Abstract
Background
The strong inter-individual pharmacokinetic variability and the narrow therapeutic window of tacrolimus (TAC) have hampered the clinical application. Gene polymorphisms play an important role in TAC pharmacokinetics. Here, we investigate the influence of genotypes of IL-10, CYP3A5, CYP2C8, and ABCB1 on dose-adjusted trough blood concentrations (the C0/D ratio) of TAC to reveal unclear genetic factors that may affect TAC dose requirements for renal transplant recipients.
Methods
Genetic polymorphisms of IL-10, CYP3A5, CYP2C8, and ABCB1 in 188 renal transplant recipients were determined using Kompetitive Allele Specific PCR (KASP). Statistical analysis was applied to examine the effect of genetic variation on the TAC C0/D at 5, 10, 15, and 30 days after transplantation.
Results
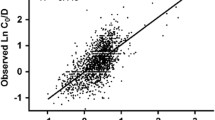
Recipients carrying the IL-10 -819C > T TT genotype showed a significantly higher TAC C0/D than those with the TC/CC genotype (p < 0.05). Additionally, the TAC C0/D values of recipients with the capacity for low IL-10 activity (-819 TT) engrafted with CYP3A5 non-expressers were higher compared to the intermediate/high activity of IL-10 -819C > T TC or CC carrying CYP3A5 expressers, and the difference was statistically significant at different time points (p < 0.05).
Conclusions
Genetic polymorphisms of IL-10 -819C > T and CYP3A5 6986A > G influence the TAC C0/D, which may contribute to variation in TAC dose requirements during the early post-transplantation period. Detecting IL-10 -819C > T and CYP3A5 6986A > G polymorphisms may allow determination of individualized tacrolimus dosage regimens for renal transplant recipients during the early post-transplantation period.

Similar content being viewed by others
Change history
24 March 2022
A Correction to this paper has been published: https://doi.org/10.1007/s43440-022-00358-z
References
Lim WH, Au E, Krishnan A, Wong G. Assessment of kidney transplant suitability for patients with prior cancers: is it time for a rethink. Transpl Int. 2019;32(12):1223–40.
Oberbauer R, Bestard O, Furian L, Maggiore U, Pascual J, Rostaing L, et al. Optimization of tacrolimus in kidney transplantation: New pharmacokinetic perspectives. Transplant Rev (Orlando). 2020;34(2):100531.
Lancia P, Jacqz-Aigrain E, Zhao W. Choosing the right dose of tacrolimus. Arch Dis Child. 2015;100(4):406–13.
Venkataramanan R, Swaminathan A, Prasad T, Jain A, Zuckerman S, Warty V, et al. Clinical pharmacokinetics of tacrolimus. Clin Pharmacokinet. 1995;29(6):404–30.
Tron C, Lemaitre F, Verstuyft C, Petitcollin A, Verdier MC, Bellissant E. Pharmacogenetics of membrane transporters of tacrolimus in solid organ transplantation. Clin Pharmacokinet. 2019;58(5):593–613.
Brunet M, van Gelder T, Åsberg A, Haufroid V, Hesselink DA, Langman L, et al. therapeutic drug monitoring of tacrolimus-personalized therapy: second consensus report. Ther Drug Monit. 2019;41(3):261–307.
Gonzales HM, McGillicuddy JW, Rohan V, Chandler JL, Nadig SN, Dubay DA, et al. A comprehensive review of the impact of tacrolimus intrapatient variability on clinical outcomes in kidney transplantation. Am J Transplant. 2020;20(8):1969–83.
Hesselink DA, Bouamar R, Elens L, van Schaik RH, van Gelder T. The role of pharmacogenetics in the disposition of and response to tacrolimus in solid organ transplantation. Clin Pharmacokinet. 2014;53(2):123–39.
Liu MZ, He HY, Zhang YL, Hu YF, He FZ, Luo JQ, et al. IL-3 and CTLA4 gene polymorphisms may influence the tacrolimus dose requirement in Chinese kidney transplant recipients. Acta Pharmacol Sin. 2017;38(3):415–23.
Mohamed ME, Schladt DP, Guan W, Wu B, van Setten J, Keating BJ, et al. Tacrolimus troughs and genetic determinants of metabolism in kidney transplant recipients: a comparison of four ancestry groups. Am J Transplant. 2019;19(10):2795–804.
Khan AR, Raza A, Firasat S, Abid A. CYP3A5 gene polymorphisms and their impact on dosage and trough concentration of tacrolimus among kidney transplant patients: a systematic review and meta-analysis. Pharmacogenom J. 2020;20(4):553–62.
Campagne O, Mager DE, Brazeau D, Venuto RC, Tornatore KM. Tacrolimus population pharmacokinetics and multiple CYP3A5 genotypes in black and white renal transplant recipients. J Clin Pharmacol. 2018;58(9):1184–95.
Campagne O, Mager DE, Tornatore KM. Population pharmacokinetics of tacrolimus in transplant recipients: what did we learn about sources of interindividual variabilities. J Clin Pharmacol. 2019;59(3):309–25.
Naushad SM, Pavani A, Rupasree Y, Hussain T, Alrokayan SA, Kutala VK. Recipient ABCB1, donor and recipient CYP3A5 genotypes influence tacrolimus pharmacokinetics in liver transplant cases. Pharmacol Rep. 2019;71(3):385–92.
Vanhove T, de Jonge H, de Loor H, Oorts M, de Hoon J, Pohanka A, et al. Relationship between in vivo CYP3A4 Activity, CYP3A5 genotype, and systemic tacrolimus metabolite/parent drug ratio in renal transplant recipients and healthy volunteers. Drug Metab Dispos. 2018;46(11):1507–13.
Wang Z, Zheng M, Yang H, Han Z, Tao J, Chen H, et al. Association of genetic variants in CYP3A4, CYP3A5, CYP2C8, and CYP2C19 with tacrolimus pharmacokinetics in renal transplant recipients. Curr Drug Metab. 2019;20(7):609–18.
de Jonge H, Kuypers DR. Pharmacogenetics in solid organ transplantation: current status and future directions. Transplant Rev (Orlando). 2008;22(1):6–20.
Rojas LE, Herrero MJ, Bosó V, García-Eliz M, Poveda JL, Librero J, et al. Meta-analysis and systematic review of the effect of the donor and recipient CYP3A5 6986A>G genotype on tacrolimus dose requirements in liver transplantation. Pharmacogenet Genom. 2013;23(10):509–17.
Zhang X, Xu J, Fan J, Zhang T, Li Y, Xie B, et al. Influence of IL-18 and IL-10 polymorphisms on tacrolimus elimination in chinese lung transplant patients. Dis Marker. 2017;2017:7834035.
Kasamatsu T, Saitoh T, Ino R, Gotoh N, Mitsui T, Shimizu H, et al. Polymorphism of IL-10 receptor β affects the prognosis of multiple myeloma patients treated with thalidomide and/or bortezomib. Hematol Oncol. 2017;35(4):711–8.
Kim DH, Lee NY, Sohn SK, Baek JH, Kim JG, Suh JS, et al. IL-10 promoter gene polymorphism associated with the occurrence of chronic GVHD and its clinical course during systemic immunosuppressive treatment for chronic GVHD after allogeneic peripheral blood stem cell transplantation. Transplantation. 2005;79(11):1615–22.
Kalsotra A, Anakk S, Brommer CL, Kikuta Y, Morgan ET, Strobel HW. Catalytic characterization and cytokine mediated regulation of cytochrome P450 4Fs in rat hepatocytes. Arch Biochem Biophys. 2007;461(1):104–12.
Zhang X, Wang Z, Fan J, Liu G, Peng Z. Impact of interleukin-10 gene polymorphisms on tacrolimus dosing requirements in Chinese liver transplant patients during the early posttransplantation period. Eur J Clin Pharmacol. 2011;67(8):803–13.
Li D, Lu W, Zhu JY, Gao J, Lou YQ, Zhang GL. Population pharmacokinetics of tacrolimus and CYP3A5, MDR1 and IL-10 polymorphisms in adult liver transplant patients. J Clin Pharm Ther. 2007;32(5):505–15.
Li D, Zhu JY, Gao J, Wang X, Lou YQ, Zhang GL. Polymorphisms of tumor necrosis factor-alpha, interleukin-10, cytochrome P450 3A5 and ABCB1 in Chinese liver transplant patients treated with immunosuppressant tacrolimus. Clin Chim Acta. 2007;383(1–2):133–9.
Li CJ, Li L, Lin L, Jiang HX, Zhong ZY, Li WM, et al. Impact of the CYP3A5, CYP3A4, COMT, IL-10 and POR genetic polymorphisms on tacrolimus metabolism in Chinese renal transplant recipients. PLoS ONE. 2014;9(1):e86206.
Genvigir F, Nishikawa AM, Felipe CR, Tedesco-Silva H Jr, Oliveira N, Salazar A, et al. Influence of ABCC2, CYP2C8, and CYP2J2 polymorphisms on tacrolimus and mycophenolate sodium-based treatment in brazilian kidney transplant recipients. Pharmacotherapy. 2017;37(5):535–45.
Deng R, Liao Y, Li Y, Tang J. Association of CYP3A5, CYP2C8, and ABCB1 polymorphisms with early renal injury in chinese liver transplant recipients receiving tacrolimus. Transplant Proc. 2018;50(10):3258–65.
Hu R, Barratt DT, Coller JK, Sallustio BC, Somogyi AA. CYP3A5*3 and ABCB1 61A>G significantly influence dose-adjusted trough blood tacrolimus concentrations in the first three months post-kidney transplantation. Basic Clin Pharmacol Toxicol. 2018;123(3):320–6.
Provenzani A, Santeusanio A, Mathis E, Notarbartolo M, Labbozzetta M, Poma P, et al. Pharmacogenetic considerations for optimizing tacrolimus dosing in liver and kidney transplant patients. World J Gastroenterol. 2013;19(48):9156–73.
Ponticelli C, Arnaboldi L, Moroni G, Corsini A. Treatment of dyslipidemia in kidney transplantation. Expert Opin Drug Saf. 2020;19(3):257–67.
Degraeve AL, Moudio S, Haufroid V, Chaib Eddour D, Mourad M, Bindels LB, et al. Predictors of tacrolimus pharmacokinetic variability: current evidences and future perspectives. Expert Opin Drug Metab Toxicol. 2020;16(9):769–82.
Yamada T, Zhang M, Masuda S. Significance of ethnic factors in immunosuppressive therapy management after organ transplantation. Ther Drug Monit. 2020;42(3):369–80.
Jouve T, Noble J, Rostaing L, Malvezzi P. An update on the safety of tacrolimus in kidney transplant recipients, with a focus on tacrolimus minimization. Expert Opin Drug Saf. 2019;18(4):285–94.
Schutte-Nutgen K, Tholking G, Suwelack B, Reuter S. Tacrolimus—pharmacokinetic considerations for clinicians. Curr Drug Metab. 2018;19(4):342–50.
Thölking G, Reuter S. Commentary: the clinical impact of the C0/D ratio and the CYP3A5 genotype on outcome in tacrolimus treated kidney transplant recipients. Front Pharmacol. 2021;12:603345.
Buendía JA, Halac E, Bosaleh A, Garcia de Davila MT, Imvertasa O, Bramuglia G. Frequency of CYP3A5 genetic polymorphisms and tacrolimus pharmacokinetics in pediatric liver transplantation. pharmaceutics. 2020;12(9):898–905.
Zhang M, Tajima S, Shigematsu T, Fu R, Noguchi H, Kaku K, et al. Donor CYP3A5 gene polymorphism alone cannot predict tacrolimus intrarenal concentration in renal transplant recipients. Int J Mol Sci. 2020;21(8):2976–87.
Mendrinou E, Mashaly ME, Al Okily AM, Mohamed ME, Refaie AF, Elsawy EM, et al. CYP3A5 gene-guided tacrolimus treatment of living-donor Egyptian kidney transplanted patients. Front Pharmacol. 2020;11:1218.
Suetsugu K, Mori Y, Yamamoto N, Shigematsu T, Miyamoto T, Egashira N, et al. Impact of CYP3A5, POR, and CYP2C19 polymorphisms on trough concentration to dose ratio of tacrolimus in allogeneic hematopoietic stem cell transplantation. Int J Mol Sci. 2019;20(10):2413–29.
Nakamura T, Fukuda M, Matsukane R, Suetsugu K, Harada N, Yoshizumi T, et al. Influence of POR*28 polymorphisms on CYP3A5*3-associated variations in tacrolimus blood levels at an early stage after liver transplantation. Int J Mol Sci. 2020;21(7):2287–304.
Ling J, Dong LL, Yang XP, Qian Q, Jiang Y, Zou SL, et al. Effects of CYP3A5, ABCB1 and POR*28 polymorphisms on pharmacokinetics of tacrolimus in the early period after renal transplantation. Xenobiotica. 2020;50(12):1501–9.
Chang WS, Liao CH, Tsai CW, Hu PS, Wu HC, Hsu SW, et al. The role of IL-10 promoter polymorphisms in renal cell carcinoma. Anticancer Res. 2016;36(5):2205–9.
Thakkinstian A, Dmitrienko S, Gerbase-Delima M, McDaniel DO, Inigo P, Chow KM, et al. Association between cytokine gene polymorphisms and outcomes in renal transplantation: a meta-analysis of individual patient data. Nephrol Dial Transplant. 2008;23(9):3017–23.
Xiong J, Wang Y, Zhang Y, Nie L, Wang D, Huang Y, et al. Lack of association between interleukin-10 gene polymorphisms and graft rejection risk in kidney transplantation recipients: a meta-analysis. PLoS ONE. 2015;10(6):e0127540.
Mu HJ, Xie P, Chen JY, Gao F, Zou J, Zhang J, et al. Association of TNF-α, TGF-β1, IL-10, IL-6, and IFN-γ gene polymorphism with acute rejection and infection in lung transplant recipients. Clin Transplant. 2014;28(9):1016–24.
Bogacz A, Polaszewska A, Bartkowiak-Wieczorek J, Tejchman K, Dziewanowski K, Ostrowski M, et al. The effect of genetic variations for interleukin-10 (IL-10) on the efficacy of immunosuppressive therapy in patients after kidney transplantation. Int Immunopharmacol. 2020;89(Pt A):107059.
Gorski JC, Hall SD, Becker P, Affrime MB, Cutler DL, Haehner-Daniels B. In vivo effects of interleukin-10 on human cytochrome P450 activity. Clin Pharmacol Ther. 2000;67(1):32–43.
Oetting WS, Wu B, Schladt DP, Guan W, Remmel RP, Dorr C, et al. Attempted validation of 44 reported SNPs associated with tacrolimus troughs in a cohort of kidney allograft recipients. Pharmacogenomics. 2018;19(3):175–84.
Iwasaki K. Metabolism of tacrolimus (FK506) and recent topics in clinical pharmacokinetics. Drug Metab Pharmacokinet. 2007;22(5):328–35.
Zhang T, Liu Y, Zeng R, Ling Q, Wen P, Fan J, et al. Association of donor small ubiquitin-like modifier 4 rs237025 genetic variant with tacrolimus elimination in the early period after liver transplantation. Liver Int. 2018;38(4):724–32.
Ou B, Liu Y, Zhang T, Sun Y, Chen J, Peng Z. TLR9 rs352139 genetic variant promotes tacrolimus elimination in chinese liver transplant patients during the early posttransplantation period. Pharmacotherapy. 2019;39(1):67–76.
Elens L, van Schaik RH, Panin N, de Meyer M, Wallemacq P, Lison D, et al. Effect of a new functional CYP3A4 polymorphism on calcineurin inhibitors’ dose requirements and trough blood levels in stable renal transplant patients. Pharmacogenomics. 2011;12(10):1383–96.
Pallet N, Jannot AS, El Bahri M, Etienne I, Buchler M, de Ligny BH, et al. Kidney transplant recipients carrying the CYP3A4*22 allelic variant have reduced tacrolimus clearance and often reach supratherapeutic tacrolimus concentrations. Am J Transplant. 2015;15(3):800–5.
Acknowledgements
This work was supported by the China Post doctoral Science Foundation (NO: 2019M662207), Anhui Natural Science Fund Project (1908085QH363).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
We confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: Due to an error in the affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, Z., Cheng, X., Zhang, L. et al. The impact of IL-10 and CYP3A5 gene polymorphisms on dose-adjusted trough blood tacrolimus concentrations in early post-renal transplant recipients. Pharmacol. Rep 73, 1418–1426 (2021). https://doi.org/10.1007/s43440-021-00288-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43440-021-00288-2