Abstract
Objectives
To identify the potential opportunities and risks around future UK regulatory reform of medical devices.
Design
A mixed methods approach, comprising a rapid literature review, one-to-one, semi-structured interviews with key stakeholders, a multidisciplinary stakeholder workshop, and a post-workshop survey.
Setting
United Kingdom.
Participants
32 key stakeholders across the medical device sector were identified both from the public and private sectors.
Results
Opportunities relating to regulatory independence were identified, including the potential to create and implement a regulatory framework that ensures availability of medical devices; innovation and investment potential; and safety to the citizens of the UK. The most significant risks identified included threats to the safety of individual patients and the wider health system arising from the delay in awaiting regulatory approval due to the shortage of approved bodies; and reduced competitiveness of UK market and device manufacturers. Recommendations were identified to mitigate risks, centred on harnessing broader cross-sector collaborations, promoting patient and public partnership, and maximizing international engagement.
Conclusions
The UK’s medical device sector is at a time-critical juncture to construct a regulatory framework to navigate its exit of Europe and respond to Europe's transition to new medical device regulations whilst also addressing the ongoing demand for rapid approval for new devices in response to the global pandemic. Investment, capacity-building, and international engagement will play a central role in mitigating risks and maximizing opportunities for medical device regulation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Medical devices are an essential and increasing part of healthcare delivery. Their applications are hugely diverse, with both hardware (including apparatus, appliances, and instruments) and software (including artificial intelligence as a medical device (AIaMD)) applications [1]. The medical device sector is a highly innovative space, as exemplified during the pandemic when innovators and manufacturers responded with new products ranging from personal protective equipment to in vitro diagnostics to machine learning (ML)-based imaging analytics. This rapid innovation can bring significant societal benefits—directly to individual patients, to population health, and to system efficiencies—but needs to be set within regulatory safeguards that ensure they meet acceptable standards of safety, quality and effectiveness.
Innovation, and regulatory reform to support innovation, is a central part of the UK Government's strategy to respond to the opportunities and challenges arising from leaving the European Union (EU) [2]. As with other sectors, regulatory reform on medical devices can bring new opportunities. It is projected that streamlining the pathway from innovation to market will not only bring benefit to patients sooner, but also accelerate growth in the medical device sector (which currently employs 102,800 people, with an annual turnover of £7.7 billion) [3]. However, medical device companies operate within a global market, and regulatory divergence may result in barriers to the import and export of medical devices, and pose a risk to patients’ health [4].
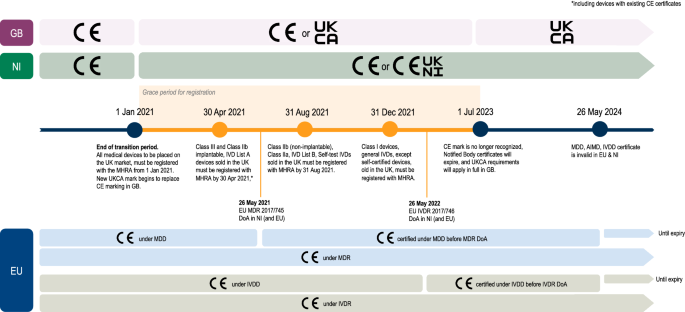
In preparation for the post-Brexit regime, the UK Government introduced the new UK Conformity Assessed (UKCA; and UKNI for Northern Ireland) mark as a replacement for the existing Conformitè Europëenne (CE) marking system for medical devices being placed on the market in Great Britain [5]. The EU CE mark will continue to be recognised in Great Britain until 30 June 2023, after which all medical devices on the market will require a UKCA mark.
In 2019, the UK government established the Regulatory Horizons Council (RHC), an independent expert committee that identifies the implications of technological innovation, and provides the government with impartial, expert advice on the regulatory reform required to support its rapid and safe introduction. From 2020 to 2021, the RHC undertook a review of the medical device sector culminating in a report submitted to the UK Government in July 2021. As part of the evidence underpinning that report, the RHC commissioned a multi-stakeholder mixed-methods research study which was undertaken by Birmingham Health Partners Centre for Regulatory Science and Innovation. This study engaged a diverse group of stakeholders to explore key questions prioritized by the RHC: First, to identify the potential opportunities and risks around future UK regulatory reform of medical devices; second, to discuss how the UK can encourage international investment, innovation, and improve safety in the medical devices through regulatory and non-regulatory change; third, to determine implications of ending the use of the EU CE mark for medical devices in Great Britain and the mitigations needed to facilitate the move to the UKCA mark; and fourth, to explore potential alternative routes to market for medical devices that are currently being used internationally that could be transposed to the UK market and regulatory system.
Methods
Study Design
A mixed methods approach was used to collate multiple sources of evidence and representative stakeholder views. This comprised: (i) a rapid literature review; (ii) one-on-one, semi-structured interviews with stakeholders; (iii) a multidisciplinary stakeholder workshop to review initial findings and discuss areas of agreement and disagreement; and (iv) a post-workshop survey to further explore areas of contention identified during the workshop. An independent advisory committee was appointed comprising representatives from Innovate UK, British In Vitro Diagnostics Association, and the Association of British HealthTech Industries.
This study was approved by the ethical review committee at the University of Birmingham, UK (ERN_20-1852).
Rapid Literature Review
A rapid literature review was conducted on 08 January 2021. PubMed and Google Scholar were used to search published literature and Google Search Engine was used to search grey literature to find relevant literature under two themes: (i) reported implications of ending the use of the EU CE mark for medical devices in Great Britain and (ii) potential alternative routes to the UK market for medical devices.
Composite and extended terms containing the roots “medical” and “device*” were combined with terms relating to the transition to the UKCA mark, such as “Brexit”, “EU CE” “UKCA” (Online Appendix 1). The first 100 citations from Google Scholar and Google Search Engine were screened. Citations were independently screened by two co-investigators according to pre-defined inclusion and exclusion criteria (Online Appendix 1). Disagreements were resolved via consensus.
Qualitative Research
Semi-structured Interviews
Prospective participants were provided with the electronic participant information sheet and consent form (Online Appendix 2). We used a maximum variation sample of a heterogeneous group of stakeholders, primarily focusing on those involved in the life cycle of a medical device, both from the public and private sectors (including: medical device regulation consultants, representatives from small to medium enterprise or start ups, providers of medical device testing services, academics, clinicians, UK Government bodies, trade association or industry groups, patient and public partners and large enterprise). We approached prospective interviewees from December 2020 to February 2021 by email. Participants were recruited until a point of saturation was reached, i.e. when the additional interviewees provided no further information.
Multidisciplinary Stakeholder Workshop
All 30 participants involved in the online interviews and 19 further participants identified via stakeholders’ recommendations, were invited to an online workshop to explore areas of concern or contention highlighted by the literature review and stakeholder interviews. The workshop was conducted via video-conferencing (MS Teams, Microsoft) on 09 February 2021.
Post-workshop Survey
A post-workshop anonymized online survey (Online Appendix 3) was distributed via Qualtrics Survey Software between 19 February 2021 and 05 March 2021. The survey was designed to provide workshop attendees with an opportunity to identify any additional concerns that might have arisen since the workshop, and contribute additional feedback.
Selection of Study Participants
To provide robust, multi-stakeholder, cross-sector input, to inform recommendations for the UK Government around medical devices regulatory reform that maximizes opportunity and minimizes risks, we recruited stakeholders likely to give in-depth information on regulations around medical devices, without any geographical restriction. The project team worked with the independent advisory committee to identify a list of stakeholders to contact and supplemented this list by: (i) searching publicly available listings from the relevant UK Government bodies, industry-specific organizations, and academic institutions, and (ii) snowball sampling from interviewees. Representatives from the UK Medicines and Healthcare products Regulatory Agency (MHRA) were not recruited so as to maintain independence.
Data Collection
We performed online interviews via video-conferencing (MS Teams, Microsoft) between 04 January 2021 and 02 February 2021. A total of 30 one-on-one, semi-structured interviews were conducted based on an interview guide with open-ended questions on the stakeholders’ assessment of current UK medical device regulatory framework, as well as their expectations and concerns regarding the reforms. We pilot-tested the interview guides with members of the advisory committee to ensure the questions were appropriately framed and addressed all the relevant aspects of the subject (Online Appendix 4).
Data Analysis
The interview and workshop narratives relevant to our research questions were identified and analyzed thematically using the framework approach [6, 7]. This method allowed a comprehensive review of descriptive narratives that was driven by stakeholders’ original accounts and literature review. The interviews were transcribed, reviewed, and coded independently by JDH and HI, using the stakeholder interview questions as an initial thematic framework. Textual codes were grouped into clusters around similar and interrelated concepts and a matrix of recurrent themes were identified and systemically analysed within Google Sheets. Key statements from interviews and the workshop are reported verbatim to respect the integrity of the statements without any bias. The inductive coding process was reviewed internally by OLA, and any disagreements about the coding were discussed and resolved with third reviewers (MJC, EM, AKD).
Results
Themes were initially explored in rapid literature reviews where there was pre-existing literature to support this, followed by the qualitative studies; themes which did not have significant pre-existing literature (either by their nature or recency) were explored through the qualitative studies. The breadth of views and typical representative views are reported.
Rapid Literature Review
The rapid literature review identified a total of 307 non-duplicate articles under the theme, Key implications of the transition to the UKCA or UKNI mark. Following title and abstract screening, 119 papers were removed, and 108 articles were reviewed in full text. A full-text screening resulted in the exclusion of 77 articles resulting in a total of 31 articles included in the final review for this theme. Reasons for exclusions were that articles did not relate to medical devices or in vitro diagnostic devices; published before 31 December 2019; or were reporting factual information of the transition to the UKCA mark (Online Appendix 5). A total of 270 non-duplicate titles from studies of any format, were identified for the theme: Evaluating alternative regulatory frameworks or regulatory components for potential adoption by the UK. 112 were excluded after title and abstract screening and 158 articles were reviewed in detail. Of these, 135 did not meet the inclusion criteria, specifically the papers did not report on the new, alternative or international routes to the UK market. Overall, 23 articles were included in the final review for this theme (Online Appendix 6).
Stakeholder Group Representation for Qualitative Studies
A total of 32 key stakeholders across the medical device sector participated in the study (30 in qualitative interviews and 26 in the consensus meeting). Of the 32 participants, 30 were from the UK, one was from mainland Europe (Germany), and one was from the USA. This distribution reflects the UK context of this work (Table 1).
Theme 1: Potential Opportunities and Risks Around Future UK Regulatory Reform of Medical Devices
Based on literature review findings and interview responses, we categorized a range of opportunities and risks around future UK regulatory reform of medical devices into four key areas: (i) patient and public access to high quality medical devices; (ii) international investment and innovation; (iii) patient and user safety; and (iv) global standing in regulation of the life sciences sector (Table 2).
Patient and Public Access to High Quality Medical Devices
Overall, stakeholders believed that the UK regulatory reforms present a timely opportunity to enhance patient and public access to high-quality and safe medical devices by streamlining the regulatory approval process that aligns with the interest of the UK public. A representative view was that “there is too much noise (in the European Committee), as regulations need to work and be relevant to 27 different member states and their national health strategies”. Their view was that a UK-oriented regulatory system could produce the best outcomes for the UK National Health Service (NHS), life sciences sector and internal market. However, many stakeholders highlighted that regulatory divergence from the international regulatory framework is likely to increase the burden on the regulatory authorities and device companies. Participants were concerned that this, in turn, would increase the delay and cost in placing the devices in the UK market, and would ultimately decrease the availability of medical devices for UK patients.
International Investment and Innovation
With regards to potential opportunities to accelerate the adoption of cutting-edge technology and ensure innovation in the UK, stakeholders expressed health data as the “most obvious and the strongest asset, provided by the NHS”. Respondents, mainly from the small to medium enterprises (SMEs), were of the opinion that by leveraging the development of data-driven devices and enhancing scalability of collaboration with NHSx, NHS Digital (organizations now absorbed into NHS England and NHS Improvement), and other organizations responsible for the digital services delivery, the UK will foster a globally competitive environment for the medical device start-ups. One common view was that the UK should prioritize its regulatory resources and implement a specialist regulatory system to safely support the creation of the more innovative and complex medical devices over “run-of-the-mill” devices to secure its competitiveness in the global market.
Patient and User Safety
Participants across all stakeholder groups agreed on the need for greater involvement of public and patient advocates in regulatory activities across the lifetime of a medical device. Stakeholders suggested several ways to promote patient and user safety, including the use of patient-centred clinical data to demonstrate device compliance, a stronger vigilance, and post-market surveillance process, coupled with a publicly funded and accessible database on medical devices. Stakeholders raised concern over implementation of a more relaxed regulation relative to the current regulation. The prevailing concerns over such regulatory “shortcuts” were of compromising the overall quality of medical devices, which, in turn, may incur considerable societal cost, as well as potential harm to patient safety.
A widely shared opinion suggested that there is an opportunity for the new UK regulatory system to “be a ‘halfway house’ between EU Medical Device Directive 93/42/EEC (MDD) and Medical Device Regulation 2017/745 (MDR) that brings in safety aspects of MDR but keeps the lighter touch of MDD”, to encourage a system that is efficient enough for investors and innovators to use it, whilst safe enough to protect patients and users.
Global Standing in Regulation of Life Sciences Sector
Stakeholders, regardless of their role and experience, agreed on maximising both new and existing collaboration with international standards and other international regulatory regimes, to identify where the UK’s domestic regulatory system could be made more streamlined and effective. The concept of regulatory harmonisation with wider international standards, including the US, Commonwealth countries, individual EU member states, and elsewhere, was also highly valued, and was suggested as a key element for the UK “to preserve the leading role in shaping global regulatory sciences”.
Theme 2: Regulatory and Non-regulatory Changes in the UK to Encourage International Investment, Innovation, and Improve Patient Safety
Stakeholders were invited to identify the regulatory and non-regulatory changes in the UK that would improve international investment, innovation, and patient safety (Table 3).
How Can the UK Encourage International Investment in the Medical Device Area?
Stakeholders regarded alignment with international regulatory authorities as desirable, and a key component of attracting international investment in the medical devices area. A typical view was “it is important for the (UK) regulatory processes to open up more than one market” with an emphasis from all stakeholder groups on encouraging greater access to international markets. The following ways of increasing entry to the international market were proposed: aligning the UK conformity assessment with the EU CE; optimising global regulatory harmonisation; and agreeing on a mutual recognition with other countries (the Commonwealth countries and the US were particularly highlighted). One stakeholder critically noted that a regulatory ecosystem that encourages and supports companies specialising in high-risk devices from the earliest stage, would lower the risk of nonconformance, offer investor confidence and, thereby, drive international investment in the industry.
Participants also provided several non-regulatory measures for the purpose of encouraging investment. These included but were not limited to: (i) a greater clarity around new regulations; (ii) investment incentives, such as grants, tax credits, and subsidized manufacturing infrastructures; (iii) optimization of the NHS data access and procurement process; (iv) international collaborations between academia and industry. Such elements were commonly identified as non-regulatory practice to encourage innovation.
How Can the UK Encourage Innovation in the Medical Device Area?
With reference to promoting innovation, stakeholders expressed the need to decrease the burden involved in acquisition of clinical evidence for regulatory approval and health technology assessment (HTA) decisions. Some interviews centred on the debate on guiding developers to understand the end-user needs in the context of providing target product profile and publicly funded horizon scanning. Furthermore, respondents promoted the idea of adopting a regulatory pathway similar to the Humanitarian Device Exemption and Breakthrough Device Designation of the US FDA to provide incentives for development of devices for the management of rare or life-threatening conditions; it should be noted that the MHRA do in fact have an exceptional use route on humanitarian grounds.
How Can the UK Improve Safety in the Medical Device Area?
Regarding improvement in patient safety, the area most strongly highlighted was the need to improve post-market surveillance (PMS). Respondents suggested that systematic monitoring and analysis of user complaints may provide early warning of possible device-related harms. Another suggestion to enhance PMS was to introduce a post-approval transition period to gather further evidence on safety before deploying and scaling up for routine clinical use. Additionally, a publicly accessible, national database of registered medical devices using unique device identifiers to “promote transparency around the evidence for effectiveness and safety of medical devices” was proposed.
The majority of the respondents maintained that patient and public involvement in the design and development of clinical trials of medical devices would best capture and address users’ needs and enhance safety. Representatives from patient and public partners highlighted the growing recognition of validated patient-reported outcomes as a means of involving “patients in a meaningful manner.”
Theme 3: Key Implications of the Transition to the UKCA or UKNI Mark
Stakeholders identified that the end to the use of the EU CE mark for medical devices in the UK and the move to the UKCA mark (or UKNI mark) pose unique implications for different stakeholder groups. Implications and mitigations were considered according to: (i) those most relevant to regulators, (ii) those most relevant to medical device companies; (iii) and those most relevant to patients and the public (Table 4).
Of 26 workshop attendees, a total of 16 stakeholders completed the anonymized post-workshop survey. 56.2% (9/16) of respondents said that the medical device industry is able to meet the new UKCA mark requirements by the deadline of 01 July 2023. Respondents held contrasting opinions over the degree of continued regulatory alignment in the current requirements for the UKCA mark with the EU CE mark requirements. Whilst some assumed that there would be a high degree of similarity between the two requirements that should minimize the regulatory burden on device manufactures, others criticized the potential disparity and the delay in providing the full details of the UKCA requirements. Stakeholders also expressed concerns that this deadline can only be met with “practical support from the Government” and said that it was essential that “any changes made to the UK regulations are agreed and communicated by the end of 2021 at the latest”. Additionally, half the survey respondents (8/16) strongly agreed that the UKCA deadline of 01 July 2023 poses a potential risk to being able to provide new and existing devices to the patients in the UK (Online Appendix 7).
Theme 4: Evaluating Alternative Regulatory Frameworks or Regulatory Components for Potential Adoption by the UK
Finally, three regulatory systems in operation internationally were identified as particularly relevant to the regulation of medical devices in the UK. This included the Medical Device Single Audit Programme (MDSAP) [8], U.S. Food and Drug Administration (FDA), and Mutual Recognition Agreements (MRAs). Provided that these systems are not mutually exclusive, stakeholders proposed the idea of the UK choosing to adopt certain aspects or regulatory stages of each system.
Participants suggested that joining the MDSAP as a Participating Country would reduce the regulatory burden and facilitate a more efficient route to international markets, by satisfying quality management system (QMS) requirements across multiple authorities. With regard to MDSAP, MHRA is currently an Official Observer and is considering becoming a member. The main area of criticism in this regard was inadequate coverage of QMS requirements over the entire regulatory approval process. Concerns were expressed that additional QMS activities and harmonization of technical documentation, especially around Software as a Medical Device (SaMD), may be required to fulfil the regulatory requirements of specific participating jurisdictions.
Implementation of a more extensive equivalence-based approval process that is similar to 510(k) of US FDA were suggested as an alternative route to increase the speed, number, and the diversity of devices made available in the UK. The 510(k) pathway’s efficiency is achieved by granting regulatory clearance from low-to-moderate risk devices through demonstration of substantial ‘equivalence’ to a legally marketed predicate device.
In contrast, some stakeholders expressed the fear that the lack of clear consensus on how much divergence is permitted before a device is no longer equivalent to its predicate may result in negative implications on patient safety.
Stakeholder engagement identified that establishing MRAs in conformity assessment certificates of medical devices will address the anticipated capacity gap in UK registration, which can promote efficiency in both the UK’s regulatory system and the regulatory systems of its international counterparts. It was highlighted that this would allow MHRA to a proportionate allocation of regulatory resources towards medical devices with potentially higher public health risk or those with a higher public interest. However, industry is concerned about the further delay involved in building the trust that is required to negotiate bilateral or multilateral MRAs.
Discussion
This mixed methods study of stakeholder views provides a series of important insights into the potential diverse impacts of future UK regulatory reform in the medical device sector. Analysis of the literature review, semi-structured interviews, workshop, and post-workshop survey identified a number of major themes in which UK regulatory reforms are seen as a balance between opportunities of regulatory independence and the risk of regulatory divergence.
Theme 1 addressed the opportunity and risk of the current and evolving scenario, specifically the opportunities and risk around future UK regulatory reform in the medical device sector. In response, stakeholder insights highlighted the following priority areas: (i) patient and public access to high quality medical devices; (ii) international investment and innovation; (iii) patient and user safety; and (iv) global standing in regulation of the life sciences sector.
Theme 2 invited stakeholders to use their expertise and experience to address these opportunities and risks, specifically to consider the regulatory and non-regulatory changes in the UK to encourage international investment, innovation, and improve patient safety. This elicited a broad range of suggestions but with recurrent themes including: clarity and certainty (the regulatory requirements are known, well-communicated, and any change is provided with due warning); alignment or mutual recognition (reducing friction in export and import), streamlining (e.g. through regulation into health technology assessment and through to procurement), and efficient, digitally enhanced safety monitoring (to improve safety, reduce burden, and potentially enable earlier deployment).
Theme 3 highlighted specific considerations related to the transition to the UKCA and UKNI marks and noted that the risks and mitigations are distinct for regulators, manufacturers, and the patients and public.
Theme 4 highlighted opportunities to learn from or align to examples of best practice internationally. There was broad consensus that there is value in participating in international initiatives such as MDSAP and seeking to reduce friction in import and export through alignment or mutual recognition agreements (Fig. 1).
The findings of our study need to be set in the context of the wider, rapidly evolving environment in which the UK’s medical devices sector sits. There are a number of major drivers that shape this environment, many of which are dynamic, and some of which may change rapidly. Two recent and now immutable agents of change for the medical device sector are: first, the transition by the EU from Directives (Medical Device Directive [9], In Vitro Diagnostic Directive [10] and the Active Implantable Medical Device Directive [11]) to the Regulations (Medical Device Regulation (MDR) [12] and In Vitro Diagnostic Regulation (IVDR) [13]; and second, the choice by the UK to leave the EU and establish regulatory independence (including the UK CA mark and the UKNI mark) [14]. This regulatory independence leads to the much-increased influence of a third factor: UK government policy and its potential effect on UK regulation. Previously UK regulatory policy had to align to wider EU decisions and any change was cautious and highly sign-posted [15]. This brought advantages to the device sector of relative certainty and longer-term stability, but disadvantages of poor responsiveness to new challenges, and an inability to tune regulation to the opportunities and requirements of the UK. In contrast, the UK government could now bring sweeping changes to the regulatory framework as it seeks to strongly pursue its own agenda, which is currently strongly pro-innovation.
In February 2021, the UK Government commissioned a Taskforce on Innovation Growth and Regulatory Reform (TIGRR) to “identify and develop proposals across a range of areas that will drive innovation, growth and competitiveness through regulatory reform. ” [16] This reported to the UK government on 16 June 2021, and included a number of specific recommendations relevant to the UK medical devices sector. The view of the reports was that “In establishing our own regulatory framework the UK can now set our own Innovative Medical Devices regime to support this growing sector and anticipate the growing use of software and AI in medical devices. A framework of regulated digital products and devices needs a robust quality system for data management as part of the approval. This could be supplemented with some form of post-marketing surveillance (PMS) as one would see with traditional regulated medical devices.” The report was welcomed by the Prime Minister, and provides an indicator of the UK’s current direction of travel even if not all the specific recommendations survive the journey from report to policy to action.
Our study noted a number of themes that align with the findings and recommendations of the TIGRR report [16], as well as the more recent Life Science Vision for the UK [17], notably to build on some of the unique strengths of the NHS and the opportunity to more effectively use NHS data (including through greater centralisation). Unlike the TIGRR report, our study provides qualitative evidence from diverse stakeholders that recognizes some of the challenges that need to be overcome including current fragmentation into data silos, non-interoperability between datasets [18], and ambiguous data ownership [19] that exist within the current health and legal system. As highlighted in the TIGRR report and echoed by the participants in our study, leveraging existing assets and setting out a “new ambitious regulatory framework”, including approaches such as the Accelerated Access Pathway, will enable a timely response to innovative technology, including AI and ML components. This, alongside a more proportionate, targeted system-based approach, would offer the UK improved capacity and the flexibility to rapidly adapt to the future advancement of digital technologies. Aspirations such as these will require collaborative partnership across the industry and the UK Government.
An important balance to the emphasis on innovation and growth of the TIGRR report, is the Independent Medicines and Medical Devices Safety Review (IMMDSR) chaired by Baroness Cumberlege [20]. This report puts the emphasis firmly on patient safety, an important theme arising from our study. The IMMDSR also highlights the contribution that greater patient and public involvement can make to this. As with our study and the TIGRR report, it highlights the potential for more effective use of NHS data to detect safety issues early and to ensure that all parts of the health system including regulatory components are joined up. In line with our study and the TIGRR report, it also highlights the value of patient-reported outcomes and indeed the potential for collection to be enhanced through digital applications. The inclusion of meaningful, measurable data that is directly relevant to end-users, such as patient-reported outcomes, would maximize the chances that all important safety issues are captured during clinical investigation of devices.
One important difference between the findings of our study and the TIGRR report, is around international engagement. The TIGRR report is focused on UK leadership on the global stage. For our participants, this opportunity was recognized, but even higher priorities for international engagement were avoiding import/export friction through alignment (or mutual recognition), adoption of international technical specifications wherever possible, and ensuring that patient safety was not compromised through lower standards; leadership was considered desirable particularly in those areas where no existing models were considered adequate (such as AIaMD). Nationally, close collaboration from different sectors of the system and stakeholder groups can build synergy and accelerate the future of innovation in the UK [21]. This effort, coupled with global partnership and leadership has the potential to better understand which markets or innovation spaces are most vital and helpful to the UK, and ultimately pursue the goal of developing a robust regulatory regime for medical devices that prioritizes patient safety.
To our knowledge, this is the first published study to have assessed the in-depth stakeholder perspectives around the UK regulatory reforms for medical devices. Our findings are based on the recruitment of a diverse set of stakeholder groups representative of those involved in medical device sectors. The study shares limitations of qualitative approaches in general, principally the non-generalizability of study findings. However, the qualitative approach focusing on the depth was suitable for our study since it enabled us to explore, describe, and analyze sensitive issues related to the new regulatory reforms in the UK medical device sector. Though we interviewed a wide range of participants across eight different stakeholder groups, the small number of respondents in some categories made comparison across groups difficult. The interviews and workshop were undertaken during the Covid-19 pandemic, and this may have influenced responses from some individuals through ‘recency bias’, however given that learning from pandemic is an important, global, and ongoing phenomenon we would argue that this enhances rather than reduces the validity of our findings.
Our study highlights that we are at a critical juncture in the life of the UK’s medical device sector. Already responding to the requirements of transitioning from the EU directives to regulations, the medical device sector is challenged by the rapid pivot to an emerging UK-specific regulatory framework under the Medicines and Medical Devices Act 2021 [22], whilst also navigating a global pandemic. Our study provides additional evidence that stakeholders in the medical device sector recognize the significant opportunities that this brings, but also are concerned about the risks to patients and to the industry arising from the current transition, particularly over potential regulatory divergence. They call for a range of mitigations including investment, capacity-building, and international partnership which would enable the UK device industry to be supported and indeed strengthened, and to ensure that patients can still be provided with the medical devices they need.
References
GHTF Study Group. Information Document Concerning the Definition of the Term “Medical Device” [Internet]. The Global Harmonization Task Force; 2005 May. Available from: http://www.imdrf.org/docs/ghtf/final/sg1/technical-docs/ghtf-sg1-n29r16-2005-definition-medical-device-050520.pdf
Department for Business, Energy & Industrial Strategy. Life science sector data, 2020 [Internet]. GOV.UK; 2021 [cited 2021 Jul 26]. Available from: https://www.gov.uk/government/publications/life-science-sector-data-2020
Bioscience and Health Technology Sector Analysis 2019. Office for National Statistics; 2020.
McHale JV. Health law, Brexit and medical devices: a question of legal regulation and patient safety. Med Law Int. 2018;18(2–3):195–215.
UK Conformity Assessment [Internet]. GOV.UK. 2021 [cited 2021 Jul 2]. Available from: https://www.gov.uk/guidance/uk-conformity-assessment#combining-eu-and-uk-certification
Pope C, Ziebland S, Mays N. Qualitative research in health care. Analysing qualitative data. BMJ. 2000;320(7227):114–6.
Ritchie J, Lewis J, Nicholls CM, et al. Qualitative Research Practice: A Guide for Social Science Students and Researchers. London: SAGE; 2013. p. 456.
Medical Device Single Audit Program (MDSAP) [Internet]. International Medical Device Regulators Forum. [cited 2021 Jul 2]. Available from: http://www.imdrf.org/workitems/wi-mdsap.asp
Directive 93/42/EEC [Internet]. Jun 14, 1993. Available from: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX:31993L0042
Directive 98/79/EC [Internet]. Oct 27, 1998. Available from: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A31998L0079
Directive 90/385/EEC. Jun 20, 1990.
Regulation (EU) 2017/745 [Internet]. Apr 5, 2017. Available from: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32017R0745
Regulation (EU) 2017/746. Apr 5, 2017.
Regulating Medical Devices in the UK [Internet]. [cited 2021 Jul 26]. Available from: https://www.gov.uk/guidance/regulating-medical-devices-in-the-uk
Chai J. Regulation of medical devices in the European Union. J Leg Med. 2000;21(4):537–56.
Smith ID, Villiers T, Freeman G. Taskforce on Innovation, Growth and Regulatory Reform Independent Report. 2021 Jun.
Department for Business, Energy & Industrial Strategy. Life Sciences Vision. 2021 Jul.
Asthana S, Jones R, Sheaff R. Why does the NHS struggle to adopt eHealth innovations? A review of macro, meso and micro factors. BMC Health Serv Res. 2019;19(1):984.
Ballantyne A, Stewart C. Big data and public-private partnerships in healthcare and research. Asian Bioethics Rev. 2019;11(3):315–26.
First Do No Harm: The Report of the IMMDS Review. Department of Health and Social Care; 2020 Jul.
Cruz Rivera S, Torlinska B, Marston E, et al. Advancing UK regulatory science strategy in the context of global regulation: a stakeholder survey. Ther Innov Regul Sci. 2021;55(4):646–55.
Medicines and Medical Devices Act 2021 [Internet]. [cited 2021 Jul 26]. https://bills.parliament.uk/bills/2700/publications
Acknowledgements
The Regulatory Horizons Council is an independent committee that identifies the implications of technological innovation and provides the UK Government with impartial and expert advice on the regulatory reform required to support its rapid and safe introduction.
Funding
This work was supported by Research England’s Quality-related Research (QR) Strategic Priorities Fund.
Author information
Authors and Affiliations
Contributions
JEDH and HI had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. The authors confirm contribution to the paper as follows: Study conception and design: EM, AKD and MJC; Data acquisition, analysis, or interpretation: All authors; Drafting of the manuscript: JEDH; Critical revision of the manuscript for important intellectual content: EM, AKD and MJC. Obtained funding: EM, AKD and MJC. Supervision: OLA, EM, AKD and MJC. All authors reviewed and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
OLA receives funding from the NIHR Birmingham Biomedical Research Centre (BRC), NIHR Applied Research Centre (ARC), West Midlands at the University of Birmingham and University Hospitals Birmingham NHS Foundation, Innovate UK (part of UK Research and Innovation), Gilead Sciences Ltd, and Janssen Pharmaceuticals, Inc. OLA declares personal fees from Gilead Sciences Ltd, GlaxoSmithKline (GSK) and Merck outside the submitted work. MC is Director of the Birmingham Health Partners Centre for Regulatory Science and Innovation, Director of the Centre for Patient-Reported Outcomes Research and is a National Institute for Health Research (NIHR) Senior Investigator. She receives funding from the NIHR Birmingham Biomedical Research Centre, the NIHR Surgical Reconstruction and Microbiology Research Centre and NIHR ARC West Midlands at the at the University of Birmingham and University Hospitals Birmingham NHS Foundation Trust, Health Data Research UK, Innovate UK (part of UK Research and Innovation), Macmillan Cancer Support, UCB and GSK Pharma. MC has received personal fees from Astellas, Aparito Mltd, CIS Oncology, Takeda, Merck, Daiichi Sankyo, Glaukos, GSK and the Patient-Centred Outcomes Research Institute (PCORI) outside the submitted work. AD is employed by University Hospitals Birmingham NHS Foundation Trust. He is also a Member of the Regulatory Horizons Council, Honorary Professor at University of Birmingham and Artificial Intelligence Theme Lead for the Birmingham Health Partners Centre for Regulatory Science and Innovation. His work undertaken on behalf of the Regulatory Horizons Council is funded by the Department for Business, Energy and Industrial Strategy. His research outside the submitted work is supported by grant funding from University Hospitals Birmingham NHSFT, NIHR HTA awards, the NIHR Biomedical Research Centre (Moorfields Eye Hospital NHSFT/University College London), Health Data Research UK, Medical Research Council and the Wellcome Trust. The views expressed in this article are those of the author(s) and not necessarily those of the NIHR, the Department of Health and Social Care or the Department of Business, Energy and Industrial Strategy. Other authors declare no competing interests.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Han, J.E.D., Ibrahim, H., Aiyegbusi, O.L. et al. Opportunities and Risks of UK Medical Device Reform. Ther Innov Regul Sci 56, 596–606 (2022). https://doi.org/10.1007/s43441-022-00394-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43441-022-00394-0