Abstract
Background
Hyperactivation of blood platelets is an essential factor in the pathomechanism of diabetes-evoked angiopathies. The aim of this work was to investigate whether blood platelets hyperactivation resulting from type 2 diabetic hyperglycaemia-increased pyruvate dehydrogenase complex activity and excessive acetyl-CoA accumulation may be brought to the normal range by the enzyme inhibitors.
Methods
Platelets were isolated from the blood of 9 type 2 diabetic patients and 10 healthy donors. Effects of 3-bromopyruvate and 3-nitropropionate on pyruvate dehydrogenase complex (PDHC) and succinate dehydrogenase activities, as well as levels of acetyl-CoA, ATP, thiobarbituric acid reactive species and aggregation were assessed in non-activated and thrombin-activated platelets.
Results
In type 2 diabetic patients fasting plasma glucose and fructosamine levels were 61 and 64% higher, respectively, than in the healthy group (p < 0.001). In non-activated diabetic platelets PDHC activity, PDHC-E2, acetyl-CoA and ATP levels were 66, 70, 68 and 60%, higher, respectively, than in platelets from healthy controls (p < 0.01). 3-bromopyruvate (0.1 mM) decreased pyruvate dehydrogenase activity in healthy and diabetic platelets by 42% and 59%, respectively. Similar inhibitory effects were observed for acetyl-CoA and ATP levels, aggregation and TBARS accumulation rates. Succinate dehydrogenase activity was inhibited by 3-nitropropionate (10 mM) to 38 and 41% of control values in healthy and diabetic platelets, respectively, but affected neither function nor acetyl-CoA metabolism in platelets of both groups.
Conclusions
These data indicate that inhibition of pyruvate dehydrogenase excessive activity in diabetic platelets by 3-bromopyruvate may normalise their functional parameters through adjustment of acetyl-CoA/ATP levels to control values.
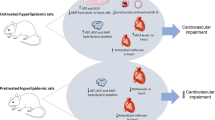
Graphic abstract
Platelets from blood of diabetic patients display higher activities of pyruvate dehydrogenase complex (PDHC), higher levels of dihydrolipoate transacetylase (DLAT, E2 subunit of PDHC) as well as higher levels of acetyl-CoA yielding greater ATP/ADP accumulation than in platelets of normoglycemic subjects. Therefore, in diabetic platelets, thrombin caused higher release of ATP/ADP triggering excessive production of reactive oxygen species (ROS) and stronger aggregation compared to control platelets. In diabetic platelets, relative excess of DLAT in PDHC made them highly susceptible to 3-bromopyruvate (3BrP) inhibition. Resulting limitation of acetyl-CoA provision by 3-BrP normalised activity of diabetic platelets.







Similar content being viewed by others
Abbreviations
- 3-BrP:
-
3-Bromopyruvate
- 3-NP:
-
3-Nitropropionic acid
- PDHC:
-
Pyruvate dehydrogenase complex
- TBARS:
-
Thiobarbituric acid reactive species
- TCA:
-
Tricarboxylic acid cycle
References
Vazzana N, Ranalli P, Cuccurullo Ch, Davì G. Diabetes mellitus and thrombosis. Thromb Res. 2012;129:371–7.
Kakouros N, Rade J, Kourliouros A, Resar J. Platelet function in patients with diabetes mellitus: from a theoretical to a practical perspective. Int J Endocrinol. 2011;2011:1–14.
Keating FK, Whitaker DA, Sobel BE, Schneider DJ. Augmentation of inhibitory effects of glycoprotein IIb-IIIa antagonists in patients with diabetes. Thromb Res. 2004;113:27–34.
Liani R, Halvorsen B, Sestili S, Handberg A, Santilli F, Vazzana N, et al. Plasma levels of soluble CD36, platelet activation, inflammation and oxidative stress are increased in type 2 diabetic patients. Free Radic Biol Med. 2012;14:1–7.
Thaning P, Bune L, Hellsten Y, Pilegaard H, Saltin B, Rosenmmeier B. Attentuated purinergic receptor function in patients with type 2 diabetes. Diabetes. 2010;59:182–9.
Xia W, Li Y, Wang B, Chen J, Wang X, Sun Q, et al. Enhanced store-operated calcium entry in platelets is associated with peripheral artery disease in type 2 diabetes. Cell Physiol Biochem. 2015;37:1945–55.
Michno A, Skibowska A, Raszeja-Specht A, Cwikowska J, Szutowicz A. The role of adenosine triphosphate- citrate lyase in the metabolism of acetyl-CoA and function of blood platelets in diabetes mellitus. Metabolism. 2004;53:66–72.
Michno A, Raszeja-Specht A, Jankowska-Kulawy A, Pawełczyk T, Szutowicz A. Effect of L-carnitine on acetyl-CoA content and activity of blood platelets in healthy and diabetic persons. Clin Chem. 2005;51:1673–82.
Michno A, Bielarczyk H, Pawełczyk T, Jankowska-Kulawy A, Klimaszewska A, Szutowicz A. Alterations of adenine nucleotide metabolism and function of blood platelets in patients with diabetes. Diabetes. 2007;56:462–7.
Karunarathne W, Ku CJ, Spence DM. The dual nature of extracellular ATP as a concentration-dependent platelet P2X1 agonist and antagonist. Integr Biol. 2009;1:655–63.
Ghoshal K, Bhattacharyya M (2014) Overview of platelet physiology: its hemostatic and nonhemostatic role in disease pathogenesis. Sci World J 2014: Article ID 781857
Storey RF. Pharmacology and clinical trials of reversibly-binding P2Y12 inhibitors. Thromb Haemost. 2011;105(suppl. 1):75–81.
Blache D, Bourdon E, Salloigon P, Lucci G, Ducoroy P, Petit JM, et al. Glycated albumin with loss fatty acid binding capacity contributes to enhanced arachidonate oxygenation and platelet hyperactivity: relevance in patients with type 2 diabetes. Diabetes. 2015;64:960–72.
Craik JD, Stewart M, Cheeseman C. GLUT-3 (brain-type) glucose transporter polypeptides in human blood platelets. Thromb Res. 1995;79:461–9.
Lowe PN, Perham RN. Bromopyruvate as an active-site directed inhibitor of the pyruvate dehydrogenase multienzyme complex from Escherichia coli. Biochemistry. 1984;23:91–7.
Burton MD, Nouri M, Kazemi H. Acetylcholine and central respiratory control: perturbations of acetylcholine synthesis in the isolated brainstem of the neonatal rat. Brain Res. 1995;670:39–47.
Vali M, Liapi E, Kowalski J, Hong K, Khwaja A, Torbenson MS, et al. Intra-arterial therapy with a new potent inhibitor of tumor metabolism (3-bromopyruvate): identification of therapeutic dose and method of injection in an animal model of liver cancer. J Vasc Interv Radiol. 2007;18:95–101.
Apfel MA, Ikeda BH, Speckhard DC, Frey PA. Escherichia coli pyruvate dehydrogenase complex. Thiamin pyrophosphate-dependent inactivation by 3-bromopyruvate. J Biol Chem. 1984;10:2905–9.
Bielarczyk H, Szutowicz A. Evidence for the regulatory function of synaptoplasmic acetyl-CoA in acetylcholine synthesis in nerve endings. Biochem J. 1989;262:377–80.
Dutschke K, Nitsch RM, Hoyer S. Short-term mental activation accelerates the age-related decline of high-energy phosphates in rat cerebral cortex. Arch Gerontol Geriatr. 1994;19:43–51.
Ricny J, Tucek S. Acetyl coenzyme A and acetylcholine in slices of rat caudate nuclei incubated in the presence of metabolic inhibitors. J Biol Chem. 1981;256:4919–23.
Dell’Antone P. Targets of 3-bromopyruvate, a new, energy depleting, anticancer agent. Med Chem. 2009;5:491–6.
Cradci S, Desideri E, Ciriolo MR. Targeting aerobic glycolysis: 3-bromopyruvate as a promising anticancer drug. J Bioenerg Biomembr. 2012;44:17–29.
Galina A. Mitochondria: 3-bromopyruvate vs. mitochondria? A small molecule that attacs tumors by targeting their bioenergetic diversity. Int J Biochem Cell Biol. 2014;54:266–71.
El Sayed SM, Abou El-Magd RM, Shishido Y, Chung SP, Diem TH, Sakai T, et al. 3-Bromopyruvate antagonizes effects of lactate and pyruvate, synergizes with citrate and exerts novel anti-glioma effects. J Bioenerg Biomembr. 2012;44:61–79.
Huang LS, Sun G, Cobessi D, Wang AC, Shen JT, Tung EY, et al. 3-nitropropionic acid is a suicide inhibitor of mitochondrial respiration that, upon oxidation by complex II, forms a covalent adduct with a catalytic base arginine in the active site of the enzyme. J Biol Chem. 2006;281:5965–72.
Pass MA, Carlisle CH, Reuhl KR. 3-nitropropionic acid toxicity in cultured murine embryonal carcinoma cells. Nat Toxins. 1994;2:386–94.
Szutowicz A, Stępień M, Piec G. Determination of pyruvate dehydrogenase and acetyl-CoA synthetase activities using citrate synthase. Anal Biochem. 1981;115:81–7.
Glock GE, McLean P. Further studies on the properties and assay of glucose 6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase of rat liver. Biochem J. 1953;55:400–8.
Pawełczyk T, Angielski S. Cooperation of Ca and pH in regulation of the activity of the 2-oxoglutarate dehydrogenase complex and its components from bovine kidney cortex. Acta Biochim Pol. 1984;3:289–305.
De Villafranca GW, Haines VE. Paramyosin from arthropod cross-striated muscle. Comp Biochem Physiol. 1974;47:9–26.
Veeger C, Vartanian Der, Zeylemeker WP. Succinate dehydrogenase. In: Lowenstein JM, editor. Methods in enzymol, vol. 13. New York: Academic Press; 1969. p. 106–16.
Gorman MW, Marble DR, Ogimoto K, Feigl EO. Measurement of adenine nucleotides in plasma. Luminescence. 2003;18:173–81.
Panse M, Black H, Fuster W, Mest H. An improved malonyl dialdehyde assay for estimation of thromboxane synthetase activity in washed human blood platelets. Prostaglandins. 1985;30:1031–8.
Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54.
Ferroni P, Basili A, Davi G. Platelet activation in type 2 diabetes mellitus. J Thromb Haemost. 2004;2:1282–91.
Guo X, Wu J, Du J, Ran J, Xu J. Platelets of type 2 diabetic patients are characterized by high ATP content and low mitochondrial membrane potential. Platelets. 2009;20:588–93.
Jedlitschky G, Cattaneo M, Lubenow LE, Riosskopf D, Lecchi A, Artoni A, et al. Role of MRP4 (ABCC4) in platelet adenine nucleotide storage. Evidence from patients with delta-storage pool deficiencies. Am J Pathol. 2010;176:1097–103.
Akkerman JWN, Rijksen G, Gorter G, Staal GEJ. Platelet functions and energy metabolism in a patient with hexokinase deficiency. Blood. 1984;63:147–53.
Winocour PD. Platelet abnormalities in diabetes mellitus. Diabetes. 1992;41:26–31.
Winocour PD. Platelet turnover in advanced diabetes. Eur J Clin Invest. 1994;24:34–7.
Szutowicz A, Tomaszewicz M, Jankowska A, Kisielevski Y. Acetylcholine synthesis in nerve terminals of diabetic rats. NeuroReport. 1994;5:2421–4.
Umegaki H. Neurodegeneration in diabetes mellitus. Adv Exp Med Biol. 2012;65:724–5.
Acknowledgements
This work was supported by Medical University of Gdansk projects, ST-57, MN0116.
Author information
Authors and Affiliations
Contributions
AM was responsible for conceptualization, validation, investigation, data curation, formal analysis, writing, supervision and project administration. KG was responsible for investigation and resources. HB was responsible for data curation, validation, and funding acquistation. MZ was resoponsible for statistical analysis. AS was responsible for conceptualization, formal analysis , writing and supervision.
Corresponding author
Ethics declarations
Conflict of interest
All the co-authors of the manuscript contributed to the presented manuscript. There are no circumstances that present a potential conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Michno, A., Grużewska, K., Bielarczyk, H. et al. Inhibition of pyruvate dehydrogenase complex activity by 3-bromopyruvate affects blood platelets responses in type 2 diabetes. Pharmacol. Rep 72, 225–237 (2020). https://doi.org/10.1007/s43440-019-00005-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43440-019-00005-0