Abstract
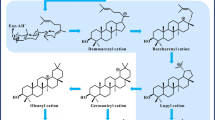
Betulinic acid (BA), a penta-cyclic triterpenoid found as a ubiquitous secondary metabolite throughout the plant kingdom, has aroused tremendous interests due to its different pharmacological properties, which lead to large market demand. However, the content of BA in plant is low for phytoextraction. Although chemical semi-synthesis or biotransformation of BA from betulin with high conversion efficiency is achieved, it still relies on phytoextraction from the bark of medicinal trees. To circumvent this issue, the biotechnological synthesis of BA in engineered yeasts has been developed. In this review, the pharmacological properties of BA are first summarized, including antitumor, anti-HIV, antiprotozoal, anti-inflammatory, apoptosis activator and anti-metabolic syndrome. Then, the traditional phytoextraction, semi-synthesis and biotechnological synthesis of BA are discussed. Particularly, current advances in its biotechnological synthesis and strategies to improve BA production are focused. Moreover, potential strategies for further promotion of BA yield, including the introduction of artificial isopentenol utilization pathway, semi-rational mutagenesis of lupeol synthase and cytochrome P450, and subcellular morphology and compartmentalization, are discussed.








Similar content being viewed by others
References
Xiao SL, Tian ZY, Wang YF, Si LL, Zhang LH, Zhou DM. Recent progress in the antiviral activity and mechanism study of pentacyclic triterpenoids and their derivatives. Med Res Rev. 2018;38(3):951–76. https://doi.org/10.1002/med.21484.
Czarnotta E, Dianat M, Korf M, Granica F, Merz J, Maury J, et al. Fermentation and purification strategies for the production of betulinic acid and its lupane-type precursors in Saccharomyces cerevisiae. Biotechnol Bioeng. 2017;114(11):2528–38. https://doi.org/10.1002/bit.26377.
Retzlaff F. Ueber herba gratiolae. Arch Pharm. 1902;240(8):561–8. https://doi.org/10.1002/ardp.19022400802.
Chen GG, Lai P. Targeting cancer by betulin and betulinic acid. Dordrecht: Springer; 2012.
Zuco V, Supino R, Righetti SC, Cleris L, Formelli F. Selective cytotoxicity of betulinic acid on tumor cell lines, but not on normal cells. Cancer Lett. 2002;175(1):17–25. https://doi.org/10.1016/S0304-3835(01)00718-2.
Zhao Y, Chen CH, Morris-Natschke SL, Lee KH. Design, synthesis, and structure activity relationship analysis of new betulinic acid derivatives as potent HIV inhibitors. Eur J Med Chem. 2021;215: 113287. https://doi.org/10.1016/j.ejmech.2021.113287.
Cichewicz RH, Kouzi SA. Chemistry, biological activity, and chemotherapeutic potential of betulinic acid for the prevention and treatment of cancer and HIV infection. Med Res Rev. 2004;24(1):90–114. https://doi.org/10.1002/med.10053.
Cunha AB, Batista R, Castro MA, David JM. Chemical strategies towards the synthesis of betulinic acid and its more potent antiprotozoal analogues. Molecules. 2021;26(4):1081. https://doi.org/10.3390/molecules26041081.
Ou ZP, Zhao J, Zhu LJ, Huang L, Ma YR, Ma CY, et al. Anti-inflammatory effect and potential mechanism of betulinic acid on λ-carrageenan-induced paw edema in mice. Biomed Pharmacother. 2019;118: 109347. https://doi.org/10.1016/j.biopha.2019.109347.
Laavola M, Haavikko R, Hamalainen M, Leppanen T, Nieminen R, Alakurtti S, et al. Betulin derivatives effectively suppress inflammation in vitro and in vivo. J Nat Prod. 2016;79(2):274–80. https://doi.org/10.1021/acs.jnatprod.5b00709.
Kumar P, Bhadauria AS, Singh AK, Saha S. Betulinic acid as apoptosis activator: molecular mechanisms, mathematical modeling and chemical modifications. Life Sci. 2018;209:24–33. https://doi.org/10.1016/j.lfs.2018.07.056.
Kim J, Lee YS, Kim CS, Kim JS. Betulinic acid has an inhibitory effect on pancreatic lipase and induces adipocyte lipolysis. Phytother Res. 2012;26(7):1103–6. https://doi.org/10.1002/ptr.3672.
Trumbull ER, Bianchi E, Eckert DJ, Wiedhopf RM, Cole JR. Tumor inhibitory agents from Vauquelinia corymbosa (Rosaceae). J Pharm Sci US. 2010;65(9):1407–8. https://doi.org/10.1002/jps.2600650938.
Pisha E, Chai H, Lee I-S, Chagwedera TE, Farnsworth NR, Cordell GA, et al. Discovery of betulinic acid as a selective inhibitor of human melanoma that functions by induction of apoptosis. Nat Med. 1995;1(10):1046–51. https://doi.org/10.1038/nm1095-1046.
Coricovac D, Dehelean CA, Pinzaru I, Mioc A, Aburel OM, Macasoi I, et al. Assessment of betulinic acid cytotoxicity and mitochondrial metabolism impairment in a human melanoma cell line. Int J Mol Sci. 2021;22(9):4870. https://doi.org/10.3390/ijms22094870.
Chiu CF, Chang HY, Huang CY, Mau CZ, Kuo TT, Lee HC, et al. Betulinic acid affects the energy-related proteomic profiling in pancreatic ductal adenocarcinoma cells. Molecules. 2021;26(9):2482. https://doi.org/10.3390/molecules26092482.
Chintharlapalli S, Papineni S, Ramaiah SK, Safe S. Betulinic acid inhibits prostate cancer growth through inhibition of specificity protein transcription factors. Cancer Res. 2007;67(6):2816–23. https://doi.org/10.1158/0008-5472.CAN-06-3735.
Lee J-W, Choi Y-J, Kim S-I, Lee S-Y, Kang S-S, Kim N-H, et al. Betulinic acid inhibits LPS-induced MMP-9 expression by suppressing NF-kB activation in BV2 microglial cells. Biomol Ther. 2011;19(4):431–7. https://doi.org/10.4062/biomolther.2011.19.4.431.
Fujioka T, Kashiwada Y, Kashiwada RE, Cosentino LM, Ballas LM, Jiang JB, et al. Anti-AIDS agents, 11. Betulinic acid and platanic acid as anti-HIV principles from Syzigium claviflorum, and the anti-HIV activity of structurally related triterpenoids. J Nat Prod. 1994;57(2):243–7. https://doi.org/10.1021/np50104a008.
Domínguez-Carmona DB, Escalante-Erosa F, García-Sosa K, Ruiz-Pinell G, Gutierrez-Yapu D, Chan-Bacab MJ, et al. Antiprotozoal activity of betulinic acid derivatives. Phytomedicine. 2010;17(5):379–82. https://doi.org/10.1016/j.phymed.2009.08.002.
Amiri S, Dastghaib S, Ahmadi M, Mehrbod P, Khadem F, Behrouj H, et al. Betulin and its derivatives as novel compounds with different pharmacological effects. Biotechnol Adv. 2020;38: 107409. https://doi.org/10.1016/j.biotechadv.2019.06.008.
Bildziukevich U, Özdemir Z, Wimmer Z. Recent achievements in medicinal and supramolecular chemistry of betulinic acid and its derivatives. Molecules. 2019;24(19):3546. https://doi.org/10.3390/molecules24193546.
Suresh C, Zhao H, Gumbs A, Chetty CS, Bose HS. New ionic derivatives of betulinic acid as highly potent anti-cancer agents. Bioorg Med Chem Lett. 2012;22(4):1734–8. https://doi.org/10.1016/j.bmcl.2011.12.102.
Zhang X, Hu JY, Chen Y. Betulinic acid and the pharmacological effects of tumor suppression (Review). Mol Med Rep. 2016;14(5):4489–95. https://doi.org/10.3892/mmr.2016.5792.
Sousa JLC, Freire CSR, Silvestre AJD, Silva AMS. Recent developments in the functionalization of betulinic acid and its natural analogues: a route to new bioactive compounds. Molecules. 2019;24(2):355. https://doi.org/10.3390/molecules24020355.
Hordyjewska A, Ostapiuk A, Horecka A, Kurzepa J. Betulin and betulinic acid: triterpenoids derivatives with a powerful biological potential. Phytochem Rev. 2019;18(3):929–51. https://doi.org/10.1007/s11101-019-09623-1.
Ali-Seyed M, Jantan I, Vijayaraghavan K, Bukhari SN. Betulinic acid: recent advances in chemical modifications, effective delivery, and molecular mechanisms of a promising anticancer therapy. Chem Biol Drug Des. 2016;87(4):517–36. https://doi.org/10.1111/cbdd.12682.
Mueller D, Triebel S, Rudakovski O, Richling E. Influence of triterpenoids present in apple peel on inflammatory gene expression associated with inflammatory bowel disease (IBD). Food Chem. 2013;139(1–4):339–46. https://doi.org/10.1016/j.foodchem.2013.01.101.
Pai SR, Joshi RK. Distribution of betulinic acid in plant kingdom. Plant Science Today. 2014;1(3):103–7. https://doi.org/10.14719/pst.2014.1.3.58.
Pezzuto JM, Kim D. Improved methods of manufacturing betulinic acid [P]. WO9843936. 1998. October 8.
Stork G, Uyeo S, Wakamatsu T, Grieco PA, Labovitz J. Total synthesis of lupeol. J Am Chem Soc. 1971;93(19):4945–7. https://doi.org/10.1021/ja00748a068.
Surendra K, Corey EJ. A short enantioselective total synthesis of the fundamental pentacyclic triterpene lupeol. J Am Chem Soc. 2009;131(39):13928–9. https://doi.org/10.1021/ja906335u.
Co M, Koskela P, Eklund-Åkergren P, Srinivas K, King JW, Sjöberg PJR, et al. Pressurized liquid extraction of betulin and antioxidants from birch bark. Green Chem. 2009;11(5):668–74. https://doi.org/10.1039/b819965e.
Kim DSHI, Chen ZD, Nguyen VT, Pezzuto JM, Qiu SH, Lu ZZ. A concise semi-synthetic approach to betulinic acid from betulin. Synth Commun. 1997;27(9):1607–12. https://doi.org/10.1080/00397919708006099.
Ressmann AK, Kremsmayr T, Gaertner P, Zirbs R, Bica K. Toward a benign strategy for the manufacturing of betulinic acid. Green Chem. 2017;19(4):1014–22. https://doi.org/10.1039/c6gc02641a.
Csuk R, Schmuck K, Schäfer R. A practical synthesis of betulinic acid. Tetrahedron Lett. 2006;47(49):8769–70. https://doi.org/10.1016/j.tetlet.2006.10.004.
Kumar D, Dubey KK. An efficient process for the transformation of betulin to betulinic acid by a strain of Bacillus megaterium. 3 Biotech. 2017;7(3):157. https://doi.org/10.1007/s13205-017-0759-9.
Wu JN, Niu YW, Bakur A, Li H, Chen QH. Cell-free production of pentacyclic triterpenoid compound betulinic acid from betulin by the engineered Saccharomyces cerevisiae. Molecules. 2017;22(7):1075. https://doi.org/10.3390/molecules22071075.
Puder CH, Graef H, Thumerer MJ, Heitzmann M, Process for the extraction of betulinic acid [P]. US20070149490A1. 2007. June 28.
Kim J, Baidoo EEK, Amer B, Mukhopadhyay A, Adams PD, Simmons BA, et al. Engineering Saccharomyces cerevisiae for isoprenol production. Metab Eng. 2021;64:154–66. https://doi.org/10.1016/j.ymben.2021.02.002.
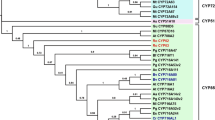
Huang JJ, Zha WL, An TY, Dong H, Huang Y, Wang D, et al. Identification of RoCYP01 (CYP716A155) enables construction of engineered yeast for high-yield production of betulinic acid. Appl Microbiol Biotechnol. 2019;103(17):7029–39. https://doi.org/10.1007/s00253-019-10004-z.
Yu Y, Rasool A, Liu HR, Lv B, Chang PC, Song H, et al. Engineering Saccharomyces cerevisiae for high yield production of α-amyrin via synergistic remodeling of α-amyrin synthase and expanding the storage pool. Metab Eng. 2020;62:72–83. https://doi.org/10.1016/j.ymben.2020.08.010.
Gowers GOF, Chee SM, Bell D, Suckling L, Kern M, Tew D, et al. Improved betulinic acid biosynthesis using synthetic yeast chromosome recombination and semi-automated rapid LC-MS screening. Nat Commun. 2020;11(1):868. https://doi.org/10.1038/s41467-020-14708-z.
Wang D, Liu Y, Xu J-Y, Wang JH, Dai ZB, Zhang XL, et al. Construction of efficient yeast cell factories for production of ginsenosides precursor dammarenediol-II. Acta Pharm Sin B. 2018;53(8):1233–41. https://doi.org/10.16438/j.0513-4870.2018-0503.
Mcgarvey DJ, Croteau R. Terpenoid metabolism. Plant Cell. 1995;7(7):1015–26. https://doi.org/10.1105/tpc.7.7.1015.
Zhu ZT, Du MM, Gao B, Tao XY, Zhao M, Ren YH, et al. Metabolic compartmentalization in yeast mitochondria: burden and solution for squalene overproduction. Metab Eng. 2021;68:232–45. https://doi.org/10.1016/j.ymben.2021.10.011.
Zhao YJ, Fan JJ, Wang C, Feng XD, Li C. Enhancing oleanolic acid production in engineered Saccharomyces cerevisiae. Bioresour Technol. 2018;257:339–43. https://doi.org/10.1016/j.biortech.2018.02.096.
Abe I, Rohmer M, Prestwich GD. Enzymatic cyclization of squalene and oxidosqualene to sterols and triterpenes. Chem Rev. 1993;93(6):2189–206. https://doi.org/10.1021/cr00022a009.
Suzuki H, Fukushima EO, Umemoto N, Ohyama K, Seki H, Muranaka T. Comparative analysis of CYP716A subfamily enzymes for the heterologous production of C-28 oxidized triterpenoids in transgenic yeast. Plant Biotechnol. 2018;35(2):131–9. https://doi.org/10.5511/plantbiotechnology.18.0416a.
Husselstein-Muller T, Schaller H, Benveniste P. Molecular cloning and expression in yeast of 2,3-oxidosqualene-triterpenoid cyclases from Arabidopsis thaliana. Plant Mol Biol. 2001;45:75–92. https://doi.org/10.1023/A:1006476123930.
Fukushima EO, Hikaru S, Kiyoshi O, Eiichiro O, Naoyuki U, Masaharu M, et al. CYP716A subfamily members are multifunctional oxidases in triterpenoid biosynthesis. Plant Cell Physiol. 2011;52(12):2050–61. https://doi.org/10.1093/pcp/pcr146.
Huang LL, Li J, Ye HC, Li CF, Wang H, Liu BY, et al. Molecular characterization of the pentacyclic triterpenoid biosynthetic pathway in Catharanthus roseus. Planta. 2012;236(5):1571–81. https://doi.org/10.1007/s00425-012-1712-0.
Sun J, Zhang CB, Nan WH, Li DS, Ke D, Lu WY. Glycerol improves heterologous biosynthesis of betulinic acid in engineered Yarrowia lipolytica. Chem Eng Sci. 2019;196:82–90. https://doi.org/10.1016/j.ces.2018.10.052.
Li J, Zhang YS. Increase of betulinic acid production in Saccharomyces cerevisiae by balancing fatty acids and betulinic acid forming pathways. Appl Microbiol Biotechnol. 2014;98(7):3081–9. https://doi.org/10.1007/s00253-013-5461-1.
Jin CC, Zhang JL, Song H, Cao YX. Boosting the biosynthesis of betulinic acid and related triterpenoids in Yarrowia lipolytica via multimodular metabolic engineering. Microb Cell Fact. 2019;18(1):77. https://doi.org/10.1186/s12934-019-1127-8.
Li J, Zhang Y. Modulating betulinic acid production in Saccharomyces cerevisiae by managing the intracellular supplies of the co-factor NADPH and oxygen. J Biosci Bioeng. 2015;119(1):77–81. https://doi.org/10.1016/j.jbiosc.2014.06.013.
Qiao WB, Zhou ZL, Liang Q, Mosongo I, Li CF, Zhang YS. Improving lupeol production in yeast by recruiting pathway genes from different organisms. Sci Rep. 2019;9:2992. https://doi.org/10.1038/s41598-019-39497-4.
Zhou C, Li J, Li CF, Zhang YS. Improvement of betulinic acid biosynthesis in yeast employing multiple strategies. BMC Biotechnol. 2016;16(1):59. https://doi.org/10.1186/s12896-016-0290-9.
Dai ZB, Liu Y, Zhang XN, Shi MY, Wang BB, Wang D, et al. Metabolic engineering of Saccharomyces cerevisiae for production of ginsenosides. Metab Eng. 2013;20:146–56. https://doi.org/10.1016/j.ymben.2013.10.004.
Dai ZB, Wang BB, Liu Y, Shi MY, Wang D, Zhang XN, et al. Producing aglycons of ginsenosides in bakers’ yeast. Sci Rep. 2014;4:3698. https://doi.org/10.1038/srep03698.
Burg JS, Espenshade PJ. Regulation of HMG-CoA reductase in mammals and yeast. Prog Lipid Res. 2011;50(4):403–10. https://doi.org/10.1016/j.plipres.2011.07.002.
Huang Y-Y, Jian X-X, Lv Y-B, Nian K-Q, Gao Q, Chen J, et al. Enhanced squalene biosynthesis in Yarrowia lipolytica based on metabolically engineered acetyl-CoA metabolism. J Biotechnol. 2018;281:106–14. https://doi.org/10.1016/j.jbiotec.2018.07.001.
Shi YS, Wang D, Li RS, Huang LQ, Dai ZB, Zhang XL. Engineering yeast subcellular compartments for increased production of the lipophilic natural products ginsenosides. Metab Eng. 2021;67:104–11. https://doi.org/10.1016/j.ymben.2021.06.002.
Vickers CE, Williams TC, Peng B, Cherry J. Recent advances in synthetic biology for engineering isoprenoid production in yeast. Curr Opin Chem Biol. 2017;40:47–56. https://doi.org/10.1016/j.cbpa.2017.05.017.
Herrera JBR, Bartel B, Wilson WK, Matsuda SPT. Cloning and characterization of the Arabidopsis thaliana lupeol synthase gene. Phytochemistry. 1998;49(7):1905–11. https://doi.org/10.1016/S0031-9422(98)00366-5.
Shibuya M, Zhang H, Endo A, Shishikura K, Kushiro T, Ebizuka Y. Two branches of the lupeol synthase gene in the molecular evolution of plant oxidosqualene cyclases. Eur J Biochem. 1999;266(1):302–7. https://doi.org/10.1046/j.1432-1327.1999.00875.x.
Nguyen T-D, MacNevin G, Ro D-K. De novo synthesis of high-value plant sesquiterpenoids in yeast. Method Enzymol. 2012;517:261–78. https://doi.org/10.1016/B978-0-12-404634-4.00013-9.
Kushiro T, Shibuya M, Ebizuka Y. Cryptic regiospecificity in deprotonation step of triterpene biosynthesis catalyzed by new members of lupeol synthase. Tetrahedron Lett. 1999;40(30):5553–6. https://doi.org/10.1016/S0040-4039(99)01035-7.
Spanova M, Zweytick D, Lohner K, Klug L, Daum G. Influence of squalene on lipid particle/droplet and membrane organization in the yeast Saccharomyces cerevisiae. BBA-Mol Cell Biol L. 2012;1821(4):647–53.
Chen C, Song KN, Zhang YZ, Chu CJ, Fan BY, Song Y, et al. Biotransformation of betulinic acid by Circinella muscae and Cunninghamella echinulata to discover anti-inflammatory derivatives. Phytochemistry. 2021;182: 112608. https://doi.org/10.1016/j.phytochem.2020.112608.
Chatzivasileiou AO, Ward V, Edgar SM, Stephanopoulos G. Two-step pathway for isoprenoid synthesis. Proc Natl Acad Sci USA. 2018;116(2):506–11. https://doi.org/10.1073/pnas.1812935116.
Dyga M, Keller A, Hasse H. Vapor–liquid equilibria and chemical equilibria in the system (formaldehyde + water + isoprenol). Ind Eng Chem Res. 2021;60(11):4471–83. https://doi.org/10.1021/acs.iecr.1c00168.
Liu H, Chen SL, Xu JZ, Zhang WG. Dual regulation of cytoplasm and peroxisomes for improved α-farnesene production in recombinant Pichia pastoris. ACS Synth Biol. 2021;10(6):1563–73. https://doi.org/10.1021/acssynbio.1c00186.
Clomburg JM, Qian S, Tan ZG, Cheong S, Gonzalez R. The isoprenoid alcohol pathway, a synthetic route for isoprenoid biosynthesis. Proc Natl Acad Sci USA. 2019;116(26):12810–5. https://doi.org/10.1073/pnas.1821004116.
Lund S, Hall R, Williams GJ. An artificial pathway for isoprenoid biosynthesis decoupled from native hemiterpene metabolism. ACS Synth Biol. 2019;8(2):232–8. https://doi.org/10.1021/acssynbio.8b00383.
Ward VCA, Chatzivasileiou AO, Stephanopoulos G. Cell free biosynthesis of isoprenoids from isopentenol. Biotechnol Bioeng. 2019;116(12):3269–81. https://doi.org/10.1002/bit.27146.
Ma XQ, Liang H, Pan QC, Prather KLJ, Sinskey AJ, Stephanopoulos G, et al. Optimization of the isopentenol utilization pathway for isoprenoid synthesis in Escherichia coli. J Agric Food Chem. 2022;70(11):3512–20. https://doi.org/10.1021/acs.jafc.2c00014.
Luo ZB, Liu N, Lazar Z, Chatzivasileiou A, Ward V, Chen J, et al. Enhancing isoprenoid synthesis in Yarrowia lipolytica by expressing the isopentenol utilization pathway and modulating intracellular hydrophobicity. Metab Eng. 2020;61:344–51. https://doi.org/10.1016/j.ymben.2020.07.010.
Zhang XY, Zhu KX, Shi H, Wang X, Zhang Y, Wang F, et al. Engineering Escherichia coli for effective and economic production of cis-abienol by optimizing isopentenol utilization pathway. J Clean Prod. 2022;351: 131310. https://doi.org/10.1016/j.jclepro.2022.131310.
Ruf A, Muller F, D’Arcy B, Stihle M, Kusznir E, Handschin C, et al. The monotopic membrane protein human oxidosqualene cyclase is active as monomer. Biochem Biophys Res Commun. 2004;315(2):247–54. https://doi.org/10.1016/j.bbrc.2004.01.052.
Thoma R, Schulz-Gasch T, D’Arcy B, Benz J, Aebi J, Dehmlow H, et al. Insight into steroid scaffold formation from the structure of human oxidosqualene cyclase. Nature. 2004;432(7013):118–22. https://doi.org/10.1038/nature02993.
Wu T, Ye LJ, Zhao DD, Li SW, Li QY, Zhang BL, et al. Engineering membrane morphology and manipulating synthesis for increased lycopene accumulation in Escherichia coli cell factories. 3 Biotech. 2018;8(6):269. https://doi.org/10.1007/s13205-018-1298-8.
Arendt P, Miettinen K, Pollier J, De Rycke R, Callewaert N, Goossens A. An endoplasmic reticulum-engineered yeast platform for overproduction of triterpenoids. Metab Eng. 2017;40:165–75. https://doi.org/10.1016/j.ymben.2017.02.007.
Ma T, Shi B, Ye ZL, Li XW, Liu M, Chen Y, et al. Lipid engineering combined with systematic metabolic engineering of Saccharomyces cerevisiae for high-yield production of lycopene. Metab Eng. 2019;52:134–42. https://doi.org/10.1016/j.ymben.2018.11.009.
Grewal PS, Samson JA, Baker JJ, Choi B, Dueber JE. Peroxisome compartmentalization of a toxic enzyme improves alkaloid production. Nat Chem Biol. 2021;17(1):96–103. https://doi.org/10.1038/s41589-020-00668-4.
Dusseaux S, Wajn WT, Liu Y, Ignea C, Kampranis SC. Transforming yeast peroxisomes into microfactories for the efficient production of high-value isoprenoids. Proc Natl Acad Sci USA. 2020;117(50):31789–99. https://doi.org/10.1073/pnas.2013968117.
Dueber JE, Wu GC, Malmirchegini GR, Moon TS, Petzold CJ, Ullal AV, et al. Synthetic protein scaffolds provide modular control over metabolic flux. Nat Biotechnol. 2009;27(8):753–9. https://doi.org/10.1038/nbt.1557.
Funding
This work was supported by the National Key R&D Program of China (2018YFA0901700), the Natural Science Foundation of Jiangsu Province (BK20202002) and the Postgraduate Research and Practice Innovation Program of Jiangsu Province (KYCX18_1789).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Rights and permissions
About this article
Cite this article
Wu, Y., Yuan, Z. & Rao, Y. Current advances in the biotechnological synthesis of betulinic acid: new findings and practical applications. Syst Microbiol and Biomanuf 3, 179–192 (2023). https://doi.org/10.1007/s43393-022-00111-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43393-022-00111-w