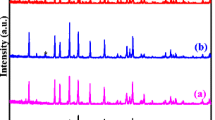
Abstract
Stable solid electrolytes have been the subject of intense research due to the growing demand for energy storage and portable energy sources in rechargeable Li-ion batteries. Sodium (NA) super (S) ionic (I) conductor (CON) abbreviated as NASICON are suitable for use as solid electrolytes due to their excellent ionic conductivity and chemical stability. We have reported the effect of partial doping of Sr at Li and Zr4+ at Ti4+ position on the structure and ionic conductivity of composition Li0.4Sr0.3Ti2(PO4)3 (LSTP). The composition with formula Li0.4Sr0.3Ti2−xZrx(PO4)3 (x = 0.0, 0.5, 2.0) were prepared by solid state reaction method. The phase and crystal structure were examined using X-ray diffraction (XRD) patterns. The compositions were in rhombohedral phase with the space group R \(\overline{3}\) c. The doping of Zr4+ in LSTP causes the peaks in the XRD spectra for doped composition (x = 0.5, 2.0) to move towards lower angle. The displacement of the XRD peaks towards lower angle side show the expansion in the lattice of the Zr doped compositions Li0.4Sr0.3Ti1.5Zr0.5(PO4)3 and Li0.4Sr0.3Zr2(PO4)3. The presence of stretching and bending modes of PO4 groups in the prepared compositions was identified by FTIR spectra. The enhanced bulk conductivity of 4.32 × 10–5 S/cm for Zr doped composition Li0.4Sr0.3Ti1.5Zr0.5(PO4)3 and 3.20 × 10–5 S/cm for composition Li0.4Sr0.3Zr2(PO4)3 is observed at 25 °C. The modulus peaks in the Zr doped composition Li0.4Sr0.3Ti1.5Zr0.5(PO4)3 is displaced towards higher frequencies at 450 °C, indicating the presence of the thermally produced dielectric relaxation phenomenon and charge carrier hopping. The cole cole plot consisting of one semicircle in case of Li0.4Sr0.3Ti1.5Zr0.5(PO4)3 discusses the contribution of bulk and grain boundary resistances and is fitted by equivalent circuit consisting of CPE and R element joined in parallel.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Ceramic based solid electrolytes are widely known for their excellent temperature reliability. Several ceramic materials have been studied, including sulphide [1, 2], Li7La3Zr2O12 [3, 4], Li2/3−3xLa3xTiO3 [5, 6], and NASICON structured materials [7]. Sulphide-type electrolytes show high ionic conductivity of ~ 10−2 S/cm [1]. Emission of hazardous gas (H2S) from the sulfide type materials negatively impacts future research applications due to the reaction with H2O and O2 [2]. The garnet type compositions (Li7La3Zr2O12) exhibit high ionic conductivity but due to the structural positions of atoms and dendrite formation limits its performance significantly [3, 4]. The perovskite type material (Li2/3−3xLa3xTiO3) exhibits a bulk conductivity of around 10−3 S/cm, but total conductivity is poor due to the large boundary resistance. It also has poor interfacial contact and Ti4+ reduction [5]. The NASICON structured lithium-ion conductors Li1+xAlxM2−x(PO4)3 (M=Ti, Ge) [8] have almost similar type of drawbacks in comparison to garnet and perovskite structured materials [3,4,5]. However, the rigid framework of the NASICON materials is more suitable for the migration of ions and exhibits a high conductivity of 10−4 S/cm at room temperature [7]. Compared to other ceramic electrolytes, it is reliable in ambient circumstances and permits charge carriers to migrate in all three dimensions [8]. The typical formula for the initially synthesized NASICON materials is Na1+xZr2P3−xSixO12 (0 ≤ x ≤ 3) [9]. Following the synthesis of NASICON materials, several phosphate-related compositions are produced by partially substituting tetravalent cations with trivalent [10], tetravalent [11], and pentavalent [12] cations. Many researchers have shown their interest for employing these materials as solid electrolytes or cathode materials in lithium batteries [13,14,15,16]. In phosphates materials, substitution/doping at heterovalent cations site increase the ionic conductivity by causing vacancies in lithium sites or extra lithium ions to form in the interstitials [17,18,19]. Subramanian et al. has prepared LiZr2−xTix(PO4)3, Li1+xScxTi2−x(PO4)3 and Li1+xInxHf2x(PO4)3 compositions by solid state method and investigated the ionic conductivity using the complex impedance approach and discovered that it was correlated with the bottleneck size and stoichiometry [20]. Two phase transitions were discovered in LiZr2(PO4)3. LiZr2(PO4)3 has a monoclinic structure at room temperature, but between 25 to 60 °C, it transforms into a rhombohedral structure with the space group R \(\overline{3}\) c. At 280 °C, the structure maintained its rhombohedral structure with large range of mobility of Li ions [21]. The structure of LISICON materials AxBy(PO4)3 are connected by corners of PO4 tetrahedra and MO6 octahedra. The framework is made up of infinite ribbons which result from this linkage that are joined together, at right angles to the c direction, by PO4 tetrahedra. The Li+ ions hold two locations in the conduction pathways: the M1 site (one per formula) is coordinated by a trigonal antiprism of oxygens, and the M2 site (three per formula) has eightfold coordination that is distorted. The M1 and M2 locations, respectively, are found within and between the ribbons [22, 23]. A series of materials were synthesized with the partial doping of M3+ in place of Ti4+ in LiTi2(PO4)3 and researchers examined that the densification of the sintered pellets was the reason for the increment in the ionic conductivity (7 × 10−4 S/cm) at 298 K of Li1.3M0.3Ti1.7(PO4)3 (M=Al and Sc) [24]. Sintering and calcination temperature significantly influences the properties of ceramic materials, particularly their density and ionic conductivity [25,26,27]. Schell et al. reported that the activation energy was found to decrease from 0.278 to 0.261 eV as the calcination temperature is increased from 750 to 900 °C for Li1.3Ti1.7Al0.3(PO4)3. Ionic conductivity of Li1.3Ti1.7Al0.3(PO4)3 gets reduced by around one order of magnitude after calcining at temperatures < 900 °C [28]. Generally, the density increases with sintering by increasing the temperature. The Li1.3Ti1.7Al0.3(PO4)3 powder has shown theoretical density of roughly 85 to 86% after sintering at 750 and 800 °C. After raising sintering temperature to 850 °C, density was found nearly 98.5% while 900 °C sintered Li1.3Ti1.7Al0.3(PO4)3 sample has shown relative densities of 94%. Li1.3Ti1.7Al0.3(PO4)3 sample shows higher density at 850 °C temperature due to the stiff agglomerates of similar small particles and which obstructs uniform densification during sintering [28]. In this study, we have examined the structural and ionic conductivity properties of Sr containing Li0.4Sr0.3Ti2−xZrx(PO4)3 (x = 0.0, 0.5, 2.0) NASICON compositions. Since Sr containing compositions are found to be responsible for the decrease in the value of dielectric constant [29], therefore we were motivated to study the larger size atom Sr2+(1.13) at Li+(0.76) site and doping of larger size atom Zr4+ (0.72) at Ti4+ (0.605) site in these compositions to improve the ionic conductivity of NASICON type compositions and make them suitable for the use in Li ion batteries as solid electrolytes.
2 Experimental
The Li containing NASICON type materials Li0.4Sr0.3Ti2-xZrx(PO4)3 (x = 0.0, 0.5, 2.0) were successfully prepared using solid state reaction method. The initial precursors taken in the stoichiometric amount were SrCO3, LiOH⋅H2O, TiO2, ZrO2 and (NH4)2HPO4. The precursors were grinded for 2 h using agate mortar and pestle. The overall reaction for the preparation of the composition is given as
The obtained mixture was then calcined at 900 °C for 12 h by maintaining the heating and cooling rate of 5°/min. Then, the calcined powders were compressed in the form of pellet having diameter of 10 mm. After that, the prepared pellets were sintered at 1000 °C for 12 h by keeping heating and cooling rate of 5°/min. The powder X-ray diffraction (XRD) technique was studied to investigate the phase and crystal symmetry of the compositions. The XRD technique was done by Rigaku X-ray diffractometer over a range of 10 to 90 degrees with a step size of 0.01 degrees using CuK1 radiation (λ = 1.5406Å) at 40 kV and 30 mA. The X-ray diffraction patterns were refined through Rietveld using the Fullprof programme. Fourier transform infrared (FTIR) technique was performed to record the FTIR spectra of the compositions, using a Perkin-Elmer spectrometer at room temperature. The KBr pellet technique was used to record the FTIR spectra of the prepared compositions [29]. Conductivity and Impedance data were recorded by using Novocontrol make impedance analyzer (Alpha-A high performance frequency analyzer) in the frequency range of 10 Hz to 10 MHz at different temperatures ranging from 25 to 450 °C. For conductivity and impedance measurements, silver was pasted on both sides of the prepared pellets to work as electrodes. The measured density was calculated using dimensions and weight of the prepared compositions. The Scanning Electron Microscope (JXA-8100) instrument with energy 15 kV was used to examine the microstructure of the prepared pellet samples. Z view software is used to fit the cole cole plots. The electrochemical characteristics of the prepared compositions are described by a resistance (R) and a constant phase element (CPE), combined in parallel. The constant phase element is expressed as ZCPE = 1/Q (iw)−n, where n < 1 and w = 2πυ denote the angular frequency. The sub-diffusive ion mobility on smaller scales is represented by CPE, whereas R stands for broad-range ionic conduction [30]. CPEel explains the phenomena of electrode blocking in the low-frequency region.
3 Results and discussion
The XRD patterns of the compositions Li0.4Sr0.3Ti2−xZrx(PO4)3 (x = 0.0, 0.5 and 2.0) shown in Fig. 1a exhibit rhombohedral symmetry with space group R \(\overline{3}\) c. The peaks of the XRD pattern of composition x = 0.0 are matched with the JCPDS (035-0754) of rhombohedral LiTi2(PO4)3 JCPDS (051-0362). However, the peaks of the XRD pattern of Zr doped composition x = 0.5 and 2.0 are well matched with the JCPDS (030-0777) of rhombohedral LiZr2(PO4)3 material. The small peaks at ~ 22.3° and 26.2° in the composition x = 0.5 and 2.0 are related to oxygen-deficient zirconium oxide ZrO0.35 phase [26]. The peaks of the XRD pattern of the compositions x = 0.5 and 2.0 move towards lower angle values with the doping of Zr at Ti site in the composition Li0.4Sr0.3Ti2(PO4)3. The peaks observed at angle ~ 22.0° in case of Li0.4Sr0.3Ti2(PO4)3 composition in the XRD were recognized as the peaks of TiP2O7/TiO2 [31]. The shifting in the peaks demonstrates the expansion in the lattice characteristics due to Zr doping. The result obtained in the XRD pattern of Fig. 1a is in correlation with the XRD pattern found in literature [8, 32]. The inset Fig. 1b and (c) of the XRD pattern indicates the zoomed part of the peaks having hkl planes (100) and (113) respectively. The study of the XRD pattern indicates that the doping of Zr at Ti site is the reason of the displacement of the peaks towards lower angle of 2θ. The XRD peaks of the composition gradually become narrow with the doping of Zr. The sharpness in the XRD peak occurs due to the size and strain effects. Williamson and Hall [33] proposed a method to determine the crystallite size and microstrain of the material. The Williamson–Hall equation is formulated as
where β denotes full width at half maximum (FWHM) of the XRD peak, k denotes the Scherrer constant, D indicates the crystallite size, λ is the wavelength of the X ray, ε denotes the lattice strain and θ indicates the Bragg angle. The linear fitting of βcosθ (on y axis) versus 4sinθ (on x axis) provide the crystallite size (kλ/D) and microstrain (ε) from the intercept and slope of the W–H plot of the prepared compositions. The calculated values of density and strain are listed in Table 1. It is found that the density of the Zr doped composition (x = 0.5, 1.0, 2.0) is low as compared to Zr free composition (x = 0.0). However, the conductivity is increased for the Zr doped composition Li0.4Sr0.3Ti1.5Zr0.5(PO4)3 (9.65 × 10–6 S/cm). The relation between density and ionic conductivity in these NASICON type materials is complex and influenced by microstructural characteristics, phase composition, defect structures and processing conditions [28]. Lower density sometimes create a more favourable microstructure for ionic transport, leading to increased conductivity despite reduced density, seen in the case of composition Li0.4Sr0.3Ti1.5Zr0.5(PO4)3. These results are similar to the obtained results for Li1.3Ti1.7Al0.3(PO4)3 [28].
Figure 2a, b show the rietveld refinement of XRD spectra of powder samples using Fullprof suite software. The refinement was carried out in order to investigate the variations in the unit cell properties and phase of the prepared compositions. The R \(\overline{3}\) c space group was preferred as a starting point for the refinement. Li atoms were not considered for the refinement due to the limited scattering of Li ions. The partial doping of Zr at the Ti site in the lattice of Li0.4Sr0.3Ti2(PO4)3 increases the cell volume (V) and lattice parameters (a and c) of the composition Li0.4Sr0.3Ti1.5Zr0.5(PO4)3 and Li0.4Sr0.3Zr2(PO4)3. The refined parameters obtained by rietveld refinement of composition Li0.4Sr0.3Ti2−xZrx(PO4)3 (x = 0.0, 0.5, 1.0 and 2.0) are shown in Table 2.
We have recorded the FTIR spectra of the prepared compositions in order to determine the local structural characteristics of these compositions [34, 35]. The FTIR spectra of compositions Li0.4Sr0.3Ti2(PO4)3, Li0.4Sr0.3Ti1.5Zr0.5(PO4)3 and Li0.4Sr0.3Zr2(PO4)3 are shown in Fig. 3. The predominant features of the IR spectra shown in Fig. 3 are the overlapping intramolecular PO43− stretching modes that range from 700 to 1400 cm−1 [14]. Normal modes of the PO43− tetrahedron produce four types of vibrations in four frequency ranges: υ1 (symmetric stretching bands, ~ 950 cm−1), υ2 (symmetric bending, ~ 400–500 cm−1), υ3 (asymmetric stretching bands, ~ 1000–1230 cm−1), and υ4 (asymmetric bending ~ 500–700 cm−1) [34,35,36,37]. The bands seen at ~ 995–989 cm−1 are related to symmetric stretching vibration modes (υ1). It is observed that the vibration mode (υ1) is not found in case of composition Li0.4Sr0.3Ti1.5Zr0.5(PO4)3. The bands observed at 1220–1035 cm−1 for the prepared compositions are associated with asymmetric stretching modes (υ3).The bands at 645–545 cm−1 are attributed with asymmetric bending modes (υ4) [36, 37].
The conductivity plots of Li0.4Sr0.3Ti2−xZrx(PO4)3 (x = 0.0, 0.5, 2.0) are shown in Fig. 4 at temperatures 25 °C, 100 °C, 350 °C and 400 °C in the frequency range 10 Hz to 10 MHz. The conductivity of all the compositions in Fig. 4a–d rises with the frequency variation and appears to coincide between 1 kHz and 10 MHz. The conductivity is almost same for all the compositions at temperature 25 °C and 100 °C in the frequency range 1 kHz–10 MHz. Due to the hopping and various modes of vibration of the ions, the ionic conductivity of the samples exhibited a distinctive behavior. In the low frequency zone, conductivity is nearly found to be frequency-independent and is equivalent to dc conductivity (in the plateau region). It is found that the ac conductivity increases with the rise in frequency. The dispersive behavior of ac conductivity is seen from low to high-frequency area at low temperatures. However, at high temperatures, the frequency-independent behavior (plateau area) in the low-frequency region coexists with a small dispersive behavior of ac conductivity, at a higher frequency side. The change from a low-frequency (plateau zone) to a high-frequency dispersive region occurs due to conductivity relaxation caused by the shift from the long-distance hopping to short-distance ionic transport [38,39,40]. The composition Li0.4Sr0.3Zr2−xTix(PO4)3 (x = 0.0, 0.5, 2.0) exhibits a rise in ac conductivity with the increase in the temperature. This proposes a negative co-efficient of resistance (NTCR) behavior in materials [38]. To understand the conduction process of Li0.4Sr0.3Zr2−xTix(PO4)3 (x = 0.0, 0.5, 2.0) compositions, frequency-dependent ac conductivity measurement were fitted with Jonscher’s power equation
where σdc, A and n denote the dc conductivity or low frequency conductivity, temperature dependent pre exponential factor and dimensionless frequency exponent respectively. The frequency exponent (n) value lies in the range from 0 to1. The given Eq. (2) is used to fit the conductivity data of the prepared compositions. The fitted values of parameter σdc and n are summarized in Table 3. The conductivity measured in the frequency independent area is temperature dependent and is referred to as dc conductivity, which is associated to Li ion movement. Govindaraj [39] identified a comparable pattern in his ac conductivity study of Na5Ti(PO4)3. The conductivity increases with the frequency in the high frequency zone, showing that the hopping of ions has relaxed in accordance with the power law, σ α ωn. The frequency coefficient parameter (n) determines the amount of inter-ion lattice interaction and divergence from the Debye behavior of the material. The exponent n fluctuates with temperature but remains within the range of 0 to 1, depending on the material. The value of n = 1 represents the Debye behavior of the material, where charge carriers interact with the lattice at zero. As temperature rises, this contact becomes stronger, causing n to drop. At high temperatures, the disordered system results in the lowest n value, indicating the strongest interaction between charge carriers and the lattice system [35].
Figure 5 shows the temperature dependence of dc conductivity (log σdc) of NASICON composition Li0.4Sr0.3Ti2−xZrx(PO4)3 (a) x = 0.0, (b) x = 0.5 (c) 2.0, indicating the existence of two different areas of conduction mechanism from temperature 273 K to 723 K. The dc conductivity follows Arrhenius equation, which can be shown as given below
where the symbols A, Ea, k and T indicate the pre exponential factor, activation energy, Boltzmann constant and temperature of the material respectively. The activation energy observed for the compositions x = 0.0, 0.5, 2.0 is in the range of 0.66–0.64 eV at high temperature region (298–473 K) and 0.20–0.23 eV at low temperature range (523–723 K).
Figure 6 indicate the real part of the modulus (M’) spectra of Ti free composition Li0.4Sr0.3Zr2(PO4)3 and Ti doped composition Li0.4Sr0.3Ti1.5Zr0.5(PO4)3 at temperature 450 °C. It was examined that the peak value of M’ is greater for Ti doped composition compared to Ti free composition. The value of M' rises as frequency rises and tends to zero at lower frequencies between 10 and 150 kHz. This is due to the minimal contribution of the effect of electrode in the material. High values of M' at high frequencies are observed, which may be connected to the nonexistence of the space charge effect [28]. The peak value of M′ occurs at frequencies of 6.6 MHz and 6.9 MHz for compositions Li0.4Sr0.3Zr2(PO4)3 and Li0.4Sr0.3Ti1.5Zr0.5(PO4)3 respectively at 450°. The smaller value of M’ in the low-frequency region suggested the charge carrier mobility over an extended region, demonstrating that ions may easily hop from one site to another over considerable distances [41, 42]. The high frequency section of the M′ peak represented the frequency zone where charged particles were physically limited to their potential wells and could move locally.
Figure 7 indicate the cole cole figures of NASICON composition (A) Li0.4Sr0.3Ti2(PO4)3, (B) Li0.4Sr0.3Ti1.5Zr0.5(PO4)3 and (C) Li0.4Sr0.3Zr2(PO4)3 at 25 °C. The characteristics of impedance are composed of grains, grain boundary and contact between sample-electrode [43]. The inset of Fig. 7A, B displays the equivalent circuit model that is utilized to fit the Cole–Cole spectra. The equivalent circuit provided the grain boundary (σgb) and bulk resistance (σb) values, which are shown in Table 4. Using equation [σ = d/R. A] and grain boundary (σgb), bulk resistance (σb) values from Table 4, we estimated the grain boundary and bulk ionic conductivities of our compositions. The presence of semicircle, tail and inflection points in the cole cole plot of Li0.4Sr0.3Ti2(PO4)3 in Fig. 7A and Li0.4Sr0.3Zr2(PO4)3 in Fig. 7C demonstrates the high frequency contribution of bulk and grain boundary resistance as well as the capacitive blocking properties of the of the samples at lower frequencies, respectively [44]. The absence of a tail in the cole cole spectra and the equivalent circuit used in Fig. 7B indicate the absence of blocking electrodes in the composition Li0.4Sr0.3Zr0.5Ti1.5(PO4)3. The bulk and grain boundary conductivity of the prepared compositions are calculated from the fitted values of grain and grain boundary resistance. The bulk conductivity observed for Zr doped samples Li0.4Sr0.3Ti1.5Zr0.5(PO4)3 (4.32 × 10–5 S/cm) and Li0.4Sr0.3Zr2(PO4)3 (3.20 × 10–5 S/cm) are higher compared to Zr free sample Li0.4Sr0.3Ti2(PO4)3 (1.89 × 10–5 S/cm). The fitted values of bulk and grain boundary resistances, bulk and total conductivity are shown in Table 4.
The cross section SEM images of prepared pellets of Li0.4Sr0.3Ti2−xZrx(PO4)3 (x = 0.0, 0.5, 2.0) are shown in Fig. 8. The composition Li0.4Sr0.3Ti2(PO4)3 (x = 0) shows grains of different diameters with large inter-grain pores. Zr doped compositions Li0.4Sr0.3Ti2−xZrx(PO4)3 (x = 0.5, 2.0) show the reduced inter-grain pores and increased grain sizes [31, 43]. The grains of the composition Li0.4Sr0.3Zr2(PO4)3 (x = 2.0) are packed and well-coalesced, with no visible inter/intra-grain pores [31].
4 Conclusion
We have prepared Sr containing NASICON compositions Li0.4Sr0.3Ti2−xZrx(PO4)3 (x = 0.0, 0.5, 2.0) and explored the structural and lithium ionic conductivity in it. Here, we have tried to improve the lithium ionic conductivity with Zr doping induced modification in the interface. The FTIR spectra demonstrated the stretching and bending modes of phosphate group. SEM images show the well grown grains in all the samples. Zr doping reduces the inter-grain pores and increases the grain sizes in Li0.4Sr0.3Ti2−xZrx(PO4)3 (x = 0.5, 2.0). The enhanced bulk conductivity 4.32 × 10–5 S/cm and 3.20 × 10–5 S/cm was observed for Zr doped composition Li0.4Sr0.3Ti1.5Zr0.5(PO4)3 and Li0.4Sr0.3Zr2(PO4)3 at 25 °C. However, the composition Li0.4Sr0.3Ti1.5Zr0.5(PO4)3 exhibited the increased total conductivity of 9.65 × 10–6 S/cm. It is anticipated that the results of this study would be beneficial for the preparation of advanced NASICON-type solid electrolytes which show high ionic conductivities. These solid electrolytes would be suitable for the applications of the storage of electrical energy in substitution of the liquid electrolytes found in Lithium ion batteries.
Data availability
Data is provided within the manuscript or supplementary information files
References
Wu J, Liu S, Han F, Yao X, Wang C. Lithium/sulfide all-solid-state batteries using sulfide electrolytes. Adv Mater. 2021. https://doi.org/10.1002/adma.202000751.
Wu J, Shen L, Zhang Z, Liu G, Wang Z, Zhou D, et al. All-solid-state lithium batteries with sulfide electrolytes and oxide cathodes. Electrochem Energy Rev. 2021. https://doi.org/10.1007/s41918-020-00081-4.
Murugan R, Thangadurai V, Weppner W. Fast lithium ion conduction in garnet-type Li7La3Zr2O12. Angew Chem Int Ed. 2007. https://doi.org/10.1002/anie.200701144.
Thangadurai V, Narayanan S, Pinzaru D. Garnet-type solid-state fast Li ion conductors for Li batteries: critical review. Chem Soc Rev. 2014. https://doi.org/10.1039/C4CS00020J.
Wu J, Chen L, Song T, Zou Z, Gao J, Zhang W, et al. A review on structural characteristics, lithium ion diffusion behavior and temperature dependence of conductivity in perovskite-type solid electrolyte Li3xLa2∕3−xTiO3. Funct Mater Lett. 2017. https://doi.org/10.1142/S179360471730002X.
Lu J, Li Y. Perovskite-type Li-ion solid electrolytes: a review. J Mater Sci Mater Electron. 2021. https://doi.org/10.1007/s10854-021-05699-8.
DeWees R, Wang H. Synthesis and properties of NASICON-type LATP and LAGP solid electrolytes. Chemsuschem. 2019. https://doi.org/10.1002/cssc.201900725.
Lakshmanan A, Gurusamy R, Venkatachalam S. Enhanced total ionic conductivity of NASICON-type solid-state electrolyte Li1+xAlxTi2−x(PO4)3. Ionics. 2023. https://doi.org/10.1007/s11581-023-05222-5.
Goodenough J B, Hong H Y P and Kafalas J A. Fast Na+-ion transport in skeleton structures. Mater Res Bull. 1976. https://doi.org/10.1016/0025-5408(76)90077-5.
Maldonado-Manso P, Losilla ER, Martínez-Lara M, Aranda MAG, Bruque S, Mouahid FE, et al. High lithium ionic conductivity in the Li1+xAlxGeyTi2−x−y(PO4)3 NASICON series. Chem Mater. 2003. https://doi.org/10.1021/cm021717j.
Aono H, Sugimoto E, Sadaoka Y, Imanaka N, Adachi G-Y. Electrical properties of sintered lithium titanium phosphate ceramics (Li1+XMXTi2−X(PO4)3, M3+ = Al3+, Sc3+, or Y3+). Chem Lett. 1990. https://doi.org/10.1246/cl.1990.1825.
Stenina I-A, Yaroslavtsev A-B. Nanomaterials for lithium-ion batteries and hydrogen energy. Pure Appl Chem. 2017;89:1185.
Li Q, Chen J, Fan L, Kong X, Lu Y. Progress in electrolytes for rechargeable Li-based batteries and beyond. Green Energy Environ. 2016. https://doi.org/10.1016/j.gee.2016.04.006.
Luo Q, Ming L, Zhang D, Wei C, Wu Z, Jiang Z, et al. Constructing Br-doped Li10SnP2S12-based all-solid-state batteries with superior performance. Energy Mater Adv. 2023. https://doi.org/10.34133/energymatadv.0065.
Dias J-A, Santagneli S-H, Messaddeq Y. Methods for lithium Ion NASICON preparation: from solid-state synthesis to highly conductive glass-ceramics. J Phys Chem C. 2020. https://doi.org/10.1021/acs.jpcc.0c07385.
Chen S, Yu C, Wei C, Jiang Z, Zhang Z, Peng L, et al. Unraveling electrochemical stability and reversible redox of Y-Doped Li2ZrCl6 solid electrolytes. Energy Mater Adv. 2023. https://doi.org/10.34133/energymatadv.0019.
Yaroslavtsev A. Modification of solid state proton conductors. Solid State Ion. 2005. https://doi.org/10.1016/j.ssi.2005.09.025.
Stenina I-A, Pinus I-Y, Yaroslavtsev A-B, Kislitsyn M-N, Arkhangelskij I-V, Zhuravlev N-A. Phase transformations and cation mobility in NASICON lithium zirconium double phosphates Li1±xZr2−xMx(PO4)3 (M = Sc, Y, In, Nb, Ta). Russ J Inorg Chem. 2005;50:906.
Pinus I-Y, Khoroshilov A-V, Gavrichev K-S, Tarasov V-P, Yaroslavtsev A-B. On cationic mobility in Nasicon phosphates LiTi2(PO4)3 and Li0.9Ti1.9Nb0.1(PO4)3. Solid State Ion. 2012. https://doi.org/10.1016/j.ssi.2012.01.035.
Subramanian M-A, Subramanian R, Clearfield A. Lithium ion conductors in the system AB(IV)2(PO4)3 (B = Ti, Zr and Hf). Solid State Ion. 1986. https://doi.org/10.1016/0167-2738(86)90179-7.
Petit D, Colomban Ph, Collin G, Boilot J-P. Fast ion transport in LiZr2(PO4)3: Structure and conductivity. Mat Res Bull. 1986. https://doi.org/10.1016/0025-5408(86)90194-7.
Aono H, Sugimoto E, Sadaoka Y, Imanaka N, Adachi G-Y. Ionic conductivity and sinterability of lithium titanium phosphate system. Solid State Ion. 1990. https://doi.org/10.1016/0167-2738(90)90282-V.
Aono H, Sugimoto E, Sadaoka Y, Imanaka N, Adachi G-Y. Ionic conductivity of solid electrolytes based on lithium titanium phosphate. J Electrochem Soc. 1990. https://doi.org/10.1149/1.2086597.
Arbi K, Tabellout M, Sanz J. NMR and electric impedance study of lithium mobility in fast ion conductors LiTi2−xZrx(PO4)3 (0 ≤ x ≤ 2). Solid State Ion. 2010. https://doi.org/10.1016/j.ssi.2009.11.010.
Stenina I, Pyrkova A, Yaroslavtsev A. NASICON-type Li1+xAlxZryTi2−x−y(PO4)3 solid electrolytes: effect of Al, Zr co-doping and synthesis method. Batteries. 2023. https://doi.org/10.3390/batteries9010059.
Rossbach A, Grieshammer N-S. Preparation, characterization and conductivity of NASICON-type Li1+xM(III)xTi2−x(PO4)3 (M(III) = Al, Cr, Fe; 0.5 ≤ x ≤ 2.0) materials via modern, scalable synthesis routes. Open Ceram. 2022. https://doi.org/10.1016/j.oceram.2022.100231.
Singh B, Kumar S, Saha N, Basu B, Gupta R. Phase stability of silver particles embedded calcium phosphate bioceramics. Bull Mater Sci. 2015;38:525–9. https://doi.org/10.1007/s12034-014-0839-y.
Schell KG, Bucharsky EC, Lemke F, Hoffmann MJ. Effect of calcination conditions on lithium conductivity in Li1.3Ti1.7Al0.3(PO4)3 prepared by sol-gel route. Ionics. 2017. https://doi.org/10.1007/s11581-016-1883-y.
Siddiqui S, Singh D, Singh P, Singh B. Structural, optical, microstructure, and giant dielectric behavior of parent, Cu and Sr doped La0.55Li0.35TiO3-δ. J Am Ceram Soc. 2023. https://doi.org/10.1111/jace.19296.
Mariappan C-R, Kumar P, Kumar A, Indris S, Ehrenberg H, Prakash G-V, et al. Ionic conduction and dielectric properties of yttrium doped LiZr2(PO4)3 obtained by a Pechinitype polymerizable complex route. Ceram Int. 2018. https://doi.org/10.1016/j.ceramint.2018.05.211.
Afif M-A, Pratiwi V-M, Noerochim L. Enhanced ionic conductivity of fluoride-doped LiTi2(PO4)3 as solid electrolyte of lithium-ion battery. Chem Eng. 2024. https://doi.org/10.4028/p-FrjE6w.
Kumar S, Balaya P. Improved ionic conductivity in NASICON-type Sr2+ doped LiZr2(PO4)3. Solid State Ion. 2016. https://doi.org/10.1016/j.ssi.2016.08.012.
Williamson G-K, Hall W-H. X-ray line broadening from filed aluminium and wolfram. Acta Metall. 1953. https://doi.org/10.1016/0001-6160(53)90006-6.
Giarola M, Sanson A, Tietz F, Pristat S, Dashjav E, Rettenwander D. Structure and vibrational dynamics of NASICON-type LiTi2(PO4)3. J Phy Chem. 2017. https://doi.org/10.1021/acs.jpcc.6b11067.
Singh B, Tandon A, Pandey AK, Singh P. Enhanced dielectric constant and structural transformation in Fe-doped hydroxyapatite synthesized by wet chemical method. J Mater Sci. 2018. https://doi.org/10.1007/s10853-018-2225-4.
Kahlaoui R, Arbi K, Jimenez R, Sobrados I, Mehnaoui M, Sanz J, et al. Synthesis, structural characterization and ionic conductivity of NASICON-type Bax/2Li1−xTi2(PO4)3 (0.4 ≤ x ≤ 1) materials. Ionics. 2017;23:837–46. https://doi.org/10.1007/s11581-016-1898-4.
Vijayan L, Govindaraj G. Structural and electrical properties of high-energy ball-milled NASICON type Li1.3Ti1.7Al0.3(PO4)2.9(VO4)0.1 ceramics. J Phys Chem Solids. 2011. https://doi.org/10.1016/j.jpcs.2011.02.001.
Sharma S-K, Mohanty H-S, Pradhan D-K, Kumar A, Shukla V-K, Singh F, Kulriya P-K. Structural, dielectric and electrical properties of pyrochlore-type Gd2Zr2O7 ceramic. J Mater Sci Mater Electron. 2020. https://doi.org/10.1007/s10854-020-04699-4.
Ortiz G-F, López M-C, Lavela P, Vidal-Abarca C, Tirado J-L. Improved lithium-ion transport in NASICON-type lithium titanium phosphate by calcium and iron doping. Solid State Ion. 2014. https://doi.org/10.1016/j.ssi.2013.09.012.
Singh B, Kumar S, Basu B, Gupta R. Enhanced ionic conduction in hydroxyapatites. Mater Lett. 2013. https://doi.org/10.1016/j.matlet.2012.12.074.
Govindaraj G, Mariappan C-R. Synthesis, characterization and ion dynamic studies of NASICON type glasses. Solid State Ion. 2002. https://doi.org/10.1016/S0167-2738(02)00005-X.
Mariappan C-R, Govindaraj G. Ac conductivity, dielectric studies and conductivity scaling of NASICON materials. Mater Sci Eng B. 2002. https://doi.org/10.1016/S0921-5107(02)00083-1.
Singh B, Kumar S, Basu B, Gupta R. Conductivity studies of silver-, potassium-, and magnesium-doped hydroxyapatite. Int J Appl Ceram Technol. 2013. https://doi.org/10.1111/ijac.12167.
Arbi K, Hoelzel M, Kuhn A, Garcia-Alvarado F, Sanz J. Structural factors that enhance lithium mobility in fast-ion Li1+xTi2–xAlx(PO4)3 (0 ≤ x ≤ 0.4) conductors investigated by neutron diffraction in the temperature range 100–500 K. Inorgan Chem. 2013. https://doi.org/10.1021/ic400577v.
Acknowledgements
The authors are grateful to Department of Science and Technology (DST) India for providing instrument grant to Center of Material Sciences, University of Allahabad under its FIST (Grant No. SR/FST/PSI-216/2016) funding scheme.
Funding
The authors did not receive support from any organization for the submitted work.
Author information
Authors and Affiliations
Contributions
S.S. wrote the main manuscript. S.S. and D.S. have collected data and plotted graphs. B.S. has helped in analysis and supervised the research work. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Siddiqui, S., Singh, D. & Singh, B. Lithium ionic conductivity and structural studies of NASICON structured Li0.4Sr0.3Zr0.5Ti1.5(PO4)3. Discov Mater 4, 18 (2024). https://doi.org/10.1007/s43939-024-00089-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43939-024-00089-3