Abstract
Influenza is an infectious acute respiratory disease with complications and a high mortality rate; the effective medicines for influenza therapy are limited. “Huanglian” or Coptidis Rhizoma, Coptis chinensis Franch., Ranunculaceae, and “ganjiang” or Zingiberis Rhizoma, Zingiber officinale Roscoe, Zingiberaceae, combination is clinically used for treating respiratory diseases. HPLC was applied for the quantification of berberine hydrochloride (1.101 mg/ml) and 6-gingerol (38.41 μg/ml) in the H2O-soluble extract of the herbal formulation. In this study, the effect of “huanglian”- “ganjiang” extract on influenza virus H1N1-induced acute pulmonary inflammation was evaluated, in addition to the investigation of its anti-influenza mechanism in a mouse model. The analyzed herbal combination inhibited the expression of cytokine IL-6 and stimulated the expression of IL-2 in the serum of influenza virus-infected mice. Meanwhile, the herbal combination downregulated the gene and protein expression levels of TLR3, TLR7, MyD88, RIG-I, MAVS, TRAF3, and NF-κB p65, which are key targets of toll-like and RIG-I-like receptor signaling pathways in mice. In addition, the herbal combination could also promote the combination of intracellular autophagosomes and lysosomes in autophagosome-lysosome formation and improve impaired fusion of autophagosomes and lysosomes by influenza virus. This study suggested that the “huanglian”- “ganjiang” extract may be a candidate therapeutic strategy for the treatment of H1N1 influenza.
Graphical Abstract

Similar content being viewed by others
Introduction
Influenza is an acute respiratory disease caused by the influenza virus infection with high incidence, short incubation period, and strong infectivity (Abdelrahman et al. 2020). According to World Health Organization, there are 3 to 5 million cases of severe illness caused by influenza worldwide, and about 290,000 to 650,000 respiratory deaths a year (Iuliano et al. 2018). However, vaccines or antiviral drugs, such as RNA polymerase inhibitors or neuraminidase inhibitors as oseltamivir, sold under the brand name tamiflu, could not work for all types of influenza viruses (Paules et al. 2017; Scorza and Pardi 2018); in addition, they show toxicity and side effects (Kumar et al. 2018). Therefore, new antiviral strategies are urgently needed for influenza therapy.
Traditional Chinese medicine has a long history of herbal use for the treatment of influenza (Rahman et al. 2022), which is based on the multi-component, multi-pathway, and multi-target mechanism of action of its formulations with low level of herbal drug resistance, and represents a source for drug development from natural resources (Zhang et al. 2018a). Traditional Chinese medicine has been used for the selection of medicinal plants with anti-viral activities against SARS (Liu et al. 2012), COVID-19 (Zhao et al. 2021b), and H1N1 (Li et al. 2016), which has attracted worldwide attention.
The herbal combination of “huanglian” or Coptidis Rhizoma, Coptis chinensis Franch., Ranunculaceae, and “ganjiang” or Zingiberis Rhizoma, Zingiber officinale Roscoe, Zingiberaceae, is a formulation derived from the multi-herb decoctions “banxia xiexin” (“banxia” or Pinelliae Rhizoma, Pinellia ternata (Thunb.) Breit, Araceae) and “gancao xiexin” (“gancao” or Glycyrrhizae Radix et Rhizoma, Glycyrrhiza uralensis Fisch., Fabaceae), both of which are classic prescriptions described in the Treatise on Febrile Diseases (Shanghan Lun) of the Han Dynasty of China compiled sometime before 220 AD (Gong et al. 2019). The herbal “huanglian”- “ganjiang” prescription is widely applied for clinical respiratory diseases treatment (Chen et al. 2019; Li et al. 2019). The active ingredient of “huanglian” is berberine (1), which can alleviate lung injury of influenza virus–infected mice as an anti-flu medicine on the TLR/RLR signaling pathways and the target genes (Yan et al. 2018). In addition, berberine appeared to be effective for treating hyperglycemia and dyslipidemia in diabetes mellitus (Khashayar et al. 2021). 6-Gingerol (2) is the active ingredient of “ganjiang” in antipyresis, analgesia, anti-inflammation, bacteriostasis, and boosting immunity (Hong et al. 2021). In previous studies, the combination of “huanglian” and “ganjiang” showed anti-influenza effect in vitro, and the virus inhibition rate was the largest when the dosage ratio of the two medicinal plants was 1:1 (Table S1). However, the action mechanism of “huanglian”- “ganjiang” combination against influenza has not been described.

When the influenza virus invades, innate immunity, as the first line of defense of the body, plays a pivotal role in resisting the virus invasion. During influenza infection, the host uses various pattern receptors, mainly toll-like receptors (TLRs) located in the cell membrane and RIG-I like receptors (RLRs) located in the cytoplasm (Zhang et al. 2015). The stress responses would be initiated when TLRs and RLRs pathways are stimulated, which are associated with adaptor proteins MyD88 and MAVS, then activate transcription factors NF-κB, and induce the expression of inflammatory cytokines, such as IL-2 and IL-6 (Zhang et al. 2015).
Autophagy is a major process of programmed cell death that degrades some intracellular proteins, damaged organelles, and invades pathogens through lysosomes, playing an important role in maintaining intracellular homeostasis and preventing viral infection (Liu et al. 2019). Influenza virus infection induces autophagy and inhibits the fusion of autophagosomes and lysosomes, resulting in the accumulation of autophagosomes (Wang et al. 2019). It causes high expression of autophagy proteins ATG5 and ATG7, and in addition, it accelerates the conversion of LC3-I into autophagy marker protein LC3-II, leading to a significant increase in the expression of LC3-II, indicating that inhibition of incomplete cell autophagy is a potential strategy to alleviate influenza virus infection (Beale et al. 2014).
In the present study, the anti-virus efficacy of the extract obtained from the “huanglian”- “ganjiang” medicinal herbal formulation was evaluated. The mechanism of action for the regulation of target protein expression was explored in vivo by the use of a pneumonia mouse and in vitro by the Madin Darby canine kidney (MDCK) epithelial cell monolayer. Thus, this study aims to provide a new way for the study of the anti-influenza potential of herbal formulations from the traditional Chinese medicine.
Materials and Methods
Plant Material
The medicinal herbs produced in the Sichuan province of China, Coptis chinensis Franch., Ranunculaceae (batch no. 20170501), and Zingiber officinale Roscoe, Zingiberaceae (batch no. 20150702), were purchased from Zhejiang Chinese Medical University Decoction Pieces Co., Ltd., Hangzhou, China. The specimens were deposited in the School of Pharmaceutical Sciences, Zhejiang Chinese Medical University, and accession numbers were assigned as follows: Coptis chinensis Franch., Ranunculaceae (no. 20190917), and Zingiber officinale Roscoe, Zingiberaceae (no. 20191209). The plants were identified by Professor Shui-li Zhang, School of Pharmaceutical Sciences, Zhejiang Chinese Medical University, China.
Extraction
“Huanglian” and “ganjiang” (3 g each) were refluxed in water (60 ml) for 2 h. After filtration, the residue was refluxed with water (48 ml) for 1 h again. Filtering and mixing the solutions, the resulting H2O-soluble extract corresponded to 0.5 g/ml, which was stored at 4 °C.
Plant Quality Control
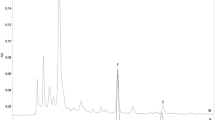
HPLC was used for quality control of the total extract. The analysis was performed in an Agilent 1260 system, including G1315D diode array detector and ChemStation chromatography workstation. Chromatographic separation was performed with an Agilent Hypersil BDS C18 column (250 mm × 4.6 mm, 5 μm) and monitored at 280 nm at 30 °C. The mobile phase was a mixture of water containing 0.3% phosphoric acid (triethylamine controlled pH 4) (A) and acetonitrile (B), and the elution method was as follows: 0–18 min (30–40% B), 18–25 min (40–57% B) with a flow rate of 1 ml/min. The injection volume was 10 μl (0.5 g/ml). Berberine hydrochloride (RT 8.18 min; purity >98%; lot: 165230–201203) and 6-gingerol (RT 22.65 min; purity >98%; lot: 0713–9906) were purchased from National Institutes for Food and Drug Control, China, and used as chemical markers.
Virus and Cells
Influenza A virus strain A/PR/8/34 (H1N1) and MDCK cells were kindly supplied by Virus Research Institute, Zhejiang Provincial Center for Disease Control and Prevention, Hangzhou, China.
Animals
Institute Cancer Research mice (120 male individuals; 18–22 g) were supplied by the Animal Experimental Center, Zhejiang Academy of Medical Sciences, China (ZSLL-2016–191). Experiments on infected animals were carried out in Zhejiang Provincial Center for Disease Control and Prevention. Mice (120) were randomly divided into 6 groups, healthy group, infection group, oseltamivir group (23 mg/kg), H2O-soluble plant extracts high dose group (Extract-H, 18 g/kg), plant extract middle dose group (Extract-M, 9 g/kg), and plant extract low dose group (Extract-L, 4.5 g/kg), 20 mice for each. Except for the healthy group, after being anesthetized with ether, the mice in each group were intranasally infected with 50 μl of 10 LD50 virus, and viral pneumonia was induced. After 2 h, oseltamivir or plant extract was administered intragastrically for 1 week. The healthy group and the infection group were fed the same volume of water.
Inhibition Rate Detection
The mice were sacrificed on the 7th day and lungs (Cui et al. 2022), and spleens and thymus were collected (Du et al. 2020). After washing in saline and dried, the samples were weighted, and the following formulae were used for index calculation:
Histology
Lung tissues were fixed with 10% formalin and dehydrated in gradient ethanol, permeabilized in xylene, and embedded into paraffin. The lung tissues were cut into 4-μm-thick sections, stained with hematoxylin/eosin, and observed under a microscope.
Viral Load
RNAs from lung tissues were extracted and amplified by RT-PCR. The primers were designed by Sangon Biotech (Shanghai) Co., Ltd. (Table S2). The relative expression level of the M1 gene (viral load of influenza virus) was calculated by the formula 2−△△Ct.
ELISA
Mouse blood was collected from the mice orbit, and the serum was obtained by centrifugation at 1369.55 × g for 15 min at 4 °C. IL-2 and IL-6 levels in serum were detected by ELISA kit.
RT-PCR
TLR3, TLR7, MyD88, RIG-I, MAVS, TRAF-3, NF-κB p65, and GAPDH primers (Table S2) were designed by JRDUN Biotechnology (Shanghai) Co. Ltd., China, and synthesized by Shanghai Generay Biotech Co. Ltd., China. Lung tissue was homogenized at 4 °C by the application of Trizol total RNA extraction protocol. Total RNA was extracted from the homogenate. Reverse transcription of RNAs was performed by the First Strand cDNA Synthesis Kit (Thermo, America). Then, the cDNA was amplified in the RT-PCR system (ABI-7300 Real-Time Detector, ABI, America). Amplification conditions were pre-denaturation at 95 °C for 2 min, 95 °C for 15 s, 55 °C for 35 s, and circulation for 40 times. The relative expression level of the target gene was calculated by the formula 2−△△Ct.
Cell Autophagy
Six groups were set as the control group, the virus model group, and the positive control group (10 mmol/ml 3-methyladenine (3-MA), 2 h pretreated cells), and plant extracts high, middle, and low dose groups (3.125, 1.56, and 0.78 mg/ml) in MDCK cell. Each group was cultured for 16 h in DMEM medium with RFP-GFP-LC3B double fluorescence plasmid. Then, the cells were inoculated with 100 median tissue culture infective doses (TCID50) of the H1N1 virus for 1 h, 100 μl/well, except for the control group (Moradi et al. 2017). Plates were washed twice with PBS. Then plant extracts were added, and cells cultivated and observed after 24 h.
Western Blotting
Tissues and cells were lysed in RIPA lysis buffer and quantified by BCA protein assay kit. After SDS-PAGE electrophoresis and semi-dry membrane transfer, 1 h blocking, TLR3 (1:500), TLR7 (1:1000), MyD88 (1:1000), RIG-I (1:1000), MAVS (1:1000), TRAF3 (1:1000), NF-κB p65 (1:1000), LC3 (1:1000), and GAPDH (1:5000) antibodies were incubated overnight, and the secondary antibodies (1:2000) were incubated in 1 h at room temperature. Immunoblotting signals were detected by ECL Western blot detection system and analyzed by Image-J software.
Statistical Analysis
All statistical computations were performed on SPSS 21.0. ANOVA, and multiple comparisons were applied. The p < 0.05 were considered statistically significant. The charts were shown in Graphpad Prism 8.
Results and Discussion
HPLC Profiling
HPLC analysis of H2O-soluble extract from the “huanglian”- “ganjiang” herbal formulation was used for the quality control of the commercial plant materials. The resulting chromatogram allowed the identification of the chemical markers used as standards (Fig. S1): compounds 1 (Rt 8.27 min) and 2 (Rt 22.66 min). In addition, the method applied allowed the quantification of berberine (1.101 mg/ml) and 6-gingerol (38.41 μg/ml) in the crude drug.
Amelioration of Pneumonia
When the body is infected with the influenza virus, pathological phenomena such as edema, inflammatory exudation, cell degeneration, death, and even shedding appear in lungs increase lung mass and lung index (Fig. 1A). The lung indexes of oseltamivir group, and the soluble extract in high and medium doses, were lower compared with the infection group (p < 0.01), and the lung index inhibition rate was higher, with 39.46, 33.37, and 16.12% in oseltamivir group, as well as extract treatments at high and medium doses, respectively.
Effect of H2O-soluble extract from the “huanglian”- “ganjiang” herbal formulation on viral pneumonia in mice infected with influenza. A Effect of the tested extract on lung index of influenza-infected mice (n = 10); B, C effect of the tested extract on immune organ index of mice infected with influenza (n = 10); D photomicrographs of lung tissue morphology of mice (200 ×) with hematoxylin/eosin stain; E effect of total extract on viral load in lung tissue (n = 3); F, G effect of total extract on serum IL-2 and IL-6 contents in influenza-infected mice (n = 10). Compared with infected group, *p < 0.05, **p < 0.01. Abbreviations: EXTRACT-H, -M, and -L = H2O-soluble extract at high, medium, and low doses
Lymphocyte proliferation and humoral immunity response occurred after infection. As the largest lymphatic organ in the human body, the spleen can identify foreign bodies, promote the proliferation and differentiation of immune cells, and initiate an immune response (Lester and Li 2014). The thymus is the central immune organ of the human body and the site for the production, differentiation, development, and maturation of T lymphocytes (Iwasaki and Medzhitov 2010). Both are closely related to the immune state of the body. The immune organ indices (spleen index and thymus index) were significantly higher (p < 0.01) with the high dose and medium dose of the tested extract (Fig. 1B, C). The results showed that extract treatments at high and medium doses had protective effects on the immune organs of mice infected by influenza virus in a dose-dependent manner.
Meanwhile, we observed that the unclear alveolar walls in the infection group were thicker than the control, with infiltrated alveoli and dilated blood vessels in the lung interstitium and consolidation lung tissues. The damages were mitigated in the oseltamivir group and tested extract groups. Moreover, the extract treatment at the high dose group showed a better effect in lung tissues for reduced lesion scope and inflammatory cell density (Fig. 1D). Pulmonary viral load indirectly reflects the extent of influenza virus replication and infection (Lalueza et al. 2019). After treatment with oseltamivir or extract by gavage, the relative expression levels of viral load in the lung tissue of mice were significantly reduced (p < 0.01). The viral load of oseltamivir group was lower than that of extract-treated groups, and the viral load of extract treatment at high doses was lower than that of extract treatment at medium and low doses (Fig. 1E).
Severe lung capillary injury and immune dysfunction caused by virus would lead to a systemic cytokine storm, which accelerates lung injury and overexpresses a variety of inflammatory factors (Shinya et al. 2012). The IL-2 level decreased in the infection group compared to the healthy group and returned when treated with the extract at the higher dose. Such treatment effect was better than that in other treated groups with the extract. Virus infection stimulated the IL-6 secretion, and was inhibited by extract, which showed a significant decrease in both the treated group with the extract at a high dose and the oseltamivir group. The results indicated that the herbal extract could significantly reduce the IL-6 and promote the IL-2 level, which regulated the synthesis and secretion of cytokines (Fig. 1F, G). The herbal formulation of Huanglian- Ganjiang crude drug combination worked in influenza-infected mice and adjusted the body function of mice by protecting the lung cells.
TLR/RLR Signaling Pathways
RT-PCR and western blotting were performed for signaling pathways analysis. The expressions of TLR3, TLR7, MyD88, RIG-I, MAVS, TRAF3, and NF-κB p65 genes in the lung were activated by virus infection both in mRNA and in protein level, especially in TLR7 and MAVS in PCR amplification, of which were more than ninefold increase. Although TRAF3 and NF-κB p65 were detected at a high level in protein, the PCR growth rates were smaller than mitochondrial antiviral-signaling (MAVS). The action of the tested extract was dose dependency; thus, the high dose displayed a better effect (Fig. 2A, B). For these results, as compared with the infected control group, the mRNA and protein expression of targets genes in the TLR/RLR signaling pathways of model mice was suppressed by the extract of the herbal formulation.
The H2O-soluble extract from the “huanglian”- “ganjiang” herbal formulation suppressed the TLRs/RLRs signaling pathways. A, B Expression levels of the mRNAs and proteins of key components of TLRs/RLRs signaling pathways of lung tissue in response to H1N1 stimulation and extract treatment (n = 3), compared with infection control, *p < 0.05, **p < 0.01. C Representative western blots of key proteins in TLRs/RLRs signaling pathways
At present, Traditional Chinese medicine anti-influenza virus mechanism focuses on TLR/RLR signaling pathways. “shixiangru” or Moslae Herba, Mosla chinensis Maxim., Lamiaceae, total flavonoids, and “huanglian xiangru decoction” (“huanglian” or Coptidis Rhizoma, Coptis chinensis Franch., Ranunculaceae. “xiangru” or Moslae Herba, Mosla chinensis Maxim., Lamiaceae) have been reported to inhibit TLR signaling pathway mice infected by influenza virus for lung protection (Wu et al. 2016; Zhang et al. 2018b). Liu Shen Wan worked on the RLR signaling pathway in the treatment of mouse pneumonia (Zhao et al. 2021a). From the results above, we proposed and confirmed that the H2O-soluble extract of the Chinese herbal formulation “huanglian”- “ganjiang” exerts anti-influenza effect by regulating the expression of key target genes and proteins of TLR and RLR signaling pathways. TLRs and RLRs are two important pattern-recognition receptors (PRRs) related to influenza invasion in innate immunity. TLR signaling pathway is for innate immunity (Duan et al. 2022); activation of both TLR and RLR signaling pathways to initiate an innate immune response requires the activation of both TRAF3 and NF-κB genes (Zou et al. 2020; Shang et al. 2021). Hence, PRRs of TLRs and RLRs form a strong innate immune signal network for influenza virus invasion resistance.
Autophagosome Elimination of Virus
Autophagy is a process in which some proteins or organelles in cells are encapsulated and transferred to lysosomes for digestion and degradation, thus maintaining cell homeostasis (Bruckova et al. 2011). We detected the programmed cell death in vitro of virus-infected MDCK cells. A reduced aggregations of green points detected in treated groups suggest that the extract inhibited the proliferation of influenza viruses in autophagosomes by inhibiting the production of autophagosomes. For the yellow spots, the accumulation occurred in the cells of the virus model group, indicating the impaired fusion of autophagosomes and lysosomes. Furthermore, red spots representing autophagosome-lysosome appeared in the 3-MA group and extract-treated groups, suggesting that the damage of the fusion process between autophagosomes and lysosomes could be reversed by the herbal extract to eliminate the intracellular influenza virus (Fig. 3A).
The H2O-soluble extract from the “huanglian”- “ganjiang” herbal formulation regulated autophagy induced by influenza virus. A Autophagy induced by the total extract against influenza virus. Under the single GFP channel, the green fluorescent aggregation points represented autophagosomes. In the GFP-RFP channel diagram, the yellow fluorescent aggregate points represented autophagosomes, and the red fluorescent aggregate points represented autophagosome-lysosome. When incomplete autophagy occurred, a yellow fluorescent aggregation point is detected. B The expression level of autophagy marker protein LC3-II/β-actin in MDCK cells induced by influenza virus (n = 3). Compared with infection control, *p < 0.05, **p < 0.01. C Western blots of autophagy marker proteins
The expression of LC3 was investigated as well. LC3-II/β-actin ratio decreased significantly after 3-MA and the extract added into medium (p < 0.05). And the decreasing trend was 3-MA >extract high dose group > extract medium dose group > extract low dose group for dose dependence (Fig. 3B, C). These results indicated that herbal extract could inhibit the expression of autophagy marker protein LC3-II, and thus inhibit autophagy to produce anti-influenza virus effects in a dose-dependent manner. In summary, the results illustrated that herbal extract could reverse the damage of the fusion process of intracellular autophagosomes and lysosomes, promote the formation of autophagosome-lysosome and then clear the influenza virus in cells, and inhibit the expression of autophagy protein LC3-II at the same time, to play a role in resisting influenza virus infection.
Conclusion
The H2O-soluble extract of the Chinese herbal formulation “huanglian”- “ganjiang” has remarkable anti-H1N1 influenza effects, by regulating the expression level of inflammatory cytokines in the body and inhibiting the expression of key target genes and proteins in the TLR/RLR signaling pathways. The herbal extract could impair the damage of the lung, reduce the inflammatory of influenza virus infection, and reverse influenza-induced incomplete autophagy. Therefore, the herbal formulation “huanglian”- “ganjiang could be a promising candidate for the treatment of H1N1 influenza and its subsequent viral pneumonia.
Data Availability
Data will be made available on request.
References
Abdelrahman Z, Li M, Wang X (2020) Comparative review of sars-cov-2, sars-cov, mers-cov, and influenza a respiratory virus. Front Immunol 11:552909. https://doi.org/10.3389/fimmu.2020.552909
Beale R, Wise H, Stuart A, Ravenhill BJ, Digard P, Randow F (2014) A lc3-interacting motif in the influenza a virus m2 protein is required to subvert autophagy and maintain virion stability. Cell Host Microbe 15:239–247. https://doi.org/10.1016/j.chom.2014.01.006
Bruckova L, Soukup T, Visek B, Moos J, Moosova M, Pavelkova J, Rezabek K, Kucerova L, Micuda S, Brcakova E, Mokry J (2011) Proliferative potential and phenotypic analysis of long-term cultivated human granulosa cells initiated by addition of follicular fluid. J Assist Reprod Genet 28:939–950. https://doi.org/10.1007/s10815-011-9617-6
Chen QQ, Shi JM, Ding Z, Xia Q, Zheng TS, Ren YB, Li M, Fan LH (2019) Berberine induces apoptosis in non-small-cell lung cancer cells by upregulating miR-19a targeting tissue factor. Cancer Manag Res 11:9005–9015. https://doi.org/10.2147/CMAR.S207677
Cui X-R, Guo Y-H, Liu Q-Q (2022) Cangma huadu granules, a new drug with great potential to treat coronavirus and influenza infections, exert its efficacy through anti-inflammatory and immune regulation. J Ethnopharmacol 287:114965. https://doi.org/10.1016/j.jep.2021.114965
Du H-X, Zhou H-F, Yang J-H, Lu Y-Y, He Y, Wan H-T (2020) Preliminary study of yinhuapinggan granule against h1n1 influenza virus infection in mice through inhibition of apoptosis. Pharm Biol 58:979–991. https://doi.org/10.1080/13880209.2020.1818792
Duan T, Du Y, Xing C, Wang HY, Wang RF (2022) Toll-like receptor signaling and its role in cell-mediated immunity. Front Immunol 13:812774. https://doi.org/10.3389/fimmu.2022.812774
Gong B, Kao Y, Zhang C, Zhao H, Sun F, Gong Z (2019) Exploring the pharmacological mechanism of the herb pair “huanglian-ganjiang” against colorectal cancer based on network pharmacology. Evid Based Complement Alternat Med 2019:2735050. https://doi.org/10.1155/2019/2735050
Hong W, Zhi FX, Kun TH, Hua FJ, Huan Ling L, Fang F, Wen C, Jie W, Yang LC (2021) 6-Gingerol attenuates ventilator-induced lung injury via anti-inflammation and antioxidative stress by modulating the pparγ/nf-κb signalling pathway in rats. Int Immunopharmacol 92:107367. https://doi.org/10.1016/j.intimp.2021.107367
Iuliano AD, Roguski KM, Chang HH, Muscatello DJ, Palekar R, Tempia S, Cohen C, Gran JM, Schanzer D, Cowling BJ, Wu P, Kyncl J, Ang LW, Park M, Redlberger-Fritz M, Yu H, Espenhain L, Krishnan A, Emukule G, van Asten L, Pereira da Silva S, Aungkulanon S, Buchholz U, Widdowson MA, Bresee JS (2018) Global Seasonal Influenza-associated Mortality Collaborator Network. Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. Lancet 391:1285–1300. https://doi.org/10.1016/S0140-6736(17)33293-2
Iwasaki A, Medzhitov R (2010) Regulation of adaptive immunity by the innate immune system. Science 327:291–295. https://doi.org/10.1126/science.1183021
Khashayar A, Bahari Z, Elliyeh M, Ghasemi M (2021) Therapeutic effects of berberine in metabolic diseases and diabetes mellitus. Rev Bras Farmacogn 31:272–281. https://doi.org/10.1007/s43450-021-00159-0
Kumar B, Asha K, Khanna M, Ronsard L, Meseko CA, Sanicas M (2018) The emerging influenza virus threat: status and new prospects for its therapy and control. Arch Virol 163:831–844. https://doi.org/10.1007/s00705-018-3708-y
Lalueza A, Folgueira D, Muñoz-Gallego I, Trujillo H, Laureiro J, Hernández-Jiménez P, Moral-Jiménez N, Castillo C, Ayuso B, Díaz-Pedroche C, Torres M, Arrieta E, Arévalo-Cañas C, Madrid O, Lumbreras C (2019) Influence of viral load in the outcome of hospitalized patients with influenza virus infection. Eur J Clin Microbiol Infect Dis 38:667–673. https://doi.org/10.1007/s10096-019-03514-1
Lester SN, Li K (2014) Toll-like receptors in antiviral innate immunity. J Mol Biol 426:1246–1264. https://doi.org/10.1016/j.jmb.2013.11.024
Li J-H, Wang R-Q, Guo W-J, Li J-S (2016) Efficacy and safety of traditional Chinese medicine for the treatment of influenza a (h1n1): a meta-analysis. J Chin Med Assoc 79:281–291. https://doi.org/10.1016/j.jcma.2015.10.009
Li Z, Liu Z, Uddandrao VVS, Ponnusamy P, Balakrishnan S, Brahmanaidu P, Vadivukkarasi S, Ganapathy S (2019) Asthma-alleviating potential of 6-gingerol: effect on cytokines, related mRNA and c-Myc, and NFAT1 expression in ovalbumin-sensitized asthma in rats. J Environ Pathol Toxicol Oncol 38:41–50. https://doi.org/10.1615/JEnvironPatholToxicolOncol.2018027172
Liu J, Wang H, Wang J, Chang Q, Hu Z, Shen X, Feng J, Zhang Z, Wu X (2019) Total flavonoid aglycones extract in radix scutellariae induces cross-regulation between autophagy and apoptosis in pancreatic cancer cells. J Ethnopharmacol 235:133–140. https://doi.org/10.1016/j.jep.2019.02.005
Liu X, Zhang M, He L, Li Y (2012) Chinese herbs combined with western medicine for severe acute respiratory syndrome (sars). Cochrane Database Syst Rev 10:CD004882. https://doi.org/10.1002/14651858.CD004882.pub3
Moradi MT, Karimi A, Rafieian-Kopaei M, Fotouhi F (2017) In vitro antiviral effects of Peganum harmala seed extract and its total alkaloids against influenza virus. Microb Pathog 110:42–49. https://doi.org/10.1016/j.micpath.2017.06.014
Paules CI, Marston HD, Eisinger RW, Baltimore D, Fauci AS (2017) The pathway to a universal influenza vaccine. Immunity 47:599–603. https://doi.org/10.1016/j.immuni.2017.09.007
Rahman MM, Bibi S, Rahaman MS, Rahman F, Islam F, Khan MS, Hasan MM, Parvez A, Hossain MA, Maeesa SK, Islam MR, Najda A, Al-malky HS, Mohamed HRH, AlGwaiz HIM, Awaji AA, Germoush MO, Kensara OA, Abdel-Daim MM, Saeed M, Kamal MA (2022) Natural therapeutics and nutraceuticals for lung diseases: traditional significance, phytochemistry, and pharmacology. Biomed Pharmacother 150:113041. https://doi.org/10.1016/j.biopha.2022.113041
Scorza FB, Pardi N (2018) New kids on the block: RNA-based influenza virus vaccines. Vaccines 6:6020020. https://doi.org/10.3390/vaccines6020020
Shang J, Zheng Y, Mo J, Wang W, Luo Z, Li Y, Chen X, Zhang Q, Wu K, Liu W, Wu J (2021) Sox4 represses host innate immunity to facilitate pathogen infection by hijacking the tlr signaling networks. Virulence 12:704–722. https://doi.org/10.1080/21505594.2021.1882775
Shinya K, Gao Y, Cilloniz C, Suzuki Y, Fujie M, Deng G, Zhu Q, Fan S, Makino A, Muramoto Y, Fukuyama S, Tamura D, Noda T, Eisfeld AJ, Katze MG, Chen H, Kawaoka Y (2012) Integrated clinical, pathologic, virologic, and transcriptomic analysis of h5n1 influenza virus-induced viral pneumonia in the rhesus macaque. J Virol 86:6055–6066. https://doi.org/10.1128/JVI.00365-12
Wang R, Zhu Y, Zhao J, Ren C, Li P, Chen H, Jin M, Zhou H (2019a) Autophagy promotes replication of influenza a virus. J Virol 93: https://doi.org/10.1128/JVI.01984-18
Wu Q-F, Zhu W-R, Yan Y-L, Zhang X-X, Jiang Y-Q, Zhang F-L (2016) Anti-H1n1 influenza effects and its possible mechanism of Huanglian Xiangru decoction. J Ethnopharmacol 185:282–288. https://doi.org/10.1016/j.jep.2016.02.042
Yan Y-Q, Fu Y-J, Wu S, Qin H-Q, Zhen X, Song B-M, Weng Y-S, Wang P-C, Chen X-Y, Jiang Z-Y (2018) Anti-influenza activity of berberine improves prognosis by reducing viral replication in mice. Phytother Res 32:2560–2567. https://doi.org/10.1002/ptr.6196
Zhang H-H, Yu W-Y, Li L, Wu F, Chen Q, Yang Y, Yu C-H (2018a) Protective effects of diketopiperazines from moslae herba against influenza a virus-induced pulmonary inflammation via inhibition of viral replication and platelets aggregation. J Ethnopharmacol 215:156–166. https://doi.org/10.1016/j.jep.2018.01.005
Zhang J, Miao J, Hou J, Lu C (2015) The effects of h3n2 swine influenza virus infection on tlrs and rlrs signaling pathways in porcine alveolar macrophages. Virol J 12:61. https://doi.org/10.1186/s12985-015-0284-6
Zhang X-X, Wu Q-F, Yan Y-L, Zhang F-L (2018b) Inhibitory effects and related molecular mechanisms of total flavonoids in mosla chinensis maxim against h1n1 influenza virus. Inflamm Res 67:179–189. https://doi.org/10.1007/s00011-017-1109-4
Zhao J, Wang Y, Huang X, Ma Q, Song J, Wu X, Zhou H, Weng Y, Yang Z, Wang X (2021a) Liu shen wan inhibits influenza virus-induced secondary staphylococcus aureus infection in vivo and in vitro. J Ethnopharmacol 277:114066. https://doi.org/10.1016/j.jep.2021a.114066
Zhao Z, Li Y, Zhou L, Zhou X, Xie B, Zhang W, Sun J (2021b) Prevention and treatment of covid-19 using traditional chinese medicine: a review. Phytomedicine 85:153308. https://doi.org/10.1016/j.phymed.2020.153308
Zou PF, Shen JJ, Li Y, Zhang ZP, Wang YL (2020) Traf3 enhances trif-mediated signaling via nf-κb and irf3 activation in large yellow croaker larimichthys crocea. Fish Shellfish Immunol 97:114–124. https://doi.org/10.1016/j.fsi.2019.12.024
Funding
This work was supported by the National Nature Science Foundation of China (81473335/H2803, 82174272) and the Zhejiang Provincial Natural Science Foundation of China (LY18H280007).
Author information
Authors and Affiliations
Contributions
YS, CY, FZ, XM, and QW conceived and designed the study. YS and CY performed animal experiments and collected data. YS, YY, FZ, and JC analyzed and interpreted the data. ZH and JH revised the manuscript. XM and QW interpreted the data and checked the revised version of the manuscript. All authors read and approved the manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
This study was approved by the Ethics Committee of The Experimental Animal Center of Zhejiang Chinese Medical University (No. ZSLL-2016–191).
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sun, Y., Yu, Cl., Yan, Yl. et al. Inhibitory Effects and Related Molecular Mechanisms of Huanglian-Ganjiang Combination Against H1N1 Influenza Virus. Rev. Bras. Farmacogn. 33, 514–522 (2023). https://doi.org/10.1007/s43450-023-00372-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43450-023-00372-z