Abstract
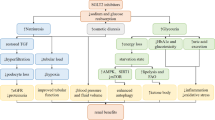
Sodium–glucose cotransporter inhibitors (SGLT2i) are a new class of anti-diabetic drugs that have beneficial cardiovascular and renal effects. These drugs decrease proximal tubular glucose reabsorption and decrease blood glucose levels as a main anti-diabetic action. Furthermore, SGLT2i decreases glomerular hyperfiltration by a tubuloglomerular feedback mechanism. However, the renal benefits of these agents are independent of glucose-lowering and hemodynamic factors, and SGLT2i also impacts the kidney structure including kidney fibrosis. Renal fibrosis is a common pathway and pathological marker of virtually every type of chronic kidney disease (CKD), and amelioration of renal fibrosis is of utmost importance to reduce the progression of CKD. Recent studies have shown that SGLT2i impact many cellular processes including inflammation, hypoxia, oxidative stress, metabolic functions, and renin–angiotensin system (RAS) which all are related with kidney fibrosis. Indeed, most but not all studies showed that renal fibrosis was ameliorated by SGLT2i through the reduction of inflammation, hypoxia, oxidative stress, and RAS activation. In addition, less known effects on SGLT2i on klotho expression, capillary rarefaction, signal transducer and activator of transcription signaling and peptidylprolyl cis/trans isomerase (Pin1) levels may partly explain the anti-fibrotic effects of SGLT2i in kidneys. It is important to remember that some studies have not shown any beneficial effects of SGLT2i on kidney fibrosis. Given this background, in the current review, we have summarized the studies and pathophysiologic aspects of SGL2 inhibition on renal fibrosis in various CKD models and tried to explain the potential reasons for contrasting findings.
Graphical abstract





Similar content being viewed by others
Data availability
The manuscript has no associated data.
Abbreviations
- AGE:
-
Advanced glycation end-products
- AKI:
-
Acute kidney injury
- α-SMA:
-
Alpha-smooth muscle actin
- CKD:
-
Chronic kidney disease
- CTGF:
-
Connective tissue growth factor
- DKD:
-
Diabetic kidney disease
- DM:
-
Diabetes mellitus
- EMT:
-
Epithelial-to-mesenchymal transition
- EPO:
-
Erythropoietin
- FN:
-
Fibronectin
- GFR:
-
Glomerular filtration rate
- HIF:
-
Hypoxia-inducible factor
- IR:
-
Ischemia–reperfusion
- MMP:
-
Matrix metalloproteinase
- MCP-1:
-
Monocyte chemoattractant protein-1
- mTOR:
-
Mammalian target of rapamycin
- PDGFB:
-
Platelet-derived growth factor subunit B
- PHD:
-
Prolyl hydroxylase domain
- ROS:
-
Reactive oxygen species
- STAT:
-
Signal transducer and activator of transcription
- SGLT2i:
-
Sodium–glucose cotransporter inhibitors
- TIMP:
-
Tissue inhibitor of metalloproteinase
- TGF-β:
-
Transforming growth factor β
- TCA:
-
Tricarboxylic acid
- TIF:
-
Tubulointerstitial fibrosis
- T2D:
-
Type 2 diabetes
- UNx:
-
Unilateral nephrectomy
- UUO:
-
Unilateral ureteric obstruction
References
Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295–306.
McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008.
Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–28.
Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323–34.
Cooper ME, Inzucchi SE, Zinman B, Hantel S, von Eynatten M, Wanner C, et al. Glucose control and the effect of empagliflozin on kidney outcomes in type 2 diabetes: an analysis from the EMPA-REG outcome trial. Am J Kidney Dis. 2019;74:713–5.
Heerspink HJ, Desai M, Jardine M, Balis D, Meininger G, Perkovic V. Canagliflozin slows progression of renal function decline independently of glycemic effects. J Am Soc Nephrol. 2017;28:368–75.
Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383:1413–24.
Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385:1451–61.
Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383:1436–46.
Neuen BL, Young T, Heerspink HJL, Neal B, Perkovic V, Billot L, et al. SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2019;7:845–54.
de Boer IH, Khunti K, Sadusky T, Tuttle KR, Neumiller JJ, Rhee CM, et al. Diabetes management in chronic kidney disease: a consensus report by the American Diabetes Association (ADA) and kidney disease: improving global outcomes (KDIGO). Kidney Int. 2022;102:974–89.
Vallon V, Thomson SC. The tubular hypothesis of nephron filtration and diabetic kidney disease. Nat Rev Nephrol. 2020;16:317–36.
Vallon V, Verma S. Effects of SGLT2 inhibitors on kidney and cardiovascular function. Annu Rev Physiol. 2021;83:503–28.
Brenner BM, Lawler EV, Mackenzie HS. The hyperfiltration theory: a paradigm shift in nephrology. Kidney Int. 1996;49:1774–7.
Denic A, Mathew J, Lerman LO, Lieske JC, Larson JJ, Alexander MP, et al. Single-nephron glomerular filtration rate in healthy adults. N Engl J Med. 2017;376:2349–57.
Fattah H, Layton A, Vallon V. How do kidneys adapt to a deficit or loss in nephron number? Physiology (Bethesda). 2019;34:189–97.
Cherney DZI, Dekkers CCJ, Barbour SJ, Cattran D, Abdul Gafor AH, Greasley PJ, et al. Effects of the SGLT2 inhibitor dapagliflozin on proteinuria in non-diabetic patients with chronic kidney disease (DIAMOND): a randomised, double-blind, crossover trial. Lancet Diabetes Endocrinol. 2020;8:582–93.
Yan H, Xu J, Xu Z, Yang B, Luo P, He Q. Defining therapeutic targets for renal fibrosis: exploiting the biology of pathogenesis. Biomed Pharmacother. 2021;143:112115.
Fine LG, Bandyopadhay D, Norman JT. Is there a common mechanism for the progression of different types of renal diseases other than proteinuria? Towards the unifying theme of chronic hypoxia. Kidney Int Suppl. 2000;75:S22–6.
Tanaka T, Nangaku M. The role of hypoxia, increased oxygen consumption, and hypoxia-inducible factor-1 alpha in progression of chronic kidney disease. Curr Opin Nephrol Hypertens. 2010;19:43–50.
Zhang L, Liu L, Bai M, Liu M, Wei L, Yang Z, et al. Hypoxia-induced HE4 in tubular epithelial cells promotes extracellular matrix accumulation and renal fibrosis via NF-κB. Faseb j. 2020;34:2554–67.
LeBleu VS, Teng Y, O’Connell JT, Charytan D, Müller GA, Müller CA, et al. Identification of human epididymis protein-4 as a fibroblast-derived mediator of fibrosis. Nat Med. 2013;19:227–31.
Chen P, Yang Q, Li X, Qin Y. Potential association between elevated serum human epididymis protein 4 and renal fibrosis: a systemic review and meta-analysis. Medicine (Baltimore). 2017;96:e7824.
Huang X, Guo X, Yan G, Zhang Y, Yao Y, Qiao Y, et al. Dapagliflozin attenuates contrast-induced acute kidney injury by regulating the HIF-1α/HE4/NF-κB pathway. J Cardiovasc Pharmacol. 2022;79:904–13.
Hodrea J, Balogh DB, Hosszu A, Lenart L, Besztercei B, Koszegi S, et al. Reduced O-GlcNAcylation and tubular hypoxia contribute to the antifibrotic effect of SGLT2 inhibitor dapagliflozin in the diabetic kidney. Am J Physiol Renal Physiol. 2020;318:F1017–29.
Li J, Liu H, Takagi S, Nitta K, Kitada M, Srivastava SP, et al. Renal protective effects of empagliflozin via inhibition of EMT and aberrant glycolysis in proximal tubules. JCI Insight. 2020;5:1.
Ndibalema AR, Kabuye D, Wen S, Li L, Li X, Fan Q. Empagliflozin protects against proximal renal tubular cell injury induced by high glucose via regulation of hypoxia-inducible factor 1-alpha. Diabetes Metab Syndr Obes. 2020;13:1953–67.
Bessho R, Takiyama Y, Takiyama T, Kitsunai H, Takeda Y, Sakagami H, et al. Hypoxia-inducible factor-1α is the therapeutic target of the SGLT2 inhibitor for diabetic nephropathy. Sci Rep. 2019;9:14754.
Ravindran S, Munusamy S. Renoprotective mechanisms of sodium–glucose co-transporter 2 (SGLT2) inhibitors against the progression of diabetic kidney disease. J Cell Physiol. 2022;237:1182–205.
Nordquist L, Friederich-Persson M, Fasching A, Liss P, Shoji K, Nangaku M, et al. Activation of hypoxia-inducible factors prevents diabetic nephropathy. J Am Soc Nephrol. 2015;26:328–38.
Jiao Y, Jiang H, Lu H, Yang Y, Zhang Y, Zhang K, et al. Deficiency of hypoxia inducible factor-1α promoted progression of diabetic nephropathy with hypertension. Exp Ther Med. 2018;16:3658–62.
Hesp AC, Schaub JA, Prasad PV, Vallon V, Laverman GD, Bjornstad P, et al. The role of renal hypoxia in the pathogenesis of diabetic kidney disease: a promising target for newer renoprotective agents including SGLT2 inhibitors? Kidney Int. 2020;98:579–89.
Xu ZH, Wang C, He YX, Mao XY, Zhang MZ, Hou YP, et al. Hypoxia-inducible factor protects against acute kidney injury via the Wnt/β-catenin signaling pathway. Am J Physiol Renal Physiol. 2022;322:F611–24.
Chen B, Brem AS, Gong R. The Janus view: dual roles for hypoxia-inducible factor in renal repair after acute kidney injury. Am J Physiol Renal Physiol. 2022;323:F1-f3.
Kanbay M, Tapoi L, Ureche C, Tanriover C, Cevik E, Demiray A, et al. Effect of sodium–glucose cotransporter 2 inhibitors on hemoglobin and hematocrit levels in type 2 diabetes: a systematic review and meta-analysis. Int Urol Nephrol. 2022;54:827–41.
Afsar B, Afsar RE, Ertuglu LA, Covic A, Kanbay M. Nutrition, immunology, and kidney: looking beyond the horizons. Curr Nutr Rep. 2022;11:69–81.
Komada T, Muruve DA. The role of inflammasomes in kidney disease. Nat Rev Nephrol. 2019;15:501–20.
Zhang H, Wang Z. Effect and regulation of the NLRP3 inflammasome during renal fibrosis. Front Cell Dev Biol. 2019;7:379.
Benetti E, Mastrocola R, Vitarelli G, Cutrin JC, Nigro D, Chiazza F, et al. Empagliflozin protects against diet-induced NLRP-3 inflammasome activation and lipid accumulation. J Pharmacol Exp Ther. 2016;359:45–53.
Ke Q, Shi C, Lv Y, Wang L, Luo J, Jiang L, et al. SGLT2 inhibitor counteracts NLRP3 inflammasome via tubular metabolite itaconate in fibrosis kidney. Faseb j. 2022;36:e22078.
Youm YH, Nguyen KY, Grant RW, Goldberg EL, Bodogai M, Kim D, et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015;21:263–9.
Wu X, Li H, Wan Z, Wang R, Liu J, Liu Q, et al. The combination of ursolic acid and empagliflozin relieves diabetic nephropathy by reducing inflammation, oxidative stress and renal fibrosis. Biomed Pharmacother. 2021;144:112267.
Wang XX, Levi J, Luo Y, Myakala K, Herman-Edelstein M, Qiu L, et al. SGLT2 protein expression is increased in human diabetic nephropathy: SGLT2 protein inhibition decreases renal lipid accumulation, inflammation, and the development of nephropathy in diabetic mice. J Biol Chem. 2017;292:5335–48.
Tang L, Wu Y, Tian M, Sjöström CD, Johansson U, Peng XR, et al. Dapagliflozin slows the progression of the renal and liver fibrosis associated with type 2 diabetes. Am J Physiol Endocrinol Metab. 2017;313:E563–76.
Terami N, Ogawa D, Tachibana H, Hatanaka T, Wada J, Nakatsuka A, et al. Long-term treatment with the sodium glucose cotransporter 2 inhibitor, dapagliflozin, ameliorates glucose homeostasis and diabetic nephropathy in db/db mice. PLoS ONE. 2014;9:e100777.
Okuma H, Mori K, Nakamura S, Sekine T, Ogawa Y, Tsuchiya K. Ipragliflozin ameliorates diabetic nephropathy associated with perirenal adipose expansion in mice. Int J Mol Sci. 2021;22:1.
Hatanaka T, Ogawa D, Tachibana H, Eguchi J, Inoue T, Yamada H, et al. Inhibition of SGLT2 alleviates diabetic nephropathy by suppressing high glucose-induced oxidative stress in type 1 diabetic mice. Pharmacol Res Perspect. 2016;4:e00239.
Ojima A, Matsui T, Nishino Y, Nakamura N, Yamagishi S. Empagliflozin, an inhibitor of sodium–glucose cotransporter 2 exerts anti-inflammatory and antifibrotic effects on experimental diabetic nephropathy partly by suppressing AGEs-receptor axis. Horm Metab Res. 2015;47:686–92.
Yao D, Wang S, Wang M, Lu W. Renoprotection of dapagliflozin in human renal proximal tubular cells via the inhibition of the high mobility group box 1-receptor for advanced glycation end products-nuclear factor-κB signaling pathway. Mol Med Rep. 2018;18:3625–30.
Abbas NAT, El Salem A, Awad MM. Empagliflozin, SGLT(2) inhibitor, attenuates renal fibrosis in rats exposed to unilateral ureteric obstruction: potential role of klotho expression. Naunyn Schmiedebergs Arch Pharmacol. 2018;391:1347–60.
Huang J, Chen Z, Li J, Chen Q, Li J, Gong W, et al. Protein kinase CK2α catalytic subunit ameliorates diabetic renal inflammatory fibrosis via NF-κB signaling pathway. Biochem Pharmacol. 2017;132:102–17.
Panchapakesan U, Pegg K, Gross S, Komala MG, Mudaliar H, Forbes J, et al. Effects of SGLT2 inhibition in human kidney proximal tubular cells—Renoprotection in diabetic nephropathy? PLoS ONE. 2013;8:e54442.
Heerspink HJL, Perco P, Mulder S, Leierer J, Hansen MK, Heinzel A, et al. Canagliflozin reduces inflammation and fibrosis biomarkers: a potential mechanism of action for beneficial effects of SGLT2 inhibitors in diabetic kidney disease. Diabetologia. 2019;62:1154–66.
Stieger N, Worthmann K, Teng B, Engeli S, Das AM, Haller H, et al. Impact of high glucose and transforming growth factor-β on bioenergetic profiles in podocytes. Metabolism. 2012;61:1073–86.
Das NA, Carpenter AJ, Belenchia A, Aroor AR, Noda M, Siebenlist U, et al. Empagliflozin reduces high glucose-induced oxidative stress and miR-21-dependent TRAF3IP2 induction and RECK suppression, and inhibits human renal proximal tubular epithelial cell migration and epithelial-to-mesenchymal transition. Cell Signal. 2020;68:109506.
Woods TC, Satou R, Miyata K, Katsurada A, Dugas CM, Klingenberg NC, et al. Canagliflozin prevents intrarenal angiotensinogen augmentation and mitigates kidney injury and hypertension in mouse model of type 2 diabetes mellitus. Am J Nephrol. 2019;49:331–42.
Tanaka S, Sugiura Y, Saito H, Sugahara M, Higashijima Y, Yamaguchi J, et al. Sodium–glucose cotransporter 2 inhibition normalizes glucose metabolism and suppresses oxidative stress in the kidneys of diabetic mice. Kidney Int. 2018;94:912–25.
Ferrannini E, Muscelli E, Frascerra S, Baldi S, Mari A, Heise T, et al. Metabolic response to sodium–glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest. 2014;124:499–508.
DeFronzo RA, Reeves WB, Awad AS. Pathophysiology of diabetic kidney disease: impact of SGLT2 inhibitors. Nat Rev Nephrol. 2021;17:319–34.
Packer M. SGLT2 inhibitors produce cardiorenal benefits by promoting adaptive cellular reprogramming to induce a state of fasting mimicry: a paradigm shift in understanding their mechanism of action. Diabetes Care. 2020;43:508–11.
Tomita I, Kume S, Sugahara S, Osawa N, Yamahara K, Yasuda-Yamahara M, et al. SGLT2 inhibition mediates protection from diabetic kidney disease by promoting ketone body-induced mTORC1 inhibition. Cell Metab. 2020;32:404-19.e6.
Hawley SA, Ford RJ, Smith BK, Gowans GJ, Mancini SJ, Pitt RD, et al. The Na+/glucose cotransporter inhibitor canagliflozin activates AMPK by inhibiting mitochondrial function and increasing cellular AMP levels. Diabetes. 2016;65:2784–94.
Han Y, Xiong S, Zhao H, Yang S, Yang M, Zhu X, et al. Lipophagy deficiency exacerbates ectopic lipid accumulation and tubular cells injury in diabetic nephropathy. Cell Death Dis. 2021;12:1031.
Zhang Z, Ni L, Zhang L, Zha D, Hu C, Zhang L, et al. Empagliflozin regulates the AdipoR1/p-AMPK/p-ACC pathway to alleviate lipid deposition in diabetic nephropathy. Diabetes Metab Syndr Obes. 2021;14:227–40.
Ding H, Jiang L, Xu J, Bai F, Zhou Y, Yuan Q, et al. Inhibiting aerobic glycolysis suppresses renal interstitial fibroblast activation and renal fibrosis. Am J Physiol Renal Physiol. 2017;313:F561–75.
Inoue MK, Matsunaga Y, Nakatsu Y, Yamamotoya T, Ueda K, Kushiyama A, et al. Possible involvement of normalized Pin1 expression level and AMPK activation in the molecular mechanisms underlying renal protective effects of SGLT2 inhibitors in mice. Diabetol Metab Syndr. 2019;11:57.
Lee YH, Kim SH, Kang JM, Heo JH, Kim DJ, Park SH, et al. Empagliflozin attenuates diabetic tubulopathy by improving mitochondrial fragmentation and autophagy. Am J Physiol Renal Physiol. 2019;317:F767–80.
Goldberg H, Whiteside C, Fantus IG. O-linked β-N-acetylglucosamine supports p38 MAPK activation by high glucose in glomerular mesangial cells. Am J Physiol Endocrinol Metab. 2011;301:E713–26.
Shibusawa R, Yamada E, Okada S, Nakajima Y, Bastie CC, Maeshima A, et al. Dapagliflozin rescues endoplasmic reticulum stress-mediated cell death. Sci Rep. 2019;9:9887.
Afsar B, Hornum M, Afsar RE, Ertuglu LA, Ortiz A, Covic A, et al. Mitochondrion-driven nephroprotective mechanisms of novel glucose lowering medications. Mitochondrion. 2021;58:72–82.
Xie Y, Jing E, Cai H, Zhong F, Xiao W, Gordon RE, et al. Reticulon-1A mediates diabetic kidney disease progression through endoplasmic reticulum–mitochondrial contacts in tubular epithelial cells. Kidney Int. 2022;102:293–306.
Siragy HM, Carey RM. Role of the intrarenal renin-angiotensin-aldosterone system in chronic kidney disease. Am J Nephrol. 2010;31:541–50.
Gross O, Schulze-Lohoff E, Koepke ML, Beirowski B, Addicks K, Bloch W, et al. Antifibrotic, nephroprotective potential of ACE inhibitor vs. AT1 antagonist in a murine model of renal fibrosis. Nephrol Dial Transpl. 2004;19:1716–23.
Rubel D, Stock J, Ciner A, Hiller H, Girgert R, Müller GA, et al. Antifibrotic, nephroprotective effects of paricalcitol versus calcitriol on top of ACE-inhibitor therapy in the COL4A3 knockout mouse model for progressive renal fibrosis. Nephrol Dial Transpl. 2014;29:1012–9.
Feldman DL, Jin L, Xuan H, Contrepas A, Zhou Y, Webb RL, et al. Effects of aliskiren on blood pressure, albuminuria, and (pro)renin receptor expression in diabetic TG(mRen-2)27 rats. Hypertension. 2008;52:130–6.
Han KH, Kang YS, Han SY, Jee YH, Lee MH, Han JY, et al. Spironolactone ameliorates renal injury and connective tissue growth factor expression in type II diabetic rats. Kidney Int. 2006;70:111–20.
Koszegi S, Molnar A, Lenart L, Hodrea J, Balogh DB, Lakat T, et al. RAAS inhibitors directly reduce diabetes-induced renal fibrosis via growth factor inhibition. J Physiol. 2019;597:193–209.
Morrissey JJ, Klahr S. Effect of AT2 receptor blockade on the pathogenesis of renal fibrosis. Am J Physiol. 1999;276:F39-45.
Crowley SD, Rudemiller NP. Immunologic effects of the renin-angiotensin system. J Am Soc Nephrol. 2017;28:1350–61.
Liu Z, Huang XR, Chen HY, Penninger JM, Lan HY. Loss of angiotensin-converting enzyme 2 enhances TGF-β/Smad-mediated renal fibrosis and NF-κB-driven renal inflammation in a mouse model of obstructive nephropathy. Lab Invest. 2012;92:650–61.
Mao N, Tan RZ, Wang SQ, Wei C, Shi XL, Fan JM, et al. Ginsenoside Rg1 inhibits angiotensin II-induced podocyte autophagy via AMPK/mTOR/PI3K pathway. Cell Biol Int. 2016;40:917–25.
Lizakowski S, Tylicki L, Renke M, Rutkowski P, Heleniak Z, Sławińska-Morawska M, et al. Aliskiren and perindopril reduce the levels of transforming growth factor-β in patients with non-diabetic kidney disease. Am J Hypertens. 2012;25:636–9.
Cuevas CA, Gonzalez AA, Inestrosa NC, Vio CP, Prieto MC. Angiotensin II increases fibronectin and collagen I through the β-catenin-dependent signaling in mouse collecting duct cells. Am J Physiol Renal Physiol. 2015;308:F358–65.
Shin SJ, Chung S, Kim SJ, Lee EM, Yoo YH, Kim JW, et al. Effect of sodium–glucose co-transporter 2 inhibitor, dapagliflozin, on renal renin-angiotensin system in an animal model of type 2 diabetes. PLoS ONE. 2016;11:e0165703.
Miyata KN, Lo CS, Zhao S, Liao MC, Pang Y, Chang SY, et al. Angiotensin II up-regulates sodium–glucose co-transporter 2 expression and SGLT2 inhibitor attenuates Ang II-induced hypertensive renal injury in mice. Clin Sci (Lond). 2021;135:943–61.
Gallo LA, Ward MS, Fotheringham AK, Zhuang A, Borg DJ, Flemming NB, et al. Once daily administration of the SGLT2 inhibitor, empagliflozin, attenuates markers of renal fibrosis without improving albuminuria in diabetic db/db mice. Sci Rep. 2016;6:26428.
Lambers Heerspink HJ, de Zeeuw D, Wie L, Leslie B, List J. Dapagliflozin a glucose-regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes Metab. 2013;15:853–62.
Tanaka H, Takano K, Iijima H, Kubo H, Maruyama N, Hashimoto T, et al. Factors affecting canagliflozin-induced transient urine volume increase in patients with type 2 diabetes mellitus. Adv Ther. 2017;34:436–51.
Takeshige Y, Fujisawa Y, Rahman A, Kittikulsuth W, Nakano D, Mori H, et al. A sodium–glucose co-transporter 2 inhibitor empagliflozin prevents abnormality of circadian rhythm of blood pressure in salt-treated obese rats. Hypertens Res. 2016;39:415–22.
Sawamura T, Karashima S, Nagase S, Nambo H, Shimizu E, Higashitani T, et al. Effect of sodium–glucose cotransporter-2 inhibitors on aldosterone-to-renin ratio in diabetic patients with hypertension: a retrospective observational study. BMC Endocr Disord. 2020;20:177.
Ryu ES, Kim MJ, Shin HS, Jang YH, Choi HS, Jo I, et al. Uric acid-induced phenotypic transition of renal tubular cells as a novel mechanism of chronic kidney disease. Am J Physiol Renal Physiol. 2013;304:F471–80.
Bailey CJ. Uric acid and the cardio-renal effects of SGLT2 inhibitors. Diabetes Obes Metab. 2019;21:1291–8.
Novikov A, Fu Y, Huang W, Freeman B, Patel R, van Ginkel C, et al. SGLT2 inhibition and renal urate excretion: role of luminal glucose, GLUT9, and URAT1. Am J Physiol Renal Physiol. 2019;316:F173–85.
Suijk DLS, van Baar MJB, van Bommel EJM, Iqbal Z, Krebber MM, Vallon V, et al. SGLT2 inhibition and uric acid excretion in patients with type 2 diabetes and normal kidney function. Clin J Am Soc Nephrol. 2022;17:663–71.
Huang F, Zhao Y, Wang Q, Hillebrands JL, van den Born J, Ji L, et al. Dapagliflozin attenuates renal tubulointerstitial fibrosis associated with type 1 diabetes by regulating STAT1/TGFβ1 signaling. Front Endocrinol (Lausanne). 2019;10:441.
Kanbay M, Demiray A, Afsar B, Covic A, Tapoi L, Ureche C, et al. Role of klotho in the development of essential hypertension. Hypertension. 2021;77:740–50.
Zhou L, Mo H, Miao J, Zhou D, Tan RJ, Hou FF, et al. Klotho ameliorates kidney injury and fibrosis and normalizes blood pressure by targeting the renin-angiotensin system. Am J Pathol. 2015;185:3211–23.
Zhang Y, Nakano D, Guan Y, Hitomi H, Uemura A, Masaki T, et al. A sodium–glucose cotransporter 2 inhibitor attenuates renal capillary injury and fibrosis by a vascular endothelial growth factor-dependent pathway after renal injury in mice. Kidney Int. 2018;94:524–35.
Gomez IG, Roach AM, Nakagawa N, Amatucci A, Johnson BG, Dunn K, et al. TWEAK-Fn14 signaling activates myofibroblasts to drive progression of fibrotic kidney disease. J Am Soc Nephrol. 2016;27:3639–52.
Shen ZJ, Hu J, Shiizaki K, Kuro-o M, Malter JS. Phosphate-induced renal fibrosis requires the prolyl isomerase pin1. PLoS ONE. 2016;11:e0150093.
Liu H, Sridhar VS, Lovblom LE, Lytvyn Y, Burger D, Burns K, et al. Markers of kidney injury, inflammation, and fibrosis associated with ertugliflozin in patients with CKD and diabetes. Kidney Int Rep. 2021;6:2095–104.
Ma Q, Steiger S, Anders HJ. Sodium glucose transporter-2 inhibition has no renoprotective effects on non-diabetic chronic kidney disease. Physiol Rep. 2017;5:1.
Droebner K, Pavkovic M, Grundmann M, Hartmann E, Goea L, Nordlohne J, et al. Direct blood pressure-independent anti-fibrotic effects by the selective nonsteroidal mineralocorticoid receptor antagonist finerenone in progressive models of kidney fibrosis. Am J Nephrol. 2021;52:588–601.
Zhang Y, Thai K, Kepecs DM, Gilbert RE. Sodium–glucose linked cotransporter-2 inhibition does not attenuate disease progression in the rat remnant kidney model of chronic kidney disease. PLoS ONE. 2016;11:e0144640.
Vallon V, Gerasimova M, Rose MA, Masuda T, Satriano J, Mayoux E, et al. SGLT2 inhibitor empagliflozin reduces renal growth and albuminuria in proportion to hyperglycemia and prevents glomerular hyperfiltration in diabetic Akita mice. Am J Physiol Renal Physiol. 2014;306:F194-204.
Gangadharan Komala M, Gross S, Mudaliar H, Huang C, Pegg K, Mather A, et al. Inhibition of kidney proximal tubular glucose reabsorption does not prevent against diabetic nephropathy in type 1 diabetic eNOS knockout mice. PLoS ONE. 2014;9:e108994.
Zeng S, Delic D, Chu C, Xiong Y, Luo T, Chen X, et al. Antifibrotic effects of low dose SGLT2 inhibition with empagliflozin in comparison to Ang II receptor blockade with telmisartan in 5/6 nephrectomised rats on high salt diet. Biomed Pharmacother. 2022;146:112606.
Tauber P, Sinha F, Berger RS, Gronwald W, Dettmer K, Kuhn M, et al. Empagliflozin reduces renal hyperfiltration in response to uninephrectomy, but is not nephroprotective in UNx/DOCA/Salt mouse models. Front Pharmacol. 2021;12:761855.
Kojima N, Williams JM, Takahashi T, Miyata N, Roman RJ. Effects of a new SGLT2 inhibitor, luseogliflozin, on diabetic nephropathy in T2DN rats. J Pharmacol Exp Ther. 2013;345:464–72.
Kojima N, Williams JM, Slaughter TN, Kato S, Takahashi T, Miyata N, et al. Renoprotective effects of combined SGLT2 and ACE inhibitor therapy in diabetic Dahl S rats. Physiol Rep. 2015;3:1.
Abdel-Wahab AF, Bamagous GA, Al-Harizy RM, ElSawy NA, Shahzad N, Ibrahim IA, et al. Renal protective effect of SGLT2 inhibitor dapagliflozin alone and in combination with irbesartan in a rat model of diabetic nephropathy. Biomed Pharmacother. 2018;103:59–66.
Jaikumkao K, Pongchaidecha A, Chueakula N, Thongnak LO, Wanchai K, Chatsudthipong V, et al. Dapagliflozin, a sodium–glucose co-transporter-2 inhibitor, slows the progression of renal complications through the suppression of renal inflammation, endoplasmic reticulum stress and apoptosis in prediabetic rats. Diabetes Obes Metab. 2018;20:2617–26.
Tahara A, Takasu T. Prevention of progression of diabetic nephropathy by the SGLT2 inhibitor ipragliflozin in uninephrectomized type 2 diabetic mice. Eur J Pharmacol. 2018;830:68–75.
Wang D, Luo Y, Wang X, Orlicky DJ, Myakala K, Yang P, et al. The sodium-glucose cotransporter 2 inhibitor dapagliflozin prevents renal and liver disease in western diet induced obesity mice. Int J Mol Sci. 2018;19:1.
Ali BH, Al Salam S, Al Suleimani Y, Al Za’abi M, Abdelrahman AM, Ashique M, et al. Effects of the SGLT-2 inhibitor canagliflozin on adenine-induced chronic kidney disease in rats. Cell Physiol Biochem. 2019;52:27–39.
Cai T, Ke Q, Fang Y, Wen P, Chen H, Yuan Q, et al. Sodium–glucose cotransporter 2 inhibition suppresses HIF-1α-mediated metabolic switch from lipid oxidation to glycolysis in kidney tubule cells of diabetic mice. Cell Death Dis. 2020;11:390.
Castoldi G, Carletti R, Ippolito S, Colzani M, Barzaghi F, Stella A, et al. Renal anti-fibrotic effect of sodium glucose cotransporter 2 inhibition in angiotensin II-dependent hypertension. Am J Nephrol. 2020;51:119–29.
Hasan R, Lasker S, Hasan A, Zerin F, Zamila M, Parvez F, et al. Canagliflozin ameliorates renal oxidative stress and inflammation by stimulating AMPK-Akt-eNOS pathway in the isoprenaline-induced oxidative stress model. Sci Rep. 2020;10:14659.
Nespoux J, Patel R, Zhang H, Huang W, Freeman B, Sanders PW, et al. Gene knockout of the Na(+)-glucose cotransporter SGLT2 in a murine model of acute kidney injury induced by ischemia-reperfusion. Am J Physiol Renal Physiol. 2020;318:F1100–12.
Yamato M, Kato N, Kakino A, Yamada KI, Inoguchi T. Low dose of sodium–glucose transporter 2 inhibitor ipragliflozin attenuated renal dysfunction and interstitial fibrosis in adenine-induced chronic kidney disease in mice without diabetes. Metabol Open. 2020;7:100049.
Castoldi G, Carletti R, Ippolito S, Colzani M, Barzaghi F, Stella A, et al. Sodium–glucose cotransporter 2 inhibition prevents renal fibrosis in cyclosporine nephropathy. Acta Diabetol. 2021;58:1059–70.
Kolkhof P, Hartmann E, Freyberger A, Pavkovic M, Mathar I, Sandner P, et al. Effects of finerenone combined with empagliflozin in a model of hypertension-induced end-organ damage. Am J Nephrol. 2021;52:642–52.
Pan X, Phanish MK, Baines DL, Dockrell MEC. High glucose-induced Smad3 linker phosphorylation and CCN2 expression are inhibited by dapagliflozin in a diabetic tubule epithelial cell model. Biosci Rep. 2021;41:1.
Ko EJ, Shin YJ, Cui S, Lim SW, Chung BH, Yang CW. Effect of dual inhibition of DPP4 and SGLT2 on tacrolimus-induced diabetes mellitus and nephrotoxicity in a rat model. Am J Transpl. 2022;22:1537–49.
Funding
None declared.
Author information
Authors and Affiliations
Contributions
BA was the owner of the project, gathered data, wrote the manuscript. REA helped to write the manuscript, reviewed, and approved the final version.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Afsar, B., Afsar, R.E. Sodium–glucose cotransporter inhibitors and kidney fibrosis: review of the current evidence and related mechanisms. Pharmacol. Rep 75, 44–68 (2023). https://doi.org/10.1007/s43440-022-00442-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43440-022-00442-4