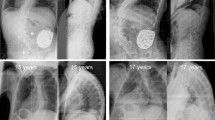
Abstract
Purpose
To assess the complication risks associated with intrathecal baclofen (ITB) pumps in cerebral palsy (CP) patients undergoing posterior spinal fusion (PSF) and to determine if timing of pump implantation before or during PSF impacts the risk of complications.
Methods
A prospectively collected multicenter database was retrospectively reviewed to identify CP patients undergoing PSF from 2008 to 2023. Patients were divided into 2 cohorts: those with an ITB pump (ITB cohort) and those without (non-ITB cohort). The ITB cohort was further categorized by placement of the pump prior to or during PSF. Cohorts were then compared in terms of postoperative complications, perioperative complications, and need for revision surgery.
Results
Four hundred six patients (ITB n = 79 [53 prior to, 26 during PSF], non-ITB n = 326) were included in this analysis. At an average follow-up of 4.0 years (range 2–10 years), there were no significant differences between the ITB and non-ITB cohorts in the rate of perioperative complications (5.0% vs 6.5%, p = 0.80), revision surgeries (2.5% vs 4.6%, p = 0.54), or any complication type, regardless of whether pumps were placed prior to or during PSF, aside from longer surgical times in the latter group.
Conclusion
Complication rates are similar for ITBs placed prior to and during PSF. Patients with spastic CP may safely be treated with ITB pumps without increased risks of complication or further reoperation/revision following PSF.
Level of evidence
Level III.
Similar content being viewed by others
Data availability
Data are available upon reasonable request.
References
Gilmartin R, Bruce D, Storrs BB, Abbott R, Krach L, Ward J, Bloom K, Brooks WH, Johnson DL, Madsen JR, McLaughlin JF, Nadell J (2000) Intrathecal baclofen for management of spastic cerebral palsy: multicenter trial. J Child Neurol 15:71–77. https://doi.org/10.1177/088307380001500201
Albright AL, Cervi A, Singletary J (1991) Intrathecal baclofen for spasticity in cerebral palsy. JAMA 265:1418–1422. https://doi.org/10.1097/00002060-200011000-00010
Stempien L, Tsai T (2000) Intrathecal baclofen pump use for spasticity: a clinical survey. Am J Phys Med Rehabil 79:536. https://doi.org/10.1097/00002060-200011000-00010
Borowski A, Littleton AG, Borkhuu B, Presedo A, Shah S, Dabney KW, Lyons S, McMannus M, Miller F (2010) Complications of intrathecal baclofen pump therapy in pediatric patients. J Pediatr Orthop 30:76. https://doi.org/10.1097/BPO.0b013e3181c6b257
Dickey MP, Rice M, Kinnett DG, Lambert R, Donauer S, Gerber MA, Staat MA (2013) Infectious complications of intrathecal baclofen pump devices in a pediatric population. Pediatr Infect Dis J 32:715. https://doi.org/10.1097/INF.0b013e318287f02a
Walter M, Altermatt S, Furrer C, Meyer-Heim A (2014) Intrathecal baclofen therapy in children with severe spasticity: outcome and complications. Dev Neurorehabil 17:368–374. https://doi.org/10.3109/17518423.2013.827256
Jones-Quaidoo SM, Yang S, Arlet V (2010) Surgical management of spinal deformities in cerebral palsy. A review. J Neurosurg Spine 13:672–685. https://doi.org/10.3171/2010.5.SPINE09669
Scannell B, Yaszay B (2015) Scoliosis, spinal fusion, and intrathecal baclofen pump implantation. Phys Med Rehabil Clin N Am 26:79–88. https://doi.org/10.1016/j.pmr.2014.09.003
Reames DL, Smith JS, Fu K-MG, Polly DWJ, Ames CP, Berven SH, Perra JH, Glassman SD, McCarthy RE, Knapp RDJ, Heary R, Shaffrey CI, Committee SRSM and M (2011) Complications in the surgical treatment of 19,360 cases of pediatric scoliosis: a review of the scoliosis research society morbidity and mortality database. Spine 36:1484. https://doi.org/10.1097/BRS.0b013e3181f3a326
Sponseller PD, Shah SA, Abel MF, Newton PO, Letko L, Marks M (2010) Infection rate after spine surgery in cerebral palsy is high and impairs results: multicenter analysis of risk factors and treatment. Clin Orthop Relat Res 468:711. https://doi.org/10.1007/s11999-009-0933-4
Yaszay B, Bartley CE, Sponseller PD, Abel M, Cahill PJ, Shah SA, Miyanji F, Samdani AF, Daquino C, Newton PO (2020) Major complications following surgical correction of spine deformity in 257 patients with cerebral palsy. Spine Deform 8:1305–1312. https://doi.org/10.1007/s43390-020-00165-7
Borowski A, Shah SA, Littleton AG, Dabney KW, Miller F (2008) Baclofen pump implantation and spinal fusion in children: techniques and complications. Spine 33:1995. https://doi.org/10.1097/BRS.0b013e31817bab42
Caird MS, Palanca AA, Garton H, Hensinger RN, Ayyangar RN, Drongowski A, Farley FA (2008) Outcomes of posterior spinal fusion and instrumentation in patients with continuous intrathecal baclofen infusion pumps. Spine 33:E94. https://doi.org/10.1097/BRS.0b013e3181642aae
Yaszay B, Scannell BP, Bomar JD, Sponseller PD, Shah SA, Asghar J, Samdani AF, Bastrom TP, Newton PO, Group HS (2015) Although inconvenient, baclofen pumps do not complicate scoliosis surgery in patients with cerebral palsy. Spine 40:E504. https://doi.org/10.1097/BRS.0000000000000811
Lins LAB, Nechyporenko AV, Halanski MA, Hetzel SJ, Noonan KJ (2020) Does an intrathecal baclofen pump impact scoliosis progression and complicate posterior spine fusion in patients with cerebral palsy? Spine Deform 8:115–121. https://doi.org/10.1007/s43390-020-00034-3
Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B (1997) Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol 39:214–223. https://doi.org/10.1111/j.1469-8749.1997.tb07414.x
Bonouvrié LA, Lagendijk KE, Beckerman H, Slot KM, van de Pol LA, Buizer AI (2022) Surgical complications of intrathecal baclofen in children: a single centre, 20-year retrospective cohort study. Eur J Paediatr Neurol 37:94–97. https://doi.org/10.1016/j.ejpn.2022.02.002
Motta F, Antonello CE (2014) Analysis of complications in 430 consecutive pediatric patients treated with intrathecal baclofen therapy: 14-year experience: clinical article. J Neurosurg Pediatr 13:301–306. https://doi.org/10.3171/2013.11.PEDS13253
Kopell BH, Sala D, Doyle WK, Feldman DS, Wisoff JH, Weiner HL (2001) Subfascial implantation of intrathecal baclofen pumps in children: technical note. Neurosurgery 49:753. https://doi.org/10.1097/00006123-200109000-00045
Gooch JL, Oberg WA, Grams B, Ward LA, Walker ML (2003) Complications of intrathecal baclofen pumps in children. Pediatr Neurosurg 39:1–6. https://doi.org/10.1159/000070870
Murphy NA, Irwin MCN, Hoff C (2002) Intrathecal baclofen therapy in children with cerebral palsy: efficacy and complications. Arch Phys Med Rehabil 83:1721–1725. https://doi.org/10.1053/apmr.2002.36068
Lipton GE, Miller F, Dabney KW, Altiok H, Bachrach SJ (1999) Factors predicting postoperative complications following spinal fusions in children with cerebral palsy. J Spinal Disord 12:197–205
Xu AL, Marrache M, Hardesty CK, Groves ML, Erickson MA, Murphy RF, Thompson GH, Sponseller PD (2022) Baclofen pump use: complications after growth-friendly instrumentation for early-onset scoliosis. J Pediatr Orthop 42:77. https://doi.org/10.1097/BPO.0000000000002035
Imerci A, Rogers KJ, Miller F, Sees JP (2020) Evaluation of risk factors for cerebrospinal leakage in pediatric patients with cerebral palsy treated with intrathecal baclofen. J Pediatr Orthop 40:e522. https://doi.org/10.1097/BPO.0000000000001472
Segal LS, Wallach DM, Kanev PM (2005) Potential complications of posterior spine fusion and instrumentation in patients with cerebral palsy treated with intrathecal baclofen infusion. Spine (Phila Pa 1976) 30:E219-224. https://doi.org/10.1097/01.brs.0000158869.90908.f8
Senaran H, Shah SA, Presedo A, Dabney KW, Glutting JW, Miller F (2007) The risk of progression of scoliosis in cerebral palsy patients after intrathecal baclofen therapy. Spine 32:2348. https://doi.org/10.1097/BRS.0b013e3181557252
Rushton PRP, Nasto LA, Aujla RK, Ammar A, Grevitt MP, Vloeberghs MH (2017) Intrathecal baclofen pumps do not accelerate progression of scoliosis in quadriplegic spastic cerebral palsy. Eur Spine J 26:1652–1657. https://doi.org/10.1007/s00586-016-4598-x
Shilt JS, Lai LP, Cabrera MN, Frino J, Smith BP (2008) The impact of intrathecal baclofen on the natural history of scoliosis in cerebral palsy. J Pediatr Orthop 28:684. https://doi.org/10.1097/BPO.0b013e318183d591
Acknowledgements
For editorial assistance, we thank Denise Di Salvo, MS, Sandra Crump, MPH, and Rachel Box, MS, in the Editorial Services group of The Johns Hopkins Department of Orthopaedic Surgery.
Funding
The Setting Scoliosis Straight Foundation provided support for the maintenance of the HARMS Study Group database. No other funding was received for the conduct of this study.
Author information
Authors and Affiliations
Consortia
Contributions
KHL, BY, MFA, SAS, AFS, PDS, HSG: Made substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work. KHL, BY, MFA, SAS, AFS, PDS, HSG: Drafted the work or revised it critically for important intellectual cont. KHL, BY, MFA, SAS, AFS, PDS, HSG: Approved the final version to be published. KHL, BY, MFA, SAS, AFS, PDS, HSG: Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflict of interest
Kenneth H. Levy: no competing interests to declare that are relevant to the content of this article. Burt Yaszay: grants from Setting Scoliosis Straight, grants and personal fees from Stryker, grants and personal fees from NuVasive, grants and personal fees from DePuy Synthes, personal fees from Globus, personal fees from Orthopediatrics, personal fees from Medtronic, personal fees from Biogen. Burt Yaszay is an editorial board member for Spine Deformity. Mark F. Abel: no competing interests to declare that are relevant to the content of this article. Suken A. Shah: grants from Setting Scoliosis Straight Foundation, grants and non-financial support from DePuy Synthes Spine. Suken A. Shah is an editorial board member for Spine Deformity. Amer F. Samdani: grants from Setting Scoliosis Straight Foundation, personal fees from DePuy Synthes Spine, personal fees from Ethicon, personal fees from Globus Medical, personal fees from Medical Device Business Services, personal fees from Mirus, personal fees from NuVasive, personal fees from Orthofix, personal fees from Stryker, personal fees from Zimmer Biomet. Amer F. Samdani is an editorial board member for Spine Deformity. Paul D. Sponseller: personal fees from DePuy Synthes Spine, personal fees from Globus, personal fees from Pacira Biosciences, personal fees from Orthopediatrics, personal fees from Journal of Bone and Joint Surgery—American Volume.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the institutional review boards of all participating institutions.
Consent to participate/publish
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Levy, K.H., Yaszay, B., Abel, M.F. et al. Baclofen pumps do not increase risk of complications following spinal fusion. Spine Deform 12, 473–480 (2024). https://doi.org/10.1007/s43390-023-00786-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43390-023-00786-8