Abstract
Magnesium alloys containing rare earth metals exhibit good creep resistance up to 300 °C and good tensile properties at ambient temperature. The high cost of rare earth has led to studies regarding the creep resistance of Mg alloys with cheap alloying elements (Sn, Ca, Si) that could be substituted for Mg-RE alloys. In this paper, the influence of Si and Al on microstructure and mechanical properties of Mg-7Sn alloy was investigated using optical (LM), scanning electron microscopy (SEM), transmission electron microscopy (TEM), X-ray diffraction analysis (XRD), tensile tests and creep tests at 200–250 °C. Microstructure of as-cast alloys consists of α-Mg matrix and intermetallic compounds at the interdendritic regions. Heat treatment consisting of solid solution treatment and ageing increases the tensile properties at ambient temperature due to the precipitation of the fine Mg2Sn phase. The creep resistance of aged Mg-7Sn alloy is poor. The addition of Si and Al to Mg-7Sn alloy has resulted in improving the creep resistance due to the refinement of Mg2Sn phase and the appearance of Mg2Si phase at the grain boundaries. The Mg-7Sn-1Si alloy exhibits better creep resistance at 200 °C than Mg-7Sn-5Si and Mg-7Sn-5Si-2Al alloys. The Mg-7Sn alloys with 5% Si have better creep properties at 250 °C in comparison to Mg-7Sn-1Si alloy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Magnesium alloys due to their low density are attractive materials for the aerospace and automotive industries [1]. Commercial Mg–Al alloys are characterized by low operating temperature (up to about 120 °C), thus intensive research is being carried out to improve the creep resistance of magnesium alloys. Increase in creep resistance of Mg–Al alloys is achieved by introducing alloying elements such as silicon [2, 3], strontium [4], calcium [3, 5] and rare earth metals [6]. These alloys can be operated up to a temperature of about 200 °C. In the last two decades, the Al-free magnesium alloys with rare earth metals, yttrium, strontium and manganese (Mg-Zn-RE, Mg-Y-Nd-Zr, Mg-Sr-Mn, and Mg-Nd-Gd-Zr) have been developed, which can be used for structural elements operating up to a temperature of 250 °C (even up to 300 °C during short-term operation) [7,8,9]. In the case of Al-free magnesium alloys good creep properties are related to the presence of the strengthening phases characterized by good thermal stability at elevated temperatures. However, these alloys are expensive due to the high cost of rare earth metals and also in some cases technological problems in casting. Thus, there is a need for an alternative alloy which has similar properties to Mg-Y-Nd-Zr and Mg-Nd-Gd-Zr alloys, but which are characterized by good casting properties and lower manufacturing costs compared to magnesium alloys consisting of rare-earth metals [10].
Silicon—that forms the Mg2Si phase in magnesium alloys—is one of the alloying elements that can favorably affect the creep resistance of magnesium alloys at low manufacturing costs. The Mg2Si phase exhibits a high melting temperature (1085 °C), high hardness, high elastic modulus and low thermal coefficient [11]. If the Si content in the magnesium alloys is below 1.34 wt%, the Mg2Si phase is formed as a result of the eutectic reaction, while above 1.34 wt%, Mg2Si primary crystals are also formed. Obviously, the presence of primary Mg2Si compound which forms the large particles in the α-Mg matrix adversely affects mechanical properties [12]. Research to modify the shape and size of primary crystals has not contributed to significant improvements in mechanical properties of Mg-Si alloys containing more than 1.34 wt% Si [11]. To increase mechanical properties at ambient and elevated temperatures of Si-rich Mg alloys, alloying elements which will promote the formation of fine precipitates strengthening of the matrix can be added. One of these elements is tin, which is readily available and forms the Mg2Sn intermetallic compound with a high melting temperature of about 770 °C. The solubility of Sn in α-Mg provides a fundamental basis for improving the mechanical properties of these alloys through ageing and has a positive effect on the mechanical properties at ambient and elevated temperatures [13, 14].
The present paper is aimed to develop as-cast Mg-Sn alloys with the addition of high content Si and investigates their microstructure, mechanical properties at ambient temperature and creep properties.
2 Experimental method
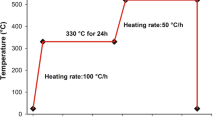
Magnesium alloys containing silicon, tin and aluminum were prepared, and its composition was analyzed by X-ray fluorescence spectroscopy (Table 1). Commercially pure Mg (99.8%), Si (99.5%), Sn (99.4%) and Al (99.6%) were used. Melting of the alloys was performed by induction melting in an alumina crucible under the protection of an argon atmosphere. The melt was maintained at 800 °C for 3 min then poured into sand moulds. T6 heat treatment (solution treatment + artificial ageing) was carried out to enhance the mechanical properties. The solution treatment was performed at 500 °C for 24 h in an argon atmosphere and water quenched to room temperature. The ageing treatment was performed at temperatures in the range between 200 °C and 250 °C from 4 to 148 h.
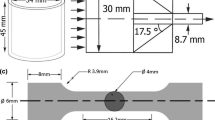
The microstructure of the alloys was analyzed by light microscopy (LM) and scanning electron microscopy (SEM) using a FE SEM Hitachi S-3400 N scanning electron microscope. Observations were used at SE (secondary electron) and BSE (back-scaterred electron) modes. Energy-dispersive X-ray spectroscopy (EDS) analysis was performed at the acceleration voltage of 15 keV. Microstructure observations were performed on the samples etched in reagent containing 3 mL HNO3 and 97 mL C2H5OH. The FEI Titan 83/300 transmission electron microscope (TEM) was also used to analyze the phase composition and chemical composition of the intermetallic phases. Thin foils were prepared by electrolytic polishing. The electrolyte composition was 5.3 g lithium chloride, 11.16 g magnesium perchlorate, 500 mL methanol, and 100 mL 2-butoxyethanol. Polishing was performed at − 45 °C and 20 V. X-ray diffraction patterns (XRD) were obtained with a JEOL JDX-7S diffractometer with a copper anode. Registration was performed by 0.02° stepwise regression for 2θ ranging from 10° to 90° 2θ. Phase identification was performed using the ICDD PDF-4 + database. Hardness measurement was carried out on Vickers hardness tester (Duramin A5) at a load of 2 kg according to ASTM E92-17 standard (ASTM E92-17. Standard Test Methods for Vickers Hardness and Knoop Hardness of Metallic Materials). Standard creep test specimens machined out from the castings in accordance with ASTM E139-11(2018) standard, were subjected to a constant load creep test at 200–250 °C at stresses of 15–60 MPa using the Zwick Kappa 50DS creep-testing machine. Creep strain was measured by extensometers which were attached directly to the gauge section of specimens. The length of the specimen was 100 mm, the gage length was 60 mm and the diameter of the reduced section was 6 mm.
3 Results and discussion
3.1 Microstructure of Mg-Si-Sn alloys
Microstructure of sand-cast Mg-7Sn, Mg-7Sn-1Si, Mg-7Sn-5Si and Mg-7Sn-5Si-2Al alloys is shown in Figs. 1 and 2. Details of the phase composition identification were presented in a previous paper [15]. The microstructure of sand-cast Mg-7Sn alloy (Figs. 1a, 2a) is composed of α-Mg magnesium matrix and eutectic Mg2Sn phase at the grain boundaries. The clear segregation of tin inside the dendrites of the α-Mg is also observed (in the dark areas visible in the LM image—Fig. 1a, increased tin content was found). The addition of 1 wt% Si to Mg-7Sn alloy (Fig. 1a, b) caused the formation of the Mg2Si phase in the form of Chinese script. The minor amounts of Sn are dissolved in the Mg2Si phase and the minor amounts of Si are dissolved in the Mg2Sn phase [15]. When the Si content is increased from 1 to 5%, substantial changes have occurred in the microstructure. In the Mg-7Sn-5Si alloy (Fig. 2c) the α-Mg matrix, eutectic Mg2Sn phase, the Chinese script type Mg2Si phase and additionally coarse primary crystals of Mg2Si compound are observed. The phase composition of Mg-7-Sn-5Si alloy did not change when 2% Al was added to alloy, because Al dissolves in the α-Mg solid solution. In the all tested alloys, the significant solute segregation in α-Mg solid solution was found. The tin content increases towards the interdendritic regions as a results of coring during solidification (LM images show the existence of diffusive dark areas and BSE images show the presence of diffusive bright areas at the dendritic and grain boundaries).
The solution heat-treatment of tested alloys at 500 °C for 24 h leads to the dissolution the eutectic Mg2Sn phase in the α-Mg solid solution and homogenization of chemical composition in the α-Mg matrix. It is obviously related to the high solubility of Sn in the Mg at 500 °C. The Mg2Si phase, which is formed as a result of the eutectic reaction and is characterized by a Chinese script morphology, undergoes spheroidization during solution heat treatment (Fig. 3a, b). Due to the very low solubility of Si in Mg, it can be expected that the diffusion of Si atoms runs along with the Mg/Mg2Si interface during spheroidization process. The diffusion of Si inside the Mg2Si particles is rather unlikely because the Mg2Si phase exists only at stoichiometric composition [16]. The Mg2Si primary crystals exhibit a high stability of shape and chemical composition during the solution heat-treatment at a temperature of 500 °C for 24 h.
During the aging of the tested alloys at 250 °C, the fine precipitates of Mg2Sn phase are formed regardless of the silicon content in the alloy (Fig. 4). The morphology of the Mg2Si primary crystals and globular Mg2Si compound remained unchanged after aging. Al in Mg-7Sn-5Si-2Al alloy does not form any phases during aging and is completely dissolved in the α-Mg solid solution. The equilibrium Mg2Sn phase is formed in the early stages of ageing and it is not preceded by the precipitation of other non-equlibrium phases. Obviously, with extending ageing time, coarsening and increase of the Mg2Sn phase content were found. The precipitation process of the Mg2Sn phase in the Mg-Sn-(X) alloys has been widely reported [17,18,19,20,21,22,23,24,25,26]. According to previous studies, it was found that lath-shaped, plate-like and polygon Mg2Sn precipitates may be formed after ageing in these alloys. Similar results were obtained in case of alloys being the subject of this paper. The lath-shaped precipitates of Mg2Sn phase dominate inside the α-Mg grains. They are mainly formed on basal planes of the α-Mg matrix (Fig. 5, type 1) and exhibit the orientation relationship: (0001)α-Mg//(110)Mg2Sn, [11,12,13,14,15,16,17,18,19,20]α-Mg//[001]Mg2Sn. For the polygonal precipitates (Fig. 5, type 2) growing in the perpendicular direction to (0001)α-Mg the orientation relationship can be described to be (2–1–10)α-Mg//(−110)Mg2Sn, [0001]α-Mg//[111]Mg2Sn. The plate-like precipitates (Fig. 5, type 3) with a hexagonal shape when the electron beam direction is parallel to [0001] α-Mg are also formed during ageing of the tested alloys. Their orientation relationship was reported in [22] and described approximately as (0001)α-Mg//(111)Mg2Sn, [2-1-10] α-Mg deviates by about 9° from [−110]Mg2Sn.
The lath-shaped (1), polygonal (2) and plate-shaped (3) of Mg2Sn precipitates observed in the Mg-7Sn-5Si-2Al alloy aged at 250 °C for 20 h (a), TEM micrograph of Mg-7Sn-5Si-2Al alloy aged at 250 °C for 20 h, which were obtained in the [0001]Mg beam direction (b), STEM micrograph of Mg-7Sn-1Si alloy aged at 250 °C/72 h registered in the [2-1-10]Mg beam direction (c), STEM image of precipitates of Mg2Sn phase in Mg-7Sn-5Si-2Al alloy aged at 250 °C for 20 h (d)
In the tested alloys the length of the lath-shaped precipitates decreases when Al and Si are added to the Mg-Sn alloys (Table 2). The addition of Al significantly decreases the size of lath-shaped Mg2Sn precipitates in Mg-7Sn-5Si alloy. However, increasing the Si content from 1 to 5% does not cause significant refinement of the Mg2Sn precipitates.
It was reported that the effect of microalloying additions on the refinement of Mg2Sn precipitates is related to the formation of clusters or minor precipitates in the α-Mg matrix, which may be effective catalysts for nucleation of the strengthening precipitates in the Mg–Sn system [14]. Silicon in the tested alloys forms the Mg2Si phase and its solubility in the α-Mg solid solution is very low (0.005 at.%) and probably Si is not able to create nano-clusters reducing the size of Mg2Sn precipitates. Microscopic observations did not allow to determine whether silicon can form nanoclusters or nanoparticles in the α-Mg matrix, but it can be seen that Si is dissolved in the Mg2Sn precipitates (Fig. 6). The incorporation of the Si atoms in the crystal structure of Mg2Sn phase may hinder its growth during ageing, thus Si may have a contribution to the refinement of Mg2Sn precipitates regardless of Si content in alloys. The Al dissolved in the α-Mg can also affect the growth rate and a number of nuclei of the Mg2Sn precipitates during aging by reducing the solubility of Sn in α-Mg or change the interfacial energy between Sn and α-Mg solid solution [23]. Thus the driving force for the nucleation of Mg2Sn precipitates will be increased and the greater the number of precipitates per unit volume can be formed. Microscopic observations (Fig. 6) also revealed that nano-clusters of Al may be formed at the surface of Mg2Sn particles and these nano-clusters may have acted as heterogeneous nucleation sites of these precipitates.
Figure 7 shows the microstructure of the peak-aged (250 °C) alloys with the revealed the α-Mg grains and Table 2 presents the results of the grain size measurements. It can be seen that the Mg-Sn alloys consisting of Si exhibit lower α-Mg grain size in comparison to Mg-Sn alloy due to the existence of the Mg2Si compound in the interdendritic regions, which hinders grain growth after casting process as well as during the solution heat treatment at 500 °C.
3.2 Mechanical properties
The sand-cast Mg-7Sn alloy exhibits low hardness (~ 45 HV). The addition of 1 wt% Si increases the hardness of Mg-Sn alloy to 51 HV due to the formation of the eutectic Mg2Si phase. When the Si content increased to 5%, an increase in hardness of up to 63 HV was noted due to the appearance of primary crystals of Mg2Si compound in the microstructure. The addition of 2 wt% Al also caused a significant increase in hardness (71 HV). The content of Mg2Si and Mg2Sn compounds in Mg-7Sn-5Si and Mg-7Sn-5Si-2Al alloys is comparable, therefore the higher hardness of Mg-7Sn-5Si-2Al is attributed to the presence of Al solute atoms in α-Mg solid solution and smaller α-Mg grain size.
The solution heat treatment at 500 °C reduced hardness of the tested alloys (Mg-7Sn alloy—40 HV, Mg-7Sn-1Si alloy—43 HV, Mg-7Sn-5Si alloy—60 HV, Mg-7Sn-5Si alloy—65 HV) as a result of the dissolution of Mg2Sn phase in the matrix, the spheroidization of eutectic Mg2Si phase and the grains growth of α-Mg phase.
It was reported that the hardness increment in binary Mg-Sn alloys is relatively low (ΔHV ≈ 10 HV) and hardness of as-cast Mg-Sn alloys aged at 200 °C is in the range from 45 to 55 HV [14, 23]. In this work, similar results of hardness increment were obtained for aged Mg-7Sn alloy (ΔHV = 11 HV) (Tab. 2). The age hardening behavior of tested alloys (Fig. 8) indicates that the silicon and aluminum additions enhance the hardness increment of Mg-7Sn alloy. The hardness growth is comparable for Mg-7Sn-1Si and Mg-7Sn-5Si alloys (ΔHV ≈ 15–16 HV), while Al caused a further increase in hardness increment (ΔHV ≈ 19 HV). Changes in the hardness of tested alloys result from the reduction of the Mg2Sn precipitates size and the increase in the number precipitates. Summary of ageing response of alloys at 250 °C is shown in Table 2.
The tensile properties including ultimate tensile strength (UTS), yield strength (YS) and elongation (ε) of as-cast and aged alloys are listed in Table 3. The sand-cast Mg-7Sn alloy exhibits the lowest tensile strength, yield strength and the best elongation. T6 heat treatment of this alloy contributes to a significant improvement of UTS, YTS and elongation, however, the YTS values are still insufficient to practical applications. The relatively low mechanical properties of the Mg-7Sn alloy result from coarse-grained structure due to the low cooling rate in the sand molds. Addition of 1 wt% silicon to Mg-7Sn alloy increased its mechanical properties, which can be explained by the reduction in α-Mg grain size and via the formation of a greater number of finer Mg2Sn precipitates. The Mg2Si eutectic phase, which has partially coagulated after solution heat treatment, should not significantly improve the strength due to its size and low efficiency in blocking dislocations. However, it may contribute to hindering grain boundary slip. Existence in the microstructure the Mg2Si primary crystals (alloys with 5%Si), which is characterized by unfavorable morphology and large size, resulted in a significant deterioration in strength, despite lower grain size of α-Mg solid solution. The Al addition promotes the improvement of mechanical properties due to the formation of the finer precipitates of the Mg2Sn phase and the solid solution strengthening. It has been shown that aluminum significantly strengthens the α-Mg solid solution due to high solubility in Mg at ambient temperature and large atomic size misfit (11%) [27].
The creep behavior of the peak-aged alloys was studied at temperatures between 200 and 300 °C under applied stresses between 15 and 60 MPa. The typical creep curves obtained at a temperature of 200 °C and a stress of 30 MPa are shown in Fig. 9. From these creep curves, the primary and secondary creep stages can be clearly distinguished. The steady-state creep rate \(\dot{\varepsilon }\) was calculated by measuring the slope of the steady-state stage of the curves and results are presented in Table 4. For comparison, the creep results of sand cast Mg-3Zn-3RE-0.7Zr (EZ33) and Mg–Al-Ca-Sr alloys are also included. The sand-cast Mg-7Sn alloy exhibits a very poor creep resistance at the stress of 30 MPa and at temperatures of 200 °C and of 250 °C, therefore this alloy was not selected to further creep tests at higher stresses and temperatures. When 1% Si is added to the Mg-7Sn alloy, a significant improvement in creep resistance at 200 °C temperature range is observed. In comparison to alloys with 5% Si (Mg-7Sn-5Si, Mg-7Sn-5Si-2Al) the Mg-7Sn-1Si alloy is characterized by better creep resistance at a temperature of 200 °C and at stresses 30–45 MPa. However, at a temperature of 250 °C the Si-rich alloys exhibit a lower creep rate and creep strain. In comparison to the sand-cast Mg–Al-Ca-Sr and EZ33 alloys, which were crept at 60 MPa, the creep properties of Mg-Sn-Si alloys tested in this work are clearly inferior.
The power-law equation is often used to evaluate creep mechanisms in magnesium alloys and it can be written as follows [2]:
where A is a constant, n is the apparent stress exponent, R is the gas constant, and Qs is the apparent activation energy.
The n and Qc parameters can be indirectly used to determine the dominant creep deformation mechanism for material in specific ranges of stress and temperature [2]. In the case of Mg-7Sn-1Si alloy, the exponent values of n were determined only in the range of 30–45 MPa due to the short duration of creep tests at 60 MPa. These values are 5.7 and 7.4 at temperatures of 200 °C and 250 °C, respectively (Table 5). Similar values of n parameters were obtained for alloys with 5% Si content, however, in the stress range of 30–60 MPa. Thus it can be concluded that the dislocation climbing and gliding can be regarded as a rate-controlling mechanism in these alloys [28,29,30,31,32,33,34].
It should be mentioned here that the n exponent values may be overestimated. Tested alloys consist of precipitates of Mg2Sn phase inside the α-Mg matrix, therefore, in determining the exponent n, the threshold stresses (σ0) should also be included in the calculation. However, correct determination of the threshold stress is possible if the experimental data covers a sufficiently large range of creep rates and in case of tested alloys the creep rate range is not large enough. On the other hand, stress exponent n is not significantly greater than expected, which may indicate that the interaction between Mg2Sn precipitates and dislocations is not strong.
The activation energy of creep deformation (Qc) for the most thermally activated processes in Mg are: lattice diffusion (135 kJ/mole), pipe diffusion (92 kJ/mole) and GB diffusion (60–80 kJ/mole). Qc values for dislocational processes in Mg alloys are dislocation climb (135 kJ/mole) and cross-slip (~ 200 kJ/mole) [10]. The activation energy (Qc) determined at 45 MPa for Mg-7Sn-1Si alloy is about 139 kJ/mole between 200 and 250 °C (Table 5), therefore, dislocation climb controlled by lattice diffusion can be the main mechanism the creep deformation in Mg-7Sn-1Si alloy at applied stresses 45 MPa and a temperature range of 200–250 °C. Activation energy Qc of Mg-7Sn-5Si alloy at these creep conditions (Table 5, Oc ≈ 123 kJ/mole) is lower than that of the Mg-7Sn-1Si alloy which may suggest a decreasing contribution of lattice diffusion and an increasing contribution of pipe diffusion to total creep deformation. The Qc value of Mg-7Sn-5Si-2Al (Table 5, Oc ≈ 87 kJ/mole) indicate that creep deformation mechanism could be dislocation climb controlled by pipe diffusion, however, the grain boundary diffusion also has a significant contribution to the total deformation of the alloy during creep in tested conditions.
The fracture surface of the alloy having a lower silicon content (Mg-7Sn-1Si) after creep at 250 °C/45 MPa (Fig. 10) exhibits ductile and brittle characteristics. The presence of dimples on the fracture surface indicates high ductility of Mg-7Sn-1Si alloy during creep under these conditions (250 °C/45 MPa). It is well known that magnesium and its alloys exhibit brittle fracture at ambient temperature due to the low number of slip systems, therefore, the domination of a ductile surface indicates the activation of additional slip systems (prismatic and pyramidal slip systems). In combination with the results of creep tests (Table 4), this leads to the conclusion that the plate-like precipitates of Mg2Sn phase within the α-Mg grains are not sufficiently effective in blocking dislocations moving in thermally activated slip systems which results from the orientation relationship: (0001)α-Mg//(110)Mg2Sn, [11,12,13,14,15,16,17,18,19,20]α-Mg//[001]Mg2Sn. Effectiveness in blocking dislocations is also reduced as a result of spheroidization of Mg2Sn precipitates at the elevated temperature (Fig. 11), thus their size and inter-particle spacing are increased. Moreover, these precipitates break during the creep (Fig. 12a) and contribute to the formation of voids within the α-Mg and, consequently, to a reduction in strength. The presence of hard Mg2Si phase at the interdendritic regions favors stress concentration at grain boundaries and nucleation of cracks at the α-Mg/Mg2Si interface (Fig. 13). These cracks propagate along the grain boundary and produce brittle fracture. In the Si-rich alloys the cracks propagate both along the grain boundaries and inside the Mg2Si primary crystals (Fig. 12b). Moreover, the fracture surfaces of the crept Si-rich alloys exhibit features of ductile fracture indicating the additional slip systems are activated.
Summarizing the results of the research on creep it can be concluded that sand-cast and heat-treated (T6) Mg-Sn alloys are characterized by low creep resistance at 200 °C. The low creep resistance of Mg alloys containing up to 5% Sn is the result of the formation of large precipitates of the equilibrium Mg2Sn phase, their unfavorable crystallographic orientation relative to the α-Mg matrix, tendency to coagulate at elevated temperature and the lack of strengthening of grain boundaries. The addition of 1% Si significantly improves creep resistance at 200 °C of Mg-Sn alloys due to the refinement of the Mg2Sn precipitates and especially the formation of the Mg2Si phase at interdendritic regions. The presence of the eutectic Mg2Si phase is conducive to strengthening the grain boundaries of the α-Mg solid solution, however, on the other hand, the solution heat treatment causes a partial spheroidization of the Mg2Si phase, which reduces the effectiveness of strengthening the grains boundaries. The main creep mechanism of the Mg-7Sn-1Si alloy is dislocation climb controlled by lattice diffusion. Increasing the silicon content to 5% resulted in improved creep resistance at 250 °C and a change in creep mechanisms. The appearance of Mg2Si primary crystals, further the refinement Mg2Sn precipitates and refinement of α-Mg grains caused that pipe diffusion has a greater contribution to controlling dislocation climb during creep at 45 MPa in the Mg7Sn-5Si alloy. In case of Mg-7Sn-5Si-2Al alloy, in which the refinement of the Mg2Sn precipitates is more significant than in the Mg-7Sn-5Si alloy, the obtained Qc value (87 kJ/mol) is between 80 kJ/mol (GBD) and 92 kJ/mol (pipe diffusion), which suggest a significant contribution of both grain boundaries diffusion and diffusion along with the dislocation cores in the creep deformation of aged Mg-7Sn-1Si alloy. The greater contribution of grain boundary diffusion may also result from the lower α-Mg grain size of the Mg-7Sn-5Si-2Al alloy.
Generally, slightly better creep resistance of Mg-7Sn-5Si-2Al alloy than Mg-7Sn-5Si is a consequence of the formation of a larger number the plate-like Mg2Sn precipitates, which are characterized by a lower size and inter-particle spacing. Moreover, precipitates of the Mg2Sn phase exhibits different morphologies (lath, polygon and plate) with different orientation relationships in the aged condition. It is possible that the Al addition promotes the formation of polygonal Mg2Sn precipitates, which possibly have better-strengthening effect than lath and plate-shaped Mg2Sn precipitates. In the studied alloy the Al, besides to such effects as refining the precipitates and partly changing their morphology, dissolves in the α-Mg matrix causing the effect of solution strengthening. However, in case of solid solution Al in Mg strengthening effects are anisotropic. According to [27] Al increases the critical resolved shear stress (CRSS) for basal slip proportional to the Al concentration and lowers the CRSS for prismatic slip, thus the strengthening effect caused by the presence of Al in Mg may not be significant at elevated temperatures.
The Mg-7Sn-1Si alloy exhibits better creep resistance at 200 °C and 30–45 MPa than Si-rich alloys, while at 250 °C creep properties of Si-rich alloys become better. It seems that the main reason is the presence of Mg2Si primary crystals contributing to the significant embrittlement of Mg alloys at ambient temperature. At 200 °C (about 0.3Tm), the plasticity of Si-rich alloys is still insufficient, which leads to the formation of cracks at the α-Mg/Mg2Sip interface. At temperatures above 250 °C, thermally stable and hard Mg2Si primary crystals inhibit displacement and rotation of α-Mg grains, and the α-Mg/Mg2Sip stress generated at the interface is less than at lower temperatures, hence the cracking process begins at a later period of creep.
In comparison to EZ33 magnesium alloy the creep properties at a temperature of 200 °C and a stress of 60 MPa of the tested Mg-Si-Sn alloys are still lower. However, at lower stresses around of 30 MPa, Mg-Sn-Si alloys may be an alternative to some alloys containing rare earth metals. At 250 °C, tested Mg-Sn-Si alloys also show clearly lower creep resistance compared to the commercial WE series magnesium alloys [35].
4 Conclusions
-
1.
Microstructure of sand-cast Mg-Sn alloys consists of α-Mg solid solution and Mg2Sn phase. Addition of Si to Mg-Sn alloy leads to the formation of eutectic Mg2Si phase and primary crystals of Mg2Si phase. The addition of 2% Al dissolved in the α-Mg matrix and does not create new intermetallic phases.
-
2.
Solution heat treatment at 500 °C for 8 h causes dissolution of the Mg2Sn phase in the α-Mg matrix. The eutectic Mg2Si phase undergoes spheroidization and the morphology of the Mg2Si primary crystals does not change during solution heat treatment at 500 °C.
-
3.
The plate-like, lath-shaped and polygonal precipitates of Mg2Sn phase are formed after ageing of these alloys at a temperature of 250 °C. The additions o Si and Al to Mg-Sn alloys promote the refinement of Mg2Sn precipitates.
-
4.
Tensile properties of as-cast alloys are low. The use of heat treatment consisting of solid solution treatment at 500 °C and aging at 250 °C increases the tensile properties.
-
5.
The sand-cast Mg-7Sn-1Si and Mg7Sn-5Si alloys exhibits good creep properties up to a temperature of 200 °C and stress of 30 MPa. Sand-cast Mg7Sn-5Si-2Al alloy has better creep resistance up to 30 MPa and 250 °C, and also shows good creep characteristics at 15 MPa and 300 °C
-
6.
The dominant creep mechanism in the Mg-7Sn-1Si alloy at stress of 45 MPa and in the temperature range 200–250 °C is the dislocation climb controlled by lattice diffusion. In the case of the Mg-7Sn-5Si alloy, the contribution of pipe diffusion increases during dislocation climb, while for the Mg-7Sn-5Si-2Al alloy, dislocation climb controlled by pipe diffusion, as well as by grain boundary diffusion appears to be the main mechanism of creep deformation.
Availability of data and material
Original data is available. Materials (alloys) are not available.
References
Mo N, Tan Q, Bermingham M, Huang Y, Dieringa H, Hort N, Zhang M-X. Current development of creep-resistant magnesium cast alloys: a review. Mater Design. 2018;155:422–42.
Luo AA. Recent magnesium alloy development for elevated temperature applications. Int Mater Rev. 2004;49:13–30.
Friedrich H, Mordike BL. Magnesium technology, metallurgy design data, applications. Berlin Heidelberg: Springer-Verlag; 2006.
Baril E, Labelle P, Pekguleryuz M. Elevated temperature Mg-Al-Sr: creep resistance, mechanical properties, and microstructure. J Met. 2003;55:34–9.
Ninomiya R, Ojiro T, Kubota K. Improved heat resistance of Mg-Al alloys by the Ca addition. Acta Metall Mater. 1995;43:669–74.
Pettersen G, Westengen H, Høier R, Lohne O. Microstructure of a pressure die cast magnesium-4wt.% aluminum alloy modified with rare earth additions. Mater Sci Eng A. 1996;207:115–20.
Podosek M, Lorimer G. The influence of intergranular microstructure of Mg-Zn-RE alloys on properties at elevated temperatures. Arch Metall. 2000;45:47–55.
P. Lyon, Processing Review for Elektron WE43, Launch of Elektron 21, Paris&London, November 2003
Celikin M, Kaya AA, Pekguleryuz M. The role of α-Mn precipitation on the creep mechanisms of Mg-Sr-Mn. Mater Sci Eng A. 2012;534:129–41.
M. Celikin, The creep behaviour of magnesium-manganese based alloys, PhD thesis, McGill University, Montréal, Canada, 2012
Zheng N, Wang HY, Gu ZH, Wang W, Jiang QC. Development of an effective modifier for hypereutectic Mg–Si alloys. J Alloys Compd. 2008;463:L1–L4.
Mirshahi F, Meratian M. High temperature tensile properties of modified Mg/Mg2Si in situ composite. Mater Des. 2012;33:557–62.
Huang Y, Dieringa H, Kainer KU, Hort N. Understanding effects of microstructural inhomogeneity on creep response—new approaches to improve the creep resistance in magnesium alloys. J Magnes Alloy. 2014;2:124–32.
Mendis CL, Bettles CJ, Gibson MA, Gorsse S, Hutchinson CR. Refinement of precipitate distributions in an age-hardenable Mg–Sn alloy through microalloying. Philos Mag Lett. 2006;86:443–56.
Rzychoń T, Dybowski B. The influence of aluminum on the microstructure and hardness of Mg-5Si-7Sn alloy. Arch Metall Mater. 2016;61:425–32.
Lu YZ, Wang QD, Zeng XQ, Zhu YP, Ding WJ. Behavior of Mg–6Al–xSi alloys during solution heat treatment at 420°C. Mater Sci Eng A. 2001;301:255–8.
Kim YK, Do Kim H, Kim WT, Kim DH. Precipitation of DO19 type metastable phase in Mg–Sn alloy. Mater Lett. 2013;113:50–3.
Mendis CL, Bettles CJ, Gibson MA, Hutchinson CR. An enhanced age hardening response in Mg–Sn based alloys containing Zn. Mater Sci Eng A. 2006;435–436:163–71.
Zhang M, Zhang W-Z, Zhu GZ. The morphology and crystallography of polygonal Mg2Sn precipitates in a Mg–Sn–Mn–Si alloy. Scr Mater. 2008;59:866–9.
Huang X, Zhang W, Ma Y, Yin M. Enhancement of hardening and thermal resistance of Mg–Sn-based alloys by addition of Cu and Al. Philos Mag Lett. 2014;94:460–9.
Sasaki TT, Elsayed FR, Nakata T, Ohkubo T, Kamado S, Hono K. Strong and ductile heat-treatable Mg–Sn–Zn–Al wrought alloys. Acta Mater. 2015;99:176–86.
Zhang M, Zhang W, Zhu G, Yu K. Crystallography of Mg2Sn precipitates in Mg-Sn-Mn-Si alloy. Trans Nonferrous Met Soc China. 2007;17:1428–32.
Elsayed FR, Sasaki TT, Mendis CL, Ohkubo T, Hono K. Compositional optimization of Mg–Sn–Al alloys for higher age hardening response. Mater Sci Eng A. 2013;566:22–9.
Behdad S, Zhou L, Henderson HB, Manuel MV, Sohn Y, Agarwal A, Boesl B. Improvement of aging kinetics and precipitate size refinement in Mg–Sn alloys by hafnium additions. Mater Sci Eng A. 2016;651:854–8.
Humaun Kabir A, Su J, Sanjari M, Jung I-H, Yue S. Age-hardening response of Mg-Al-Sn Alloys. Mater Sci Forum. 2015;828–829:250–5.
Son H, Lee J, Jeong H, Konno TJ. Effects of Al and Zn additions on mechanical properties and precipitation behaviors of Mg–Sn alloy system. Mater Lett. 2011;65:1966–9.
Caceres CH, Rovera DM. Solid solution strengthening in concentrated Mg–Al alloys. J Light Met. 2001;1:151–6.
Dieter GE, Bacon DJ. Mechanical metallurgy, vol. 3. New York: McGraw-hill; 1986.
Kassner ME, Pérez-Prado MT. Fundamentals of creep in metals and alloys. New York: Elsevier; 2004.
Watanabe H, Tsutsui H, Mukai T, Kohzu M, Tanabe S, Higashi K. Deformation mechanism in a coarse-grained Mg–Al–Zn alloy at elevated temperatures. Int J Plast. 2001;17(3):387–97.
Watanabe H, Tsutsui H, Mukai T, Kohzu M, Tanabe S, Higashi K. Effect of temperature and grain size on the dominant diffusion process for superplastic flow in an AZ61 magnesium alloy. Acta Mater. 1999;47(14):3753–8.
Somekawa H, Hirai K, Watanabe H, Takigawa Y, Higashi K. Dislocation creep behavior in Mg–Al–Zn alloys. Mater Sci Eng A. 2005;407(1–2):53–61.
Athul KR, Pillai UTS, Srinivasan A, Pai BC. A review of different creep mechanisms in Mg alloys based on stress exponent and activation energy. Adv Eng Mater. 2016;18(5):770–94.
Chelliah NM, Kraemer L, Singh H, Surappa MK, Raj R. Stress–rupture measurements of cast magnesium strengthened by in-situ production of ceramic particles. J Magnes Alloy. 2017;5(2):225–30.
Kanga YH, Wang XX, Zhang N, Yana H, Chen RS. Effect of initial temper on the creep behavior of precipitation–hardened WE43 alloy. Mater Sci Eng A. 2017;689:419–26.
Acknowledgements
This work was supported by the National Science Centre under the project UMO-2011/03/D/ST8/03869. The author is grateful to Ph.D B. Chmiela (Silesian University of Technology, Katowice, Poland) and PhD P. Skupień (IMŻ Gliwice, Poland) for their assistance and useful discussion.
Funding
This study was supported by the National Science Center Poland (Narodowe Centrum Nauki) under the Project UMO-2011/03/D/ST8/03869.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that he has no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rzychoń, T. The microstructure and creep properties of as-cast Mg-Sn-Si-(Al) magnesium alloys. Archiv.Civ.Mech.Eng 20, 97 (2020). https://doi.org/10.1007/s43452-020-00101-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43452-020-00101-6