1):430–442. https://doi.org/10.1016/j.apenergy.2011.08.009
Ki-bum Kim, Kyung-wook Choi, Young-jin Kim, Ki-hyung Lee, Kwan-soo Lee (2010). Feasibility study on a novel cooling technique using a phase change material in an automotive engine. Energy 35(1):478–484. https://doi.org/10.1016/j.energy.2009.10.015
Suman Basu, Krishnan S. Hariharan, Subramanya Mayya Kolake, Taewon Song, Dong Kee Sohn, Taejung Yeo (2016). Coupled electrochemical thermal modelling of a novel Li-ion battery pack thermal management system - Science Direct. Appl Energy 181:1–13. https://doi.org/10.1016/j.apenergy.2016.08.049
Abdelhamid ME, O’Mullane AP, Snook GA (2015) Storing energy in plastics: a review on conducting polymers & their role in electrochemical energy storage. RSC Adv 5(15):11611–11626. https://doi.org/10.1039/C4RA15947K
Abdolmaleki L, Sadrameli SM, Pirvaram A (2020) Application of environmental friendly and eutectic phase change materials for the efficiency enhancement of household freezers. Renewable Energy 145:233–241. https://doi.org/10.1016/j.renene.2019.06.035
Abedin A (2011) A critical review of thermochemical energy storage systems. Open Renew Energy J 4. https://doi.org/10.2174/1876387101004010042
Abi Mathew A, Thangavel V (2021) A novel thermal storage integrated evacuated tube heat pipe solar air heater: Energy, exergy, economic and environmental impact analysis. Sol Energy 220:828–842. https://doi.org/10.1016/j.solener.2021.03.057
Adinberg R, Zvegilsky D, Epstein M (2010) Heat transfer efficient thermal energy storage for steam generation. Energy Convers Manage 51(1):9–15. https://doi.org/10.1016/j.enconman.2009.08.006
Afroz HMM, Khan MIH (2013) Effect of Phase Change Material on Performance of a Household Refrigerator. Asian J Appl Sci 6:56–67. https://doi.org/10.3923/ajaps.2013.56.67
Agency IE (2013) Transition to sustainable buildings: strategies and opportunities to 2050, Transition to sustainable buildings: strategies and opportunities to 2050. https://doi.org/10.1787/9789264202955-en
Ahmad H, Hamza A, Ali S (2016) Energy efficiency enhancement of photovoltaics by phase change materials through thermal energy recovery. Energies 9(10):1–15. https://doi.org/10.3390/en9100782
Al-Hallaj S, Kizilel R, Lateef A, Sabbah R, Farid M, Selman JR (2005). Passive thermal management using phase change material (PCM) for EV and HEV Li-ion batteries. 2005 IEEE Vehicle Power and Propulsion Conference, Chicago, IL, USA, 2005, pp. 5. https://doi.org/10.1109/VPPC.2005.1554585
Al-Temeemi AA, Harris DJ (2004) A guideline for assessing the suitability of earth-sheltered mass-housing in hot-arid climates. Energy Buildings 36(3):251–260. https://doi.org/10.1016/j.enbuild.2003.12.005
Alva G, Lin Y, Fang G (2018) An overview of thermal energy storage systems. Energy 144:341–378. https://doi.org/10.1016/j.energy.2017.12.037
Andrepont JS (2006) Stratified Low-Temperature Fluid Thermal Energy Storage (TES) in a Major Convention District – Aging Gracefully, as Fine Wine. ASHRAE Trans 112(1):667–675
Arunachalam S (2019) Latent heat storage: container geometry, enhancement techniques, and applications—a review. J Solar Energy Eng 141(5). https://doi.org/10.1115/1.4043126
Ayyappan S, Mayilsamy K, Sreenarayanan VV (2016) Performance improvement studies in a solar greenhouse drier using sensible heat storage materials. Heat Mass Transf 52(3):459–467. https://doi.org/10.1007/s00231-015-1568-5
Badwal SP, Giddey SS, Munnings C, Bhatt AI, Hollenkamp AF (2014) Emerging electrochemical energy conversion and storage technologies. Front Chem 2:79. https://doi.org/10.3389/fchem.2014.00079
Bagherisereshki E, Tran J, Lei F, Auyeung N (2018) Investigation into SrO/SrCO 3 for high temperature thermochemical energy storage. Sol Energy 160:85–93. https://doi.org/10.1016/j.solener.2017.11.073
Bahnfleth WP, Jing S (2005) Constant flow rate charging characteristics of a full-scale stratified chilled water storage tank with double-ring slotted pipe diffusers. Appl Therm Eng 25(17–18):3067–3082. https://doi.org/10.1016/j.applthermaleng.2005.03.013
Bai F, Chen M, Song W, Feng Z, Li Y, Ding Y (2017) Thermal management performances of PCM/water cooling-plate using for lithium-ion battery module based on non-uniform internal heat source. Appl Therm Eng 126:17–27. https://doi.org/10.1016/j.applthermaleng.2017.07.141
Bales C, Jaenig D, Gantenbein P, Weber R (2007) Laboratory Prototypes of Thermo-Chemical and Sorption Storage Units: Report B3 of Subtask B. Paris: International Energy Agency, Solar Heating and Cooling Programme
Bauer T, Pfleger N, Breidenbach N, Eck M, Laing D, Kaesche S (2013) Material aspects of Solar Salt for sensible heat storage. Appl Energy 111(Nov):1114–1119. https://doi.org/10.1016/j.apenergy.2013.04.072
Beard and Jonathan (1995) Hot catalysts make for a clean cold start. New Scientist 147(1991):20–20
Beaudin M, Zareipour H, Schellenberglabe A, Rosehart W (2010) Energy storage for mitigating the variability of renewable electricity sources: An updated review. Energy Sustain Dev 14(4):302–314. https://doi.org/10.1016/j.esd.2010.09.007
Becherif M, Ramadan HS, Cabaret K, Picard F, Simoncini N, Bethoux O (2015) Hydrogen energy storage: new techno-economic emergence solution analysis. Energy Procedia 74:371–380. https://doi.org/10.1016/j.egypro.2015.07.629
Benitez-Guerrero M, Valverde JM, Sanchez-Jimenez PE, Perejon A, Perez-Maqueda LA (2018) Calcium-Looping performance of mechanically modified Al2O3-CaO composites for energy storage and CO2 capture. Chem Eng J 334:2343–2355. https://doi.org/10.1016/j.cej.2017.11.183
Besant AG, Hamidi V (2019) Technical challenges in co-location of battery storage and generation plants. J Eng 2019(18):5090–5093. https://doi.org/10.1049/joe.2018.9347
Bissell A, Gataora S, Nicholson J, Doak K (2021) Internally heated phase change material heat batteries, U.S. Patent Application No. 17/263,363
Blakers A, Stocks M, Lu B, Cheng C (2021) A review of pumped hydro energy storage. Progr Energy 3(2):022003. https://doi.org/10.1088/2516-1083/abeb5b
Bloomfield D, Fisk DJ (1977) The optimisation of intermittent heating. Build Environ 12(1):43–55. https://doi.org/10.1016/0360-1323(77)90006-3
Blüher P (1991) Latentwarmespeicher erhht den fahrkomfort und die fahrsicherheit. ATZ Automobiltechnische Zeitschrift 93(10):3–8
Bogdanović B, Reiser A, Schlichte K, Spliethoff B, Tesche B (2002) Thermodynamics and dynamics of the Mg–Fe–H system and its potential for thermochemical thermal energy storage. J Alloy Compd 345(1):77–89. https://doi.org/10.1016/S0925-8388(02)00308-0
Bohra B, Gawit N, Chavan R, Kapse R (2015) An eco-friendly and reusable heat source for self-heating food packaging. Int J Eng Res V4. https://doi.org/10.17577/IJERTV4IS050462
Boissiere B, Ansart R, Gauthier D, Flamant G, Hemati M (2015) Experimental hydrodynamic study of gas-particle dense suspension upward flow for application as new heat transfer and storage fluid. Can J Chem Eng 93(2):317–330. https://doi.org/10.1002/cjce.22087
Bondarenko VL, Ilyinskaya DN, Kazakova AA, Kozlovtsev PS, Lavrov NA, Razenko EA (2022) Hydrogen Storage. Chem Pet Eng 57(11):1026–1032. https://doi.org/10.1007/s10556-022-01041-z
Bongaarts J (2019) Intergovernmental panel on climate change special report on global warming of 1.5°C Switzerland: IPCC, 2018. Popul Dev Rev 45(1):251–252
Boonnasa S, Namprakai P (2010) The chilled water storage analysis for a university building cooling system. Appl Therm Eng 30(11):1396–1408. https://doi.org/10.1016/j.applthermaleng.2010.02.029
Borissova AV, Popov D (2020). An option for integration of Carnot Battery into a small Nuclear Power Plant. In E3S Web of Conferences Vol. 207, p. 01027. EDP Sciences. https://doi.org/10.1051/e3sconf/202020701027
Budt M, Wolf D, Span R, Yan J (2016) A review on compressed air energy storage: Basic principles, past milestones and recent developments. Appl Energy 170:250–268. https://doi.org/10.1016/j.apenergy.2016.02.108
Burch SD, Keyser MA, Colucci CP, Potter TF, Benson DK, Biel JP, Beil JP (1996). Applications and benefits of catalytic converter thermal management. SAE Fuels & Lubricants Spring Meeting, Dearborn. https://doi.org/10.4271/961134
Burch SD, Potter TF, Keyser MA, Brady MJ, Michaels KF. (1995). Reducing cold-start emissions by catalytic converter thermal management. SAE Transactions, 104:348–353. http://www.jstor.org/stable/44615090
Burmistrov I, Khanna R, Gorshkov N, Kiselev N, Artyukhov D, Boychenko E, Yudin A, Konyukhov Y, Kravchenko M, Gorokhovsky A (2022) Advances in Thermo-Electrochemical (TEC) Cell Performances for Harvesting Low-Grade Heat Energy: A Review. Sustainability 14(15):9483. https://doi.org/10.3390/su14159483
Chan CW, Ling-Chin J, Roskilly AP (2013) A review of chemical heat pumps, thermodynamic cycles and thermal energy storage technologies for low grade heat utilisation. Appl Therm Eng 50(1):1257–1273. https://doi.org/10.1016/j.applthermaleng.2012.06.041
Chao J, Xu J, Bai Z, Wang P, Wang R, Li T (2023) Integrated heat and cold storage enabled by high-energy-density sorption thermal battery based on zeolite/MgCl2 composite sorbent. J Energy Storage 64:107155. https://doi.org/10.1016/j.est.2023.107155
Chen J, Wang Y, Ma B, Guan L, Tian Z, Lin K, Zhu Y (2020) Biodegradable hollow mesoporous organosilica-based nanosystems with dual stimuli-responsive drug delivery for efficient tumor inhibition by synergistic chemo- and photothermal therapy. Appl Mater Today 19:100655. https://doi.org/10.1016/j.apmt.2020.100655
Chen L, Zhang L, Yang H, Xie M, Ye K (2022) Dynamic simulation of a Re-compressed adiabatic compressed air energy storage (RA-CAES) system. Energy 261:125351. https://doi.org/10.1016/j.energy.2022.125351
Chen X, Jin X, Ling X, Wang Y (2020) Exergy analysis of concentrated solar power plants with thermochemical energy storage based on calcium looping. ACS Sustainable Chem. Eng. 8:7928–41. https://doi.org/10.1021/acssuschemeng.0c01586
Chen Y, Zang B (2022) Analysis of alternating flux density harmonics inside the rotor of a 1 MW high-speed interior permanent magnet synchronous machine used for flywheel energy storage systems. J Energy Storage 55:105664. https://doi.org/10.1016/j.est.2022.105664
Choudhari MS, Sharma VK, Paswan M (2021) Metal hydrides for thermochemical energy storage applications. Int J Energy Res 45(10):14465–14492. https://doi.org/10.1002/er.6818
Choudhury S (2021) Flywheel energy storage systems: A critical review on technologies, applications, and future prospects. Int Transact Electr Energy Syst 31(9):e13024. https://doi.org/10.1002/2050-7038.13024
Cooper C, Sovacool BK (2013) Miracle or mirage? The promise and peril of desert energy part 1. Renewable Energy 50:820–825. https://doi.org/10.1016/j.renene.2012.07.027
D’Ans P, Courbon E, Permyakova A, Nouar F, Simonnet-Jégat C, Bourdreux F, Malet L, Serre C, Frère M, Steunou N (2019) A new strontium bromide MOF composite with improved performance for solar energy storage application. Journal of Energy Storage 25:100881. https://doi.org/10.1016/j.est.2019.100881
Dahariya S, Patel N, Egbo MK, Hwang G, Betz AR (2020) High-Pressure Pool-Boiling Heat Transfer Enhancement Mechanism on Sintered-Particle Wick Surface. Front Mech Engineering 5. https://doi.org/10.3389/fmech.2019.00071
Dannemand M, Dragsted J, Fan J, Johansen JB, Kong W, Furbo S (2016) Experimental investigations on prototype heat storage units utilizing stable supercooling of sodium acetate trihydrate mixtures. Appl Energy 169:72–80. https://doi.org/10.1016/j.apenergy.2016.02.038
Darkwa K, O’Callaghan PW (1997) Green transport technology (GTT): Analytical studies of a thermochemical store for minimising energy consumption and air pollution from automobile engines. Appl Therm Eng 17(7):603–614. https://doi.org/10.1016/S1359-4311(97)80001-4
Davidson JH, Quinnell J, Burch J, Zondag HA, Boer RD, Finck CJ, Cuypers R, Cabeza LF, Heinz A, Jahnig D (2013). Development of space heating and domestic hot water systems with compact thermal energy storage. Compact thermal energy storage: Material development for System Integration. Iea Solar Heating and Cooling
de Boer R, Haije WG, Veldhuis JBJ (2002) Determination of structural, thermodynamic and phase properties in the Na2S–H2O system for application in a chemical heat pump. Thermochim Acta 395(1):3–19. https://doi.org/10.1016/S0040-6031(02)00158-2
de Gracia A, Navarro L, Castell A, Ruiz-Pardo Á, Álvarez S, Cabeza LF (2013) Thermal analysis of a ventilated facade with PCM for cooling applications. Energy Buildings 65:508–515. https://doi.org/10.1016/j.enbuild.2013.06.032
De RK, Ganguly A (2020) Performance comparison of solar-driven single and double-effect LiBr-water vapor absorption system based cold storage. Thermal Sci Eng Progr 17:100488. https://doi.org/10.1016/j.tsep.2020.100488
Deng Y, Jiang Y, Liu J (2021) Liquid metal technology in solar power generation - Basics and applications. Sol Energy Mater Sol Cells 222:110925. https://doi.org/10.1016/j.solmat.2020.110925
Dharuman C, Arakeri JH, Srinivasan K (2006) Performance evaluation of an integrated solar water heater as an option for building energy conservation. Energy Buildings 38(3):214–219. https://doi.org/10.1016/j.enbuild.2005.05.007
Di Lauro F, Tregambi C, Montagnaro F, Salatino P, Chirone R, Solimene R (2021) Improving the performance of calcium looping for solar thermochemical energy storage and CO2 capture. Fuel 298:120791. https://doi.org/10.1016/j.fuel.2021.120791
Díaz-Heras M, Calderón A, Navarro M, Almendros-Ibáñez JA, Fernández AI, Barreneche C (2021) Characterization and testing of solid particles to be used in CSP plants: Aging and fluidization tests. Solar Energy Mater Solar Cells 219:110793. https://doi.org/10.1016/j.solmat.2020.110793
Díaz-Heras M, Moya JD, Belmonte JF, Córcoles-Tendero JI, Molina AE, Almendros-Ibáñez JA (2020) CSP on fluidized particles with a beam-down reflector: Comparative study of different fluidization technologies. Sol Energy 200:76–88. https://doi.org/10.1016/j.solener.2019.09.006
Ding B, Xu C, Liao Z, Ye F (2021) Study on long-term thermochemical thermal storage performance based on SrBr 2-expanded vermiculite composite materials. J Energy Storage 42:103081. https://doi.org/10.1016/j.est.2021.103081
Ding W, Bauer T (2021) Progress in research and development of molten chloride salt technology for next generation concentrated solar power plants. Engineering 7(3):334–347. https://doi.org/10.1016/j.eng.2020.06.027
Dirand M, Bouroukba M, Briard A-J, Chevallier V, Petitjean D, Corriou J-P (2002) Temperatures and enthalpies of (solid + solid) and (solid + liquid) transitions of n-alkanes. J Chem Thermodyn 34(8):1255–1277. https://doi.org/10.1006/jcht.2002.0978
Donkers PAJ, Pel L, Adan OCG (2016) Experimental studies for the cyclability of salt hydrates for thermochemical heat storage. J Energy Storage 5:25–32. https://doi.org/10.1016/j.est.2015.11.005
Dumont O, Frate GF, Pillai A, Lecompte S, De Paepe M, Lemort V (2020) Carnot battery technology: A state-of-the-art review. J Energy Storage 32:101756. https://doi.org/10.1016/j.est.2020.101756
Dunn R, Lovegrove K, Burgess G (2012) A review of ammonia-based thermochemical energy storage for concentrating solar power. Proc IEEE 100(2):391–400. https://doi.org/10.1109/JPROC.2011.2166529
Dunn RI, Hearps PJ, Wright MN (2012) Molten-salt power towers: newly commercial concentrating solar storage. Proc- IEEE 100(2):504–515. https://doi.org/10.1109/JPROC.2011.2163739
Dzekan D, Waske A, Nielsch K, Fähler S (2021) Efficient and affordable thermomagnetic materials for harvesting low grade waste heat. APL Mater 9(1):011105. https://doi.org/10.1063/5.0033970
Einstein P (2015). Effective Integration of Seasonal Thermal Energy Storage Systems in existing buildings. European Project 2015. www.einstein-project.eu
Elfeky KE, Ahmed N, Wang Q (2018). Numerical comparison between single PCM and multi-stage PCM based high temperature thermal energy storage for CSP tower plants. Appl Thermal Eng 139:609–622. https://doi.org/10.1016/j.applthermaleng.2018.04.122
Englmair G, Moser C, Schranzhofer H, Fan J, Furbo S (2019) A solar combi-system utilizing stable supercooling of sodium acetate trihydrate for heat storage: Numerical performance investigation. Appl Energy 242:1108–1120. https://doi.org/10.1016/j.apenergy.2019.03.125
Ervin G (1977) Solar heat storage using chemical reactions. J Solid State Chem 22(1):51–61. https://doi.org/10.1016/0022-4596(77)90188-8
Fang ZZ, Zhou C, Fan P, Udell KS, Bowman RC, Vajo JJ et al (2015) Metal hydrides based high energy density thermal battery. J Alloys Compd 645:S184–189. https://doi.org/10.1016/j.jallcom.2014.12.260
Feng PH, Zhao BC, Wang RZ (2020) Thermophysical heat storage for cooling, heating, and power generation: A review. Appl Thermal Eng 166:114728. https://doi.org/10.1016/j.applthermaleng.2019.114728
Ferone C, Colangelo F, Frattini D, Roviello G, Cioffi R, Maggio RD (2014) Finite element method modeling of sensible heat thermal energy storage with innovative concretes and comparative analysis with literature benchmarks. Energies 7(8):5291–5316. https://doi.org/10.3390/en7085291
Figgener J, Stenzel P, Kairies K-P, Linßen J, Haberschusz D, Wessels O, Robinius M, Stolten D, Sauer DU (2021) The development of stationary battery storage systems in Germany – status 2020. J Energy Storage 33:101982. https://doi.org/10.1016/j.est.2020.101982
Fopah Lele A, Kuznik F, Rammelberg HU, Schmidt T, Ruck WKL (2015) Thermal decomposition kinetic of salt hydrates for heat storage systems. Appl Energy 154:447–458. https://doi.org/10.1016/j.apenergy.2015.02.011
Fumey B, Weber R, Baldini L (2017) Liquid sorption heat storage – A proof of concept based on lab measurements with a novel spiral fined heat and mass exchanger design. Appl Energy 200:215–225. https://doi.org/10.1016/j.apenergy.2017.05.056
Furbo S (2015). Using water for heat storage in thermal energy storage (TES) systems. Advances in thermal energy storage systems, Elsevier: 31–47. https://doi.org/10.1533/9781782420965.1.31
Galione PA, Pérez-Segarra CD, Rodríguez I, Torras S, Rigola J (2015) Multi-layered solid-PCM thermocline thermal storage for CSP. Numerical evaluation of its application in a 50MWe plant. Sol Energy 119:134–150. https://doi.org/10.1016/j.solener.2015.06.029
Gao JT, Xu ZY, Wang RZ (2020) Experimental study on a double-stage absorption solar thermal storage system with enhanced energy storage density. Appl Energy 262:114476. https://doi.org/10.1016/j.apenergy.2019.114476
Gao W, Wenxian L, Liu T, Xia C (2007) An Experimental Study on the Heat Storage Performances of Polyalcohols NPG, TAM, PE, and AMPD and their Mixtures as Solid-Solid Phase-Change Materials for Solar Energy Applications. Int J Green Energy 4:301–311. https://doi.org/10.1080/15435070701332112
Gardner S (2016) European commission calls for prioritizing heating, cooling efficiency measures. international environment. Reporter 39(4):216–217
Gbenou TRS, Fopah-Lele A, Wang K (2021) Recent Status and Prospects on Thermochemical Heat Storage Processes and Applications. Entropy 23(8):953. https://doi.org/10.3390/e23080953
Giap V-T, Lee YD, Kim YS, Ahn KY (2020) A novel electrical energy storage system based on a reversible solid oxide fuel cell coupled with metal hydrides and waste steam. Appl Energy 262:114522. https://doi.org/10.1016/j.apenergy.2020.114522
Glasser L (2014) Thermodynamics of inorganic hydration and of humidity control, with an extensive database of salt hydrate pairs. J Chem Eng Data 59:526–530. https://doi.org/10.1021/je401077x
Glasser L, Jenkins HDB (2007) The thermodynamic solvate difference rule: solvation parameters and their use in interpretation of the role of bound solvent in condensed-phase solvates. Inorg Chem 46(23):9768–9778. https://doi.org/10.1021/ic701105p
Goli P, Legedza S, Dhar A, Salgado R, Renteria J, Balandin AA (2014) Graphene-enhanced hybrid phase change materials for thermal management of Li-ion batteries. J Power Sources 248:37–43. https://doi.org/10.1016/j.jpowsour.2013.08.135
Gomez JC (2011). High-temperature phase change materials (PCM) candidates for thermal energy storage (TES) applications, National Renewable Energy Lab.(NREL), Golden, CO (United States). NREL/TP-5500-51446
González-Roubaud E, Pérez-Osorio D, Prieto C (2017) Review of commercial thermal energy storage in concentrated solar power plants: Steam vs. molten salts. Renew Sustain Energy Rev 80:133–148. https://doi.org/10.1016/j.rser.2017.05.084
Gude VG (2015) Energy storage for desalination processes powered by renewable energy and waste heat sources. Appl Energy 137:877–898. https://doi.org/10.1016/j.apenergy.2014.06.061
Guillot S, Faik A, Rakhmatullin A, Lambert J, Veron E, Echegut P, Bessada C, Calvet N, Py X (2012) Corrosion effects between molten salts and thermal storage material for concentrated solar power plants. Appl Energy 94:174–181. https://doi.org/10.1016/j.apenergy.2011.12.057
Gumus M (2009) Reducing cold-start emission from internal combustion engines by means of thermal energy storage system. Appl Therm Eng 29(4):652–660. https://doi.org/10.1016/j.applthermaleng.2008.03.044
Guo J, Cai L, Chen J, Zhou Y (2016) Performance evaluation and parametric choice criteria of a Brayton pumped thermal electricity storage system. Energy 113:693–701. https://doi.org/10.1016/j.energy.2016.07.080
Guo P, Wang Y, Li J, Yuan W (2016) Thermodynamic analysis of a solar chimney power plant system with soil heat storage. Appl Therm Eng 100:1076–1084. https://doi.org/10.1016/j.applthermaleng.2016.03.008
Hadden T. (2017). Thermal storage for electric vehicle cabin heating in cold weather conditions. Hamilton, Ontario, Canada
Han R, Gao J, Wei S, Su Y, Su C, Li J, Liu Q, Qin Y (2020) High-performance CaO-based composites synthesized using a space-confined chemical vapor deposition strategy for thermochemical energy storage. Solar Energy Mater Solar Cells 206:110346. https://doi.org/10.1016/j.solmat.2019.110346
Han YM, Wang RZ, Dai YJ (2009) Thermal stratification within the water tank. Renew Sustain Energy Rev 13(5):1014–1026. https://doi.org/10.1016/j.rser.2008.03.001
Hasnain SM (1998) Review on sustainable thermal energy storage technologies, Part II: cool thermal storage. Energy Convers Manage 39(11):1139–1153. https://doi.org/10.1016/S0196-8904(98)00024-7
Hasnain SM (1998) Review on sustainable thermal energy storage technologies, Part I: Heat Storage Materials and techniques. Energy Convers Manage 39(11):1127–1138. https://doi.org/10.1016/S0196-8904(98)00025-9
Hauer, A. (2007). ADSORPTION SYSTEMS FOR TES—DESIGN AND DEMONSTRATION PROJECTS. In: Paksoy, H.Ö. (eds) Thermal Energy Storage for Sustainable Energy Consumption. NATO Science Series, vol 234. Springer, Dordrecht. https://doi.org/10.1007/978-1-4020-5290-3_25
He D-H, Di Y-Y, Tan Z-C, Yi F-F, Dan W-Y, Liu Y-P (2011) Crystal structures and thermochemistry on phase change materials (n-CnH2n+1NH3)2CuCl4(s) (n=14 and 15). Sol Energy Mater Sol Cells 95(10):2897–2906. https://doi.org/10.1016/j.solmat.2011.06.014
Hed G, Bellander R (2006) Mathematical modelling of PCM air heat exchanger. Energy Buildings 38(2):82–89. https://doi.org/10.1016/j.enbuild.2005.04.002
Helaly H, El-Sharkawy I, Kandel A, Awad M (2020) A study on thermal energy storage using open adsorption system. Bull Faculty Eng Mansoura University. 43:34–43
Hemmatian B, Heidarzadeh N, Fard GC, Maleknia L (2020) Fabrication of phase-change core/shell nanofibers based on a eutectic fatty acid mixture to control body temperature fluctuations. Mater Chem Phys 245:122738. https://doi.org/10.1016/j.matchemphys.2020.122738
Henninger S, Habib H (2009) MOFs as Adsorbents for Low Temperature Heating and Cooling Applications. J Am Chem Soc 131:2776–2777. https://doi.org/10.1021/ja808444z
Herrmann U, Kelly B, Price H (2004) Two-tank molten salt storage for parabolic trough solar power plants. Energy 29(5–6):883–893. https://doi.org/10.1016/S0360-5442(03)00193-2
Hong S, Zhang X, Kai C, Wang S (2018) Design of flow configuration for parallel air-cooled battery thermal management system with secondary vent. Int J Heat Mass Transfer 116:1204–1212. https://doi.org/10.1016/j.ijheatmasstransfer.2017.09.092
Hongois S (2011) Inter-seasonal thermal energy storage based on a thermochemical process for solar space heating of single-family houses
Stockage de chaleur inter-saisonnier par voie thermochimique pour le chauffage solaire de la maison individuelle, INSA de Lyon
Hoshi A, Mills DR, Bittar A, Saitoh TS (2005) Screening of high melting point phase change materials (PCM) in solar thermal concentrating technology based on CLFR. Sol Energy 79(3):332–339. https://doi.org/10.1016/j.solener.2004.04.023
Huang C, Wang Q, Rao Z (2015) Thermal conductivity prediction of copper hollow nanowire. Int J Therm Sci 94:90–95. https://doi.org/10.1016/j.ijthermalsci.2015.02.017
Hui L, Edem NTK, Nolwenn LP, Lingai L (2011) Evaluation of a seasonal storage system of solar energy for house heating using different absorption couples. Energy Convers Manage 52(6):2427–2436. https://doi.org/10.1016/j.enconman.2010.12.049
Hunt JD, Zakeri B, Lopes R, Barbosa PSF, Nascimento A, N. J. d. Castro, R. Brandão, P. S. Schneider and Y. Wada, (2020) Existing and new arrangements of pumped-hydro storage plants. Renewable Sustain Energy Rev 129:109914. https://doi.org/10.1016/j.rser.2020.109914
Huo D, Tian H, Shu G, Wang W (2022) Progress and prospects for low-grade heat recovery electrochemical technologies. Sustain Energy Technol Assessments 49:101802. https://doi.org/10.1016/j.seta.2021.101802
International Energy Agency I.E.A (2019). Energy Efficiency:buildings.The global exchange for energy efficiency policies,data and analysis. https://www.iea.org/topics/energyefficiency/buildings/
Incropera FP and Dewitt DP (2002). Software tools and user's guides to accompany Fundamentals of heat and mass transfer, & Introduction to heat transfer, 5th edition & Introduction to heat transfer, 4th edition
Ismail MS, Moghavvemi M, Mahlia TMI, Muttaqi KM, Moghavvemi S (2015) Effective utilization of excess energy in standalone hybrid renewable energy systems for improving comfort ability and reducing cost of energy: A review and analysis. Renew Sustain Energy Rev 42:726–734. https://doi.org/10.1016/j.rser.2014.10.051
Janssen NT, Peterson RA, Wies RW (2017) Generalized heat flow model of a forced air electric thermal storage heater core. J Thermal Sci Eng Appl 9(4):041008. https://doi.org/10.1115/1.4036366
Jemmal Y, Zari N, Maaroufi M (2017) Experimental characterization of siliceous rocks to be used as filler materials for air-rock packed beds thermal energy storage systems in concentrated solar power plants. Sol Energy Mater Sol Cells 171:33–42. https://doi.org/10.1016/j.solmat.2017.06.026
Ji Y, Wang CY (2013) Heating strategies for Li-ion batteries operated from subzero temperatures. Electrochim Acta 107:664–674. https://doi.org/10.1016/j.electacta.2013.03.147
Jian X, Lan C, Yu Q, Ma Y (2017) Prevent Thermal Runaway of Lithium-Ion Batteries with Minichannel Cooling. Appl Therm Eng 110:883–890. https://doi.org/10.1016/j.applthermaleng.2016.08.151
Jiang G, Huang J, Liu M, Cao M (2017) Experiment and simulation of thermal management for a tube-shell Li-ion battery pack with composite phase change material. Appl Therm Eng 120:1–9. https://doi.org/10.1016/j.applthermaleng.2017.03.107
Jiang K, Du X, Kong Y, Xu C, Ju X (2019) A comprehensive review on solid particle receivers of concentrated solar power. Renew Sustain Energy Rev 116:109463. https://doi.org/10.1016/j.rser.2019.109463
Johannes K, Kuznik F, Hubert J-L, Durier F, Obrecht C (2015) Design and characterisation of a high powered energy dense zeolite thermal energy storage system for buildings. Appl Energy 159:80–86. https://doi.org/10.1016/j.apenergy.2015.08.109
John E, Hale M, Selvam P (2013) Concrete as a thermal energy storage medium for thermocline solar energy storage systems. Sol Energy 96:194–204. https://doi.org/10.1016/j.solener.2013.06.033
Jung W, Park J, Won W, Lee KS (2018) Simulated moving bed adsorption process based on a polyethylenimine-silica sorbent for CO2 capture with sensible heat recovery. Energy 150:950–964. https://doi.org/10.1016/j.energy.2018.03.022
Darkwa K (1998) Thermochemical energy storage in inorganic oxides: An experimental evaluation. Appl Thermal Eng 18(6):387–400. https://doi.org/10.1016/S1359-4311(97)00052-5
Kant K, Shukla A, Smeulders DMJ, Rindt CCM (2021) Performance analysis of a K2CO3-based thermochemical energy storage system using a honeycomb structured heat exchanger. J Energy Storage 38:102563. https://doi.org/10.1016/j.est.2021.102563
Karlsson J, Wads L, Berg M (2013) A conceptual model that simulates the influence of thermal inertia in building structures. Energy Buildings 60(60):146–151. https://doi.org/10.1016/j.enbuild.2013.01.017
Karthick A, Murugavel KK, Sudalaiyandi K, Manokar AM (2020) Building integrated photovoltaic modules and the integration of phase change materials for equatorial applications. Build Serv Eng Res Technol 41(5):634–652. https://doi.org/10.1177/0143624419883
Kenisarin M, Mahkamov K (2007) Solar energy storage using phase change materials. Renew Sustain Energy Rev 11(9):1913–1965. https://doi.org/10.1016/j.rser.2006.05.005
Kenisarin M, Mahkamov K (2016) Passive thermal control in residential buildings using phase change materials. Renew Sustain Energy Rev 55:371–398. https://doi.org/10.1016/j.rser.2015.10.128
Kenisarin MM (2010) High-temperature phase change materials for thermal energy storage. Renew Sustain Energy Rev 14(3):955–970. https://doi.org/10.1016/j.rser.2009.11.011
Kenisarin MM (2014) Thermophysical properties of some organic phase change materials for latent heat storage. A review Solar Energy 107:553–575. https://doi.org/10.1016/j.solener.2014.05.001
Khalifa A, Suffer KH, Mahmoud MS (2013) A storage domestic solar hot water system with a back layer of phase change material. Exp Thermal Fluid Sci 44:174–181. https://doi.org/10.1016/j.expthermflusci.2012.05.017
Khare S, Dell’Amico M, Knight C, McGarry S (2012) Selection of materials for high temperature latent heat energy storage. Sol Energy Mater Sol Cells 107:20–27. https://doi.org/10.1016/j.solmat.2012.07.020
Khatod KJ, Katekar VP, Deshmukh SS (2022) An evaluation for the optimal sensible heat storage material for maximizing solar still productivity: A state-of-the-art review. J Energy Storage 50:104622. https://doi.org/10.1016/j.est.2022.104622
Kibria MA, Saidur R, Al-Sulaiman FA, Aziz M (2016) Development of a thermal model for a hybrid photovoltaic module and phase change materials storage integrated in buildings. Sol Energy 124:114–123. https://doi.org/10.1016/j.solener.2015.11.027
Kipouros G, Sadoway D (1987) The chemistry and electrochemistry of magnesium production. Adv Molten Salt Chem 6:127–209
Konyk A, Demchenko V (2021) Integration of Heat Storage Technologies in District Heating Systems. Rocznik Ochrona Środowiska 23:493–502. https://doi.org/10.54740/ros.2021.034
Kuitunen S, Ivi F (2018). 7th International Conference Thermal Management for EV/HEV, Berlin Thermal storage based heating system for full electric city buses. https://doi.org/10.13140/RG.2.2.24919.57766
Laing D, Bahl C, Bauer T, Fiss M, Breidenbach N, Hempel M (2012) High-Temperature Solid-Media Thermal Energy Storage for Solar Thermal Power Plants. Proc IEEE 100(2):516–524. https://doi.org/10.1109/JPROC.2011.2154290
Laing D, Bauer T, Steinmann W.-D, Lehmann D (2009). Advanced high temperature latent heat storage system-design and test results. In: The 11th international conference on thermal energy storage - Effstock 2009: Stockholm, Sweden; 2009
Laing D, Zunft S. (2015). Using concrete and other solid storage media in thermal energy storage (TES) systems. Advances in Thermal Energy Storage Systems. Woodhead Publishing, pp 65–86. https://doi.org/10.1533/9781782420965.1.65
Lajunen A, Hadden T, Hirmiz R, Cotton J, Emadi A (2017). Thermal energy storage for increasing heating performance and efficiency in electric vehicles. In 2017 IEEE Transportation Electrification Conference and Expo (ITEC) (pp. 95-100). IEEE. https://doi.org/10.1109/ITEC.2017.7993253
Lamensdorf M (1997). Flameless heater and method of making same. U.S. Patent No. 5,611,329. Washington, DC: U.S. Patent and Trademark Office
Lavine AS, Lovegrove KM, Jordan J, Anleu GB, Chen C, Aryafar H, Sepulveda A (2016) Thermochemical energy storage with ammonia: Aiming for the sunshot cost target. AIP Conference Proc 1734(1):050028. https://doi.org/10.1063/1.4949126
Lebedev VA, Amer AE (2019) Limitations of using phase change materials for thermal energy storage. IOP Confer Ser: Earth Environ Sci 378(1):012044. https://doi.org/10.1088/1755-1315/378/1/012044
Li B, Li Y, Dou Y, Wang Y, Zhao J, Wang T (2021) SiC/Mn co-doped CaO pellets with enhanced optical and thermal properties for calcium looping thermochemical heat storage. Chem Eng J 423:130305. https://doi.org/10.1016/j.cej.2021.130305
Li G, Zhang B, Li X, Zhou Y, Sun Q, Yun Q (2014) The preparation, characterization and modification of a new phase change material: CaCl2· 6H2O–MgCl2· 6H2O eutectic hydrate salt. Sol Energy Mater Sol Cells 126:51–55. https://doi.org/10.1016/j.solmat.2014.03.031
Li P, Molina E, Wang K, Xu X, Dehghani G, Kohli A, Hao Q, Kassaee MH, Jeter SM, Teja AS (2016). Thermal and Transport Properties of NaCl–KCl–ZnCl2 Eutectic Salts for New Generation High-Temperature Heat-Transfer Fluids. J Solar Energy Eng 138(5). https://doi.org/10.1115/1.4033793
Li S, Lin S, Ling Z, Fang X, Zhang Z (2020) Growth of the phase change enthalpy induced by the crystal transformation of an inorganic–organic eutectic mixture of magnesium nitrate hexahydrate–glutaric acid. Ind Eng Chem Res 59(14):6751–6760. https://doi.org/10.1021/acs.iecr.0c01029
Li W, Zhang L, Ling X (2023) Thermo-economic assessment of salt hydrate-based thermochemical heat transformer system: Heat upgrade for matching domestic hot water production. Energy Convers Manage 277:116644. https://doi.org/10.1016/j.enconman.2022.116644
Li WQ, Qu ZG, He YL, Tao YB (2014) Experimental study of a passive thermal management system for high-powered lithium ion batteries using porous metal foam saturated with phase change materials. J Power Sources 255:9–15. https://doi.org/10.1016/j.jpowsour.2014.01.006
Li X, Palazzolo A (2022) A review of flywheel energy storage systems: state of the art and opportunities. J Energy Storage 46:103576. https://doi.org/10.1016/j.est.2021.103576
Li X, Wang Z, Xu E, Ma L, Xu L, Zhao D (2019) Dynamically coupled operation of two-tank indirect TES and steam generation system. Energies 12(9):1720. https://doi.org/10.3390/en12091720
Li X, Zhang J, Liu Y, Xu Y, Cui K, Yao Z, Fu B, Song C, Shang W, Tao P, Deng T (2023) Supercooled sugar alcohols stabilized by alkali hydroxides for long-term room-temperature phase change solar-thermal energy storage. Chem Eng J 452:139328. https://doi.org/10.1016/j.cej.2022.139328
Liang T, Vecchi A, Knobloch K, Sciacovelli A, Engelbrecht K, Li Y, Ding Y (2022) Key components for Carnot Battery: Technology review, technical barriers and selection criteria. Renew Sustain Energy Rev 163:112478. https://doi.org/10.1016/j.rser.2022.112478
Ling Z, Zhang Z, Shi G, Fang X, Lei W, Gao X, Fang Y, Tao X, Wang S, Liu X (2014) Review on thermal management systems using phase change materials for electronic components, Li-ion batteries and photovoltaic modules. Renew Sustain Energy Rev 31:427–438. https://doi.org/10.1016/j.rser.2013.12.017
Liu H, Zhou F, Shi X, Sun K, Kou Y, Das P, Li Y, Zhang X, Mateti S, Chen Y, Wu Z-S, Shi Q (2023) A thermoregulatory flexible phase change nonwoven for all-season high-efficiency wearable thermal management. Nano-Micro Letters 15(1):29. https://doi.org/10.1007/s40820-022-00991-6
Liu J, Baeyens J, Deng Y, Wang X, Zhang H (2020) High temperature Mn2O3/Mn3O4 and Co3O4/CoO systems for thermo-chemical energy storage. J Environ Manage 267:110582. https://doi.org/10.1016/j.jenvman.2020.110582
Liu J, Chang Z, Wang L, Xu J, Kuang R, Wu Z (2020) Exploration of basalt glasses as high-temperature sensible heat storage materials. ACS Omega 5:19236–19246. https://doi.org/10.1021/acsomega.0c02773
Liu M, Saman W, Bruno F (2012) Review on storage materials and thermal performance enhancement techniques for high temperature phase change thermal storage systems. Renew Sustain Energy Rev 16(4):2118–2132. https://doi.org/10.1016/j.rser.2012.01.020
Luo J, Ma X (2022) An integrated strategy for the improvement of thermo-economic performance of a GWHP system. Appl Thermal Eng 213:118777. https://doi.org/10.1016/j.applthermaleng.2022.118777
Ma T, Yang H, Zhang Y, Lu L, Wang X (2015) Using phase change materials in photovoltaic systems for thermal regulation and electrical efficiency improvement: A review and outlook. Renew Sustain Energy Rev 43:1273–1284. https://doi.org/10.1016/j.rser.2014.12.003
Mahfuz M, Anisur M, Kibria M, Saidur R, Metselaar I (2014) Performance investigation of thermal energy storage system with Phase Change Material (PCM) for solar water heating application. Int Commun Heat Mass Transfer 57:132–139. https://doi.org/10.1016/j.icheatmasstransfer.2014.07.022
Mahlia TMI, Saktisahdan TJ, Jannifar A, Hasan MH, Matseelar HSC (2014) A review of available methods and development on energy storage; technology update. Renew Sustain Energy Rev 33:532–545. https://doi.org/10.1016/j.rser.2014.01.068
Malvi C, Dixon-Hardy D, Crook R (2011) Energy balance model of combined photovoltaic solar-thermal system incorporating phase change material. Sol Energy 85(7):1440–1446. https://doi.org/10.1016/j.solener.2011.03.027
Mcb A, Bn B, Mc A (2016) Heat retention of a photovoltaic/thermal collector with PCM - ScienceDirect. Sol Energy 133:533–548. https://doi.org/10.1016/j.solener.2016.04.024
Mehling H, Cabeza LF (2008). Heat and cold storage with PCM an up to date introduction into basics and applications. Heat and Mass Transfer. Springer
Mehrali M, Latibari ST, Rosen MA, Akhiani AR, Naghavi MS, Sadeghinezhad E, Metselaar HSC, Nejad MM, Mehrali M (2016) From rice husk to high performance shape stabilized phase change materials for thermal energy storage. RSC Adv 6(51):45595–45604. https://doi.org/10.1039/C6RA03721F
Meier A, Bonaldi E, Cella GM, Lipinski W, Wuillemin D, Palumbo R (2004) Design and experimental investigation of a horizontal rotary reactor for the solar thermal production of lime. Energy 29(5/6):811–821. https://doi.org/10.1016/S0360-5442(03)00187-7
Meister C, Beausoleil-Morrison I (2021) Experimental and modelled performance of a building-scale solar thermal system with seasonal storage water tank. Sol Energy 222:145–159. https://doi.org/10.1016/j.solener.2021.05.025
Mellouli S, Askri F, Edacherian A, Alqahtani T, Algarni S, Abdelmajid J, Phelan P (2018) Performance analysis of a thermal energy storage system based on paired metal hydrides for concentrating solar power plants. Appl Therm Eng 144:1017–1029. https://doi.org/10.1016/j.applthermaleng.2018.09.014
Memon, Ali S (2014) Phase change materials integrated in building walls: A state of the art review. Renew Sustain Energy Rev 31(Complete):870–906. https://doi.org/10.1016/j.rser.2013.12.042
Menéndez RP, Martínez JÁ, Prieto MJ, Barcia LÁ, Sánchez JMM (2014) A novel modeling of molten-salt heat storage systems in thermal solar power plants. Energies 7(10):6721–6740. https://doi.org/10.3390/en7106721
Mi X, Ran L, Cui H, Memon SA, Lo Y (2016) Energy and economic analysis of building integrated with PCM in different cities of China. Appl Energy 175:324–336. https://doi.org/10.1016/j.apenergy.2016.05.032
Miao Q, Zhang Y, Jia X, Li Z, Tan L, Ding Y (2021) MgSO4-expanded graphite composites for mass and heat transfer enhancement of thermochemical energy storage. Sol Energy 220:432–439. https://doi.org/10.1016/j.solener.2021.03.008
Mmia B, Akp A, Mh A, Nara C (2016) Recent progresses and achievements in photovoltaic-phase change material technology: A review with special treatment on photovoltaic thermal-phase change material systems - ScienceDirect. Energy Convers Manage 126:177–204. https://doi.org/10.1016/j.enconman.2016.07.075
Mohamed H, Brahim AB (2017) Modeling of the absorption phase of a cycle with solar absorption using the couple NH3–H2O for in-sight energy storage. Int J Hydrogen Energy 42(13):8624–8630. https://doi.org/10.1016/j.ijhydene.2016.06.183
Mohamed SA, Al-Sulaiman FA, Ibrahim NI, Zahir MH, Al-Ahmed A, Saidur R, Yılbaş BS, Sahin AZ (2017) A review on current status and challenges of inorganic phase change materials for thermal energy storage systems. Renew Sustain Energy Rev 70:1072–1089. https://doi.org/10.1016/j.rser.2016.12.012
Møller KT, Sheppard D, Ravnsbæk DB, Buckley CE, Akiba E, Li H-W, Jensen TR (2017) Complex Metal Hydrides for Hydrogen. Thermal Electrochem Energy Storage Energies 10(10):1645. https://doi.org/10.1016/j.ijhydene.2018.11.208
Mu S-Y, Guo J, Gong Y-M, Zhang S, Yu Y (2015) Synthesis and thermal properties of poly(styrene-co-acrylonitrile)-graft-polyethylene glycol copolymers as novel solid–solid phase change materials for thermal energy storage. Chin Chem Lett 26(11):1364–1366. https://doi.org/10.1016/j.cclet.2015.07.013
Murali G, Rama Krishna Reddy K, Sai Kumar MT, Sai Manikanta J, Nitish Kumar Reddy V (2020) Performance of solar aluminium can air heater using sensible heat storage. Mater Today: Proc 21:169–174. https://doi.org/10.1016/j.matpr.2019.04.213
N’Tsoukpoe KE, Liu H, Pierres NL, Luo L (2009) A review on long-term sorption solar energy storage. Renew Sustain Energy Rev 13(9):2385–2396. https://doi.org/10.1016/j.rser.2009.05.008
Najafian A, Haghighat F, Moreau A (2015) Integration of PCM in domestic hot water tanks: Optimization for shifting peak demand. Energy Build 106:59–64. https://doi.org/10.1016/j.enbuild.2015.05.036
Narayanan S, Li X, Yang S, Kim H, Umans A, McKay IS, Wang EN (2015) Thermal battery for portable climate control. Appl Energy 149:104–116. https://doi.org/10.1016/j.apenergy.2015.03.101
Neelam K, Meeta S, Onkar S, Anoop Kumar S (2022) Comparative investigation on two tank and cascade thermal storage for solar power plants. J Phys: Confer Ser 2178(1):012013. https://doi.org/10.1088/1742-6596/2178/1/012013
Nie B, Palacios A, Zou B, Liu J, Zhang T, Li Y (2020) Review on phase change materials for cold thermal energy storage applications. Renew Sustain Energy Rev 134:110340. https://doi.org/10.1016/j.rser.2020.110340
Nishioka K, Suura N, Ohno K-I, Maeda T, Shimizu M (2010) Development of Fe Base Phase Change Materials for High Temperature Using Solid-Solid Transformation. ISIJ Int 50:1240–1244. https://doi.org/10.2355/isijinternational.50.1240
Nomura T, Akiyama T (2017) High-temperature latent heat storage technology to utilize exergy of solar heat and industrial exhaust heat. Int J Energy Res 41(2):240–251. https://doi.org/10.1002/er.3611
Ogoli DM (2003) Predicting indoor temperatures in closed buildings with high thermal mass. Energy Buildings 35(9):851–862. https://doi.org/10.1016/S0378-7788(02)00246-3
Olivkar PR, Katekar VP, Deshmukh SS, Palatkar SV (2022) Effect of sensible heat storage materials on the thermal performance of solar air heaters: State-of-the-art review. Renew Sustain Energy Rev 157:112085. https://doi.org/10.1016/j.rser.2022.112085
Oskouee SS, Kamali S, Amraee T (2021) Primary frequency support in unit commitment using a multi-area frequency model with flywheel energy storage. IEEE Trans Power Syst 36(6):5105–5119. https://doi.org/10.1109/TPWRS.2021.3074634
Osuna R, Fernandez V, Romero M, Blanco M (2000). PS10: A 10 MW solar tower power plant for southern Spain[J]. Energy, 2000:386-393
Ouchani F-Z, Jbaihi O, Alami Merrouni A, Ghennioui A, Maaroufi M (2022) Geographic Information System-based Multi-Criteria Decision-Making analysis for assessing prospective locations of Pumped Hydro Energy Storage plants in Morocco: Towards efficient management of variable renewables. J Energy Storage 55:105751. https://doi.org/10.1016/j.est.2022.105751
Palacios A, Barreneche C, Navarro ME, Ding Y (2020) Thermal energy storage technologies for concentrated solar power – A review from a materials perspective. Renewable Energy 156:1244–1265. https://doi.org/10.1016/j.renene.2019.10.127
Panchal S, Khasow R, Dincer I, Agelin-Chaab M, Fowler M (2017) Thermal design and simulation of mini-channel cold plate for water cooled large sized prismatic lithium-ion battery. Appl Therm Eng 122:80–90. https://doi.org/10.1016/j.applthermaleng.2017.05.010
Pardo P, Deydier A, Anxionnaz-Minvielle Z, Rougé S, Cabassud M, Cognet P (2014) A review on high temperature thermochemical heat energy storage. Renew Sustain Energy Rev 32:591–610. https://doi.org/10.1016/j.rser.2013.12.014
Park JH, Lee J, Wi S, Jeon J, Chang SJ, Chang JD, Kim S (2019) Optimization of phase change materials to improve energy performance within thermal comfort range in the South Korean climate. Energy Buildings 185:12–25. https://doi.org/10.1016/j.enbuild.2018.12.013
Pesaran A (2001) Battery Thermal Management in EVs and HEVs: Issues and Solutions. Battery Man, 43(5):34–49
Pickard DW, Trottier RL, Lavigne PG (1994) Self-heating group meal assembly and method of using same, U.S. Patent No. 5,355,869. Washington, DC: Patent and Trademark Office
Pielichowska K, Pielichowski K (2014) Phase change materials for thermal energy storage. Prog Mater Sci 65:67–123. https://doi.org/10.1016/j.pmatsci.2014.03.005
Power R (2010). ADELE–adiabatic compressed air energy storage for electricity supply. RWE Power AG, Essen/Koln 141
Prah B, Yun R (2017) Heat Transfer and Flow Characteristics of CO2-Hydrate Mixture in Pipeline. Energy Procedia 114:6813–6823. https://doi.org/10.1016/j.egypro.2017.03.1811
Prieto C, Cabeza LF (2019) Thermal energy storage (TES) with phase change materials (PCM) in solar power plants (CSP). Concept and plant performance. Appl Energy 254:113646. https://doi.org/10.1016/j.apenergy.2019.113646
Quéré CL, Andrew RM, Friedlingstein P, Sitch S, Hauck J (2018) Global Carbon Budget 2018. Earth Syst Sci Data 10(4):2141–2194. https://doi.org/10.5194/essd-10-2141-2018
Quoilin S, Broek MVD, Declaye S, Dewallef P, Lemort V (2013) Techno-economic survey of Organic Rankine Cycle (ORC) systems. Renew Sustain Energy Rev 22:168–186. https://doi.org/10.1016/j.rser.2013.01.028
Raab S, Mangold D, Müller-Steinhagen H (2005) Validation of a computer model for solar assisted district heating systems with seasonal hot water heat store. Sol Energy 79(5):531–543. https://doi.org/10.1016/j.solener.2004.10.014
Radouane N (2022) A Comprehensive Review of Composite Phase Change Materials (cPCMs) for Thermal Management Applications, Including Manufacturing Processes, Performance, and Applications. Energies 15(21):8271. https://doi.org/10.3390/en15218271
Radziemska E (2003) The effect of temperature on the power drop in crystalline silicon solar cells. Renewable Energy 28(1):1–12. https://doi.org/10.1016/S0960-1481(02)00015-0
Rahman MA, Kim J-H, Hossain S (2022) Recent advances of energy storage technologies for grid: A comprehensive review. Energy Storage 4(6):e322. https://doi.org/10.1002/est2.322
Ramakrishnan S, Wang X, Sanjayan J, Wilson J (2017) Thermal performance of buildings integrated with phase change materials to reduce heat stress risks during extreme heatwave events. Appl Energy 194(MAY15):410–421. https://doi.org/10.1016/j.apenergy.2016.04.084
Ramanathan K, West DH, Balakotaiah V (2004) Optimal design of catalytic converters for minimizing cold-start emissions. Catal Today 98(3):357–373. https://doi.org/10.1016/j.cattod.2004.08.003
Rao Z, Zhen Q, Yong K, Li Y (2017) Thermal performance of liquid cooling based thermal management system for cylindrical lithium-ion battery module with variable contact surface. Appl Therm Eng 123:1514–1522. https://doi.org/10.1016/j.applthermaleng.2017.06.059
Ravichandran L, Rusovs D, Arjunan TV, Selvaraj V, Waran M. (2018). Experimental study of brackish water distillation in single slope solar still using sensible heat storage materials. International scientific conference RURAL DEVELOPMENT 2017:391–396. https://doi.org/10.15544/RD.2017.086
Reddy T, Norford L, Kempton W (1991) Shaving residential air-conditioner electricity peaks by intelligent use of the building thermal mass. Energy 16(7):1001–1010. https://doi.org/10.1016/0360-5442(91)90060-Y
Rehman S, Al-Hadhrami LM, Alam MM (2015) Pumped hydro energy storage system: A technological review. Renew Sustain Energy Rev 44:586–598. https://doi.org/10.1016/j.rser.2014.12.040
Reilly JJ, Wiswall RH (1968) Reaction of hydrogen with alloys of magnesium and nickel and the formation of Mg2NiH4. Inorg Chem 7(11):2254–2256. https://doi.org/10.1021/IC50069A016
Rönnebro ECE, Whyatt G, Powell M, Westman M, Zheng F, Fang ZZ (2015) Metal Hydrides for High-Temperature Power Generation. Energies 8(8):8406–8430. https://doi.org/10.3390/en8088406
Roßkopf C, Afflerbach S, Schmidt M, Görtz B, Kowald T, Linder M, Trettin R (2015) Investigations of nano coated calcium hydroxide cycled in a thermochemical heat storage. Energy Convers Manage 97:94–102. https://doi.org/10.1016/j.enconman.2015.03.034
Salatino P, Ammendola P, Bareschino P, Chirone R, Solimene R (2016) Improving the thermal performance of fluidized beds for concentrated solar power and thermal energy storage. Powder Technol 290:97–101. https://doi.org/10.1016/j.powtec.2015.07.036
Salyan S, Praveen B, Singh H, Suresh S, Reddy AS (2020) Liquid metal gallium in metal inserts for solar thermal energy storage: a novel heat transfer enhancement technique. Solar Energy Mater Solar Cells 208:110365. https://doi.org/10.1016/j.solmat.2019.110365
Samimi F, Babapoor A, Azizi M, Karimi G (2016) Thermal management analysis of a Li-ion battery cell using phase change material loaded with carbon fibers. Energy 96(Feb 1):355–371. https://doi.org/10.1016/j.energy.2015.12.064
Sanchez-Jimenez PE, Perez-Maqueda LA, Valverde JM (2014) Nanosilica supported CaO: A regenerable and mechanically hard CO2 sorbent at Ca-looping conditions. Appl Energy 118:92–99. https://doi.org/10.1016/j.apenergy.2013.12.024
Sanpasertparnich T, Idem R, Bolea I, deMontigny D, Tontiwachwuthikul P (2010) Integration of post-combustion capture and storage into a pulverized coal-fired power plant. Int J Greenhouse Gas Control 4(3):499–510. https://doi.org/10.1016/j.ijggc.2009.12.005
Sapali S, Patil P (2003). Analyses of a rock-bed-thermic oil solar energy storage system. Adv Renewable Energy Technol, Narosa Publishing House, New Delhi, pp 64–70
Sarbu I, Sebarchievici C. (2018) A comprehensive review of thermal energy storage. Sustainability 10. https://doi.org/10.3390/su10010191
Sari A, Alkan C, Bilgin C (2014) Micro/nano encapsulation of some paraffin eutectic mixtures with poly(methyl methacrylate) shell: Preparation, characterization and latent heat thermal energy storage properties. Appl Energy 136(dec31):217–227. https://doi.org/10.1016/j.apenergy.2014.09.047
Sarkar S, Mestry S, Mhaske ST (2022) Developments in phase change material (PCM) doped energy efficient polyurethane (PU) foam for perishable food cold-storage applications: A review. J Energy Storage 50:104620. https://doi.org/10.1016/j.est.2022.104620
Saw LH, Ye Y, Tay AA, Chong WT, Kuan SH, Yew MC (2016) Computational fluid dynamic and thermal analysis of Lithium-ion battery pack with air cooling. Appl Energy 177:783–792. https://doi.org/10.1016/j.apenergy.2016.05.122
Saxena A, Tirth V, Srivastava G (2014) Design and performance analysis of a solar air heater with high heat storage. Distrib Gener Altern Energy J 29(3):35–55. https://doi.org/10.1080/21563306.2014.10879016
Sayyar M, Weerasiri RR, Soroushian P, Lu J (2014) Experimental and numerical study of shape-stable phase-change nanocomposite toward energy-efficient building constructions. Energy Build 75:249–255. https://doi.org/10.1016/j.enbuild.2014.02.018
Scapino L, Zondag HA, Van Bael J, Diriken J, Rindt CCM (2017) Sorption heat storage for long-term low-temperature applications: A review on the advancements at material and prototype scale. Appl Energy 190:920–948. https://doi.org/10.1016/j.apenergy.2016.12.148
Schmidt M, Gollsch M, Giger F, Grün M, Linder M. (2016). Development of a Moving Bed Pilot Plant for Thermochemical Energy Storage with CaO/Ca(OH)2. AIP Conference Proceedings. AIP Publishing LLC, 1734(1): 050041
Shao L. (2010). Materials for energy efficiency and thermal comfort in new buildings. Materials for energy efficiency and thermal comfort in buildings, Woodhead Publ. Ltd., Oxford, UK, pp. 631–648
Sharifi NP, Shaikh A, Sakulich AR (2016) Application of phase change materials in gypsum boards to meet building energy conservation goals. Energy Buildings 138:455–467. https://doi.org/10.1016/j.enbuild.2016.12.046
Sharma A, Tyagi VV, Chen CR, Buddhi D (2009) Review on thermal energy storage with phase change materials and applications. Renew Sustain Energy Rev 13(2):318–345. https://doi.org/10.1016/j.rser.2007.10.005
Sharma RK, Ganesan P, Tyagi VV, Metselaar H, Sandaran SC (2015) Developments in organic solid–liquid phase change materials and their applications in thermal energy storage. Energy Convers Manage 95(May):193–228. https://doi.org/10.1016/j.enconman.2015.01.084
Sheppard DA, Corgnale C, Hardy B, Motyka T, Zidan R, Paskevicius M, Buckley CE (2014) Hydriding characteristics of NaMgH2F with preliminary technical and cost evaluation of magnesium-based metal hydride materials for concentrating solar power thermal storage. RSC Adv 4(51):26552–26562. https://doi.org/10.1039/C4RA01682C
Shiraki M, Yakabe H, Uchida H (2013) Efficiency Calculations for SOFC/SOEC reversible system and evaluations of performances of button-size anode-supported cell. ECS Trans 57(1):3261. https://doi.org/10.1149/05701.3261ecst
Shirazi A, Farzad M, Azadi K, He B, Rabczuk T (2016) Paraffin Nanocomposites for Heat Management of Lithium-Ion Batteries: A Computational Investigation. J Nanomater. 2016(2016):59. https://doi.org/10.1155/2016/2131946
Singh P, Sharma RK, Ansu AK, Goyal R, Sarı A, Tyagi VV (2021) A comprehensive review on development of eutectic organic phase change materials and their composites for low and medium range thermal energy storage applications. Solar Energy Mater Solar Cells 223:110955. https://doi.org/10.1016/j.solmat.2020.110955
Sjostrom S (2016) Optimizing the costs of solid sorbent-based CO2 capture process through heat integration. Ada-Es Inc, Highlands Ranch. https://doi.org/10.2172/1337015
Skrylnyk O, Courbon E, Heymans N, Frère M, Bougard J, Descy G (2017) Energy Performances of Open Sorption Reactor with Ultra-Low Grade Heat Upgrading for Thermochemical Energy Storage Applications. Energy Procedia 135:304–316. https://doi.org/10.1016/j.egypro.2017.09.522
Stack DC (2017). Conceptual design and performance characteristics of firebrick resistance-heated energy storage for industrial heat supply and variable electricity production, Massachusetts Institute of Technology
Steinmann W-D, Eck M (2006) Buffer storage for direct steam generation. Sol Energy 80(10):1277–1282. https://doi.org/10.1016/j.solener.2005.05.013
Steinmann WD. (2015). Thermal energy storage systems for concentrating solar power (CSP) technology. Advances in Thermal Energy Storage Systems, Woodhead Publishing, pp 511–531. https://doi.org/10.1533/9781782420965.4.511
Straub AP, Yip NY, Lin S, Lee J, Elimelech M (2016) Harvesting low-grade heat energy using thermo-osmotic vapour transport through nanoporous membranes. Nat Energy 1(7):16090. https://doi.org/10.1038/nenergy.2016.90
Strong C, Carrier Y, Handan Tezel F (2022) Experimental optimization of operating conditions for an open bulk-scale silica gel/water vapour adsorption energy storage system. Appl Energy 312:118533. https://doi.org/10.1016/j.apenergy.2022.118533
Sun H, Li Y, Yan X, Wang Z, Liu W (2020) CaO/CaCO3 thermochemical heat storage performance of CaO-based micrometre-sized tubular composite. Energy Convers Manage 222:113222. https://doi.org/10.1016/j.enconman.2020.113222
Sun M, Liu T, Li M, Tan J, Tian P, Wang H, Chen G, Jiang D, Liu X (2022) A deep supercooling eutectic phase change material for low-temperature battery thermal management. J Energy Storage 50:104240. https://doi.org/10.1016/j.est.2022.104240
Sunku Prasad J, Muthukumar P, Desai F, Basu DN, Rahman MM (2019) A critical review of high-temperature reversible thermochemical energy storage systems. Appl Energy 254:113733. https://doi.org/10.1016/j.apenergy.2019.113733
Suresh C, Saini RP (2020) Thermal performance of sensible and latent heat thermal energy storage systems. Int J Energy Res 44(6):4743–4758. https://doi.org/10.1002/er.5255
Tas CE, Unal H (2021) Thermally buffering polyethylene/halloysite/phase change material nanocomposite packaging films for cold storage of foods. J Food Eng 292:110351. https://doi.org/10.1016/j.jfoodeng.2020.110351
Tatsidjodoung P, Le Pierrès N, Heintz J, Lagre D, Luo L, Durier F (2016) Experimental and numerical investigations of a zeolite 13X/water reactor for solar heat storage in buildings. Energy Convers Manage 108:488–500. https://doi.org/10.1016/j.enconman.2015.11.011
Thu K, Saththasivam J, Saha BB, Chua KJ, Srinivasa Murthy S, Ng KC (2017) Experimental investigation of a mechanical vapour compression chiller at elevated chilled water temperatures. Appl Therm Eng 123:226–233. https://doi.org/10.1016/j.applthermaleng.2017.05.091
Tomassetti S, Aquilanti A, Muciaccia PF, Coccia G, Mankel C, Koenders EAB, Di Nicola G (2022) A review on thermophysical properties and thermal stability of sugar alcohols as phase change materials. J Energy Storage 55:105456. https://doi.org/10.1016/j.est.2022.105456
Tregambi C, Bareschino P, Mancusi E, Pepe F, Montagnaro F, Solimene R, Salatino P (2021) Modelling of a concentrated solar power – photovoltaics hybrid plant for carbon dioxide capture and utilization via calcium looping and methanation. Energy Convers Manage 230:113792. https://doi.org/10.1016/j.enconman.2020.113792
Tregambi C, Di Lauro F, Montagnaro F, Salatino P, Solimene R (2019) 110th Anniversary: Calcium Looping Coupled with Concentrated Solar Power for Carbon Capture and Thermochemical Energy Storage. Ind Eng Chem Res 58(47):21262–21272. https://doi.org/10.1021/acs.iecr.9b03083
Vadiee A, Martin V (2013) Thermal energy storage strategies for effective closed greenhouse design. Appl Energy 109:337–343. https://doi.org/10.1016/j.apenergy.2012.12.065
van Essen VM, Zondag HA, Gores JC, Bleijendaal LPJ, Bakker M, Schuitema R, van Helden WGJ, He Z, Rindt CCM (2009). Characterization of MgSO4 Hydrate for Thermochemical Seasonal Heat Storage. J Solar Energy Eng 131(4). https://doi.org/10.1115/1.4000275
Vargaftik N, Filippov L, Tarzimanov A, Totskii E (2020) Handbook of Thermal Conductivity of Liquids and Gases
Vasiliev LL, Burak VS, Kulakov AG, Mishkinis DA, Bohan PV (2000) Latent heat storage modules for preheating internal combustion engines: application to a bus petrol engine. Appl Therm Eng 20(10):913–923. https://doi.org/10.1016/S1359-4311(99)00061-7
Vetrovec J (2008) Engine cooling system with a heat load averaging capability. SAE paper, (2008-01), 1168
Vignarooban K, Xu X, Arvay A, Hsu K, Kannan AM (2015) Heat transfer fluids for concentrating solar power systems–a review. Appl Energy 146:383–396. https://doi.org/10.1016/j.apenergy.2015.01.125
Visnic B (2001) Thermostat, Thy Days are Numbered. Ward's Auto World 37(6):53–53
Wang C, Zhang G, Meng L, Li X, Situ W, Lv Y, Rao M (2017) Liquid cooling based on thermal silica plate for battery thermal management system. Int J Energy Res 41(15):2468–2479. https://doi.org/10.1002/er.3801
Wang M, Deng C, Wang Y, Feng X (2020) Exergoeconomic performance comparison, selection and integration of industrial heat pumps for low grade waste heat recovery. Energy Convers Manage 207:112532. https://doi.org/10.1016/j.enconman.2020.112532
Wang P, Feng X, Zhu Y, Lian J, Zhang H, Fang M (2020) Preparation and thermal properties of colloidal mixtures of capric acid and Na2HPO4·12H2O as a phase change material for energy storage. Solar Energy Mater Solar Cells 215:110636. https://doi.org/10.1016/j.solmat.2020.110636
Wang X, Dennis M, Hou L (2014) Clathrate hydrate technology for cold storage in air conditioning systems. Renew Sustain Energy Rev 36:34–51. https://doi.org/10.1016/j.rser.2014.04.032
Wang X, Zhang F, Lipiński W (2020) Carbon dioxide hydrates for cold thermal energy storage: A review. Sol Energy 211:11–30. https://doi.org/10.1016/j.solener.2020.09.035
Weber R, Dorer V (2008) Long-term heat storage with NaOH. Vacuum 82(7):708–716. https://doi.org/10.1016/j.vacuum.2007.10.018
Weinläder H, Körner W, Strieder B (2014) A ventilated cooling ceiling with integrated latent heat storage—Monitoring results. Energy Buildings 82:65–72. https://doi.org/10.1016/j.enbuild.2014.07.013
Wu W, Yang X, Zhang G, Chen K, Wang S (2017) Experimental investigation on the thermal performance of heat pipe-assisted phase change material based battery thermal management system. Energy Convers Manage 138:486–492. https://doi.org/10.1016/j.enconman.2017.02.022
Xie P, Jin L, Qiao G, Lin C, Barreneche C, Ding Y (2022) Thermal energy storage for electric vehicles at low temperatures: Concepts, systems, devices and materials. Renew Sustain Energy Rev 160:112263. https://doi.org/10.1016/j.rser.2022.112263
Xu B, Li P, Chan C (2015) Application of phase change materials for thermal energy storage in concentrated solar thermal power plants: A review to recent developments. Appl Energy 160:286–307. https://doi.org/10.1016/j.apenergy.2015.09.016
Xu J, Li Y, Wang RZ, Liu W (2014) Performance investigation of a solar heating system with underground seasonal energy storage for greenhouse application. Energy 67:63–73. https://doi.org/10.1016/j.energy.2014.01.049
Xu J, Wang R, Li Y (2014) A review of available technologies for seasonal thermal energy storage. Sol Energy 103:610–638. https://doi.org/10.1016/j.solener.2013.06.006
Xu X, Zhang X, Ji J, Fang M, Yang M, Ma K, Gao Y (2022) Comparative Analysis of Additives for Increasing Thermal Conductivity of Phase Change Materials: A Review. Energy Fuels 36(10):5088–5101. https://doi.org/10.1021/acs.energyfuels.2c00522
Yam J, Li Y, Zheng Z (2003) Nonlinear coupling between thermal mass and natural ventilation in buildings. Int J Heat Mass Transf 46(7):1251–1564. https://doi.org/10.1016/S0017-9310(02)00379-4
Yan J, Li K, Chen H, Wang Q, Sun J (2016) Experimental study on the application of phase change material in the dynamic cycling of battery pack system. Energy Convers Manage 128:12–19. https://doi.org/10.1016/j.enconman.2016.09.058
Yan J, Zhao CY (2015) Thermodynamic and kinetic study of the dehydration process of CaO/Ca(OH)2 thermochemical heat storage system with Li doping. Chem Eng Sci 138:86–92. https://doi.org/10.1016/j.ces.2015.07.053
Yang L, Li Y (2008) Cooling load reduction by using thermal mass and night ventilation. Energy Buildings 40(11):2052–2058. https://doi.org/10.1016/j.enbuild.2008.05.014
Yang S, Shao X-F, Luo J-H, Baghaei Oskouei S, Bayer Ö, Fan L-W (2023) A novel cascade latent heat thermal energy storage system consisting of erythritol and paraffin wax for deep recovery of medium-temperature industrial waste heat. Energy 265:126359. https://doi.org/10.1016/j.energy.2022.126359
Yang XH, Tan SC, Jing L (2016) Thermal management of Li-ion battery with liquid metal. Energy Convers Manage 117:577–585. https://doi.org/10.1016/j.enconman.2016.03.054
Yao L, Yang B, Cui H, Zhuang J, Ye J, Xue J (2016) Challenges and progresses of energy storage technology and its application in power systems. J Modern Power Syst Clean Energy 4(4):519–528. https://doi.org/10.1007/s40565-016-0248-x
Yu N, Wang RZ, Wang LW (2013) Sorption thermal storage for solar energy. Prog Energy Combust Sci 39(5):489–514. https://doi.org/10.1016/j.pecs.2013.05.004
Yuan C, Liu X, Wang X, Song C, Zheng H, Tian C, Gao K, Sun N, Jiang Z, Xuan Y, Ding Y (2023) Rapid and stable calcium-looping solar thermochemical energy storage via co-doping binary sulfate and Al–Mn–Fe oxides. Green Energy Environ. https://doi.org/10.1016/j.gee.2023.02.009
Yuan Y, Zhang N, Tao W, Cao X, He Y (2014) Fatty acids as phase change materials: a review. Renew Sustain Energy Rev 29:482–498
Zare M, Mikkonen KS (2023) Phase Change Materials for Life Science Applications. Adv Func Mater 33(12):2213455. https://doi.org/10.1002/adfm.202213455
Zhang F, Zhou Y, Sun W, Hou S, Yu L (2018) CO2 capture from reheating furnace based on the sensible heat of continuous casting slabs. Int J Energy Res 42(6):2273–2283. https://doi.org/10.1002/er.4020
Zhang K, Liu M, Zhao Y, Zhang S, Yan H, Yan J (2022) Thermo-economic optimization of the thermal energy storage system extracting heat from the reheat steam for coal-fired power plants. Appl Thermal Eng 215:119008. https://doi.org/10.1016/j.applthermaleng.2022.119008
Zhang X, Ameli H, Dong Z, Vecchi A, Gallego-Schmid A, Strbac G, Sciacovelli A (2022) Values of latent heat and thermochemical energy storage technologies in low-carbon energy systems: Whole system approach. J Energy Storage 50:104126. https://doi.org/10.1016/j.est.2022.104126
Zhang YN, Wang RZ, Li TX (2017) Experimental investigation on an open sorption thermal storage system for space heating. Energy 141:2421–2433. https://doi.org/10.1016/j.energy.2017.12.003
Zhang Z-Y, Xu Y-P, Yang M-L (2000) Measurement of the Thermal Conductivities of Neopentylglycol, 1,1,1-Trihydroxymethylpropane, and Their Mixture in the Temperature Range from 20 °C to Their Supermelting Temperatures. J Chem Eng Data 45(6):1060–1063. https://doi.org/10.1021/je000030h
Zhang Z, Cheng J, He X (2017) Numerical simulation of flow and heat transfer in composite PCM on the basis of two different models of open-cell metal foam skeletons. Int J Heat Mass Transf 112:959–971. https://doi.org/10.1016/j.ijheatmasstransfer.2017.05.012
Zhao D, Li Y, Dai Y, Wang R (2011) Optimal study of a solar air heating system with pebble bed energy storage. Energy Convers Manage 52(6):2392–2400. https://doi.org/10.1016/j.enconman.2010.12.041
Zhao J, Lv P, Rao Z (2017) Experimental study on the thermal management performance of phase change material coupled with heat pipe for cylindrical power battery pack. Exp Thermal Fluid Sci 82:182–188. https://doi.org/10.1016/j.energy.2021.122081
Zhao L, Luo J, Wang H, Song G, Tang G. (2016). Self-assembly fabrication of microencapsulated n-octadecane with natural silk fibroin shell for thermal-regulating textiles. Appl Therm Eng 99:495–501. https://doi.org/10.1016/j.applthermaleng.2015.12.111
Zhao Y, Zhao CY, Markides CN, Wang H, Li W (2020) Medium- and high-temperature latent and thermochemical heat storage using metals and metallic compounds as heat storage media: A technical review. Appl Energy 280:115950. https://doi.org/10.1016/j.apenergy.2020.115950
Zhou D, Zhao C-Y, Tian Y (2012) Review on thermal energy storage with phase change materials (PCMs) in building applications. Appl Energy 92:593–605. https://doi.org/10.1016/j.rser.2007.10.005
Zhu C, Huo D, Chen Q, Xue J, Shen S, Xia Y (2017) A eutectic mixture of natural fatty acids can serve as the gating material for near-infrared-triggered drug release. Adv Mater 29(40):1703702. https://doi.org/10.1002/adma.201703702
Zohuri B (2018). Hydrogen: Driving Renewable Energy. Hydrogen Energy.Hydrogen Energy: Challenges and Solutions for a Cleaner Future, 141–184
Acknowledgements
Not applicable.
Funding
Open access funding provided by Shanghai Jiao Tong University. This work was supported by National Natural Science Foundation of China (52276146) and Fundamental Research Funds for the Central Universities (DUT22YG108).
Author information
Authors and Affiliations
Contributions
MS and TL drafted the manuscript. TL, XW and ML helped to collect the literatures. GC concentrated on discussion and language. DJ conceived of the review and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The manuscript is original and not submitted to other journal. The authors consent to participate in the manuscript.
Consent for publication
The manuscript has been read and approved by all authors, and all authors agree to the publication of the manuscript.
Competing interests
The authors declare no competing financial interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sun, M., Liu, T., Wang, X. et al. Roles of thermal energy storage technology for carbon neutrality. Carb Neutrality 2, 12 (2023). https://doi.org/10.1007/s43979-023-00052-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43979-023-00052-w