Abstract
We characterize the taxonomic and functional diversity of waterbird communities in mangrove forests of 23 coastal lagoons in the southern Mexican Pacific coast, to evaluate the hypothesis of decline of taxonomic and functional richness of waterbird communities in the face of loss of natural habitat cover and increased fragmentation. We quantified patterns of land use cover, considering the heterogeneity of natural and anthropized vegetation cover as a proxy for human-caused fragmentation, and used generalized linear models to explore the relationship between these two covers with the taxonomic richness and functional richness of bird communities. Results show that both aspects of biodiversity positively relate to larger natural habitat areas, while higher fragmentation values have a negative effect on them. Our results suggest that habitat loss and fragmentation of vegetation cover negatively affect the diversity of waterbird communities and can compromise their link to ecosystem functioning processes in coastal lagoons, by decreasing their functional diversity.
Similar content being viewed by others
Introduction
Coastal ecosystems form a mosaic that links diverse types of terrestrial, marine, and inland water habitats (Sheaves 2009; Buelow and Sheaves 2015). Some of these natural systems are coastal lagoons, which link the mangrove forest habitat with estuaries, where in turn, marine and inland biotas are mixed (Buelow and Sheaves 2015). Coastal lagoons are shallow water bodies separated from the ocean by a barrier and connected to it by canals (Kjerfve 1994). In addition, coastal lagoons are frequently covered by mangrove forests (hereby mangroves), both within these bodies of water and in the periphery (Calderón et al. 2009; Rodríguez-Zúñiga et al. 2013). Though mangroves have low floristic diversity they have high structural complexity and harbor a large diversity of animal species (Nagelkerken et al. 2008; Feller et al. 2010). This biological diversity of mangroves contributes to ecosystem functioning mechanisms, like nutrient production and connectivity between inland and marine environments.
Because of their high productivity and the complex structure of the mangrove trees’ underwater roots, coastal lagoons act as nurseries for great diversity of fish and crustaceans (Robertson and Duke 1987; Nagelkerken et al. 2008, 2013; Feller et al. 2010). Hence, the aquatic ecosystem of coastal lagoons provides a fundamental source of trophic resources to animals such as waterbirds (Kutt 2007; Buelow and Sheaves 2015). Aquatic birds are one of the most conspicuous animal groups that inhabit coastal lagoons and mangroves. The mangrove forest cover offers resting sites and refuge to these organisms and provides proper nesting sites to diverse species, including several species of cormorants, ibises, herons, pelicans, and seagulls (Pfister et al. 2006; Nagelkerken et al. 2008; McFadden et al. 2016).
Several bird species make use of the mangrove habitat as a place to roost and forage, while also utilizing the tree canopies as nurseries (Pool et al. 1977; Bouillon 2011; Rodríguez-Zúñiga et al. 2013; Buelow and Sheaves 2015). Likewise, specialist bird species can take advantage of mangrove roots, along with the saltpans and creeks they create, as food sources. Indeed, it has been found that the structural complexity of mangrove forests is positively related with bird communities’ diversity (Mohd-Azlan et al. 2015). By fulfilling the ecological requirements of their diets, waterbirds become closely tied to regulation mechanisms of ecosystem functioning in coastal lagoons (Hooper et al. 2005; Whelan et al. 2016). They therefore facilitate biochemical processes, provide nutrients to primary producers, prevent eutrophication, and link other environments with the mangrove (Dobrowolski et al. 1993; Smith et al. 1999; Vanni 2002; Ligeza and Smal 2003; Buelow and Sheaves 2015; Fujita and Kameda 2016). Besides, waterbirds regulate the population size of invertebrates, fish, and amphibians and can be involved in trophic cascade phenomena (Wootton 1995; Şekercioğlu 2006). Furthermore, several species from Scolopacidae, Charadriidae, Anatidae, and Rallidae are important seed dispersers of aquatic plants (Green et al. 2016).
Unfortunately, habitat loss and fragmentation due to human impacts threaten coastal lagoons and their mangroves, which have lost 62% of their total world cover up until 2016 (Goldberg et al. 2020). In Mexico, mangrove area loss resulting from human activity has reached 20% since 1980 (CONABIO 2008; Velázquez-Salazar et al. 2021). Some processes correlated with human-mediated habitat loss, like pollution, and even overfishing, threaten mangrove biodiversity. Altogether, these factors decrease the habitat available to birds inhabiting mangrove forests within coastal lagoons (Polidoro et al. 2014). It has been reported that mangrove loss and fragmentation decrease water quality, as well as populations of crustaceans and fishes (Schaffelke et al. 2005; Tran and Fischer 2017). As a result, trophic resources exploited by waterbirds are compromised (Dobson et al. 2006; Şekercioğlu 2006). Furthermore, habitat loss can decrease the reproductive success of waterbirds by diminishing nesting area, exposing them to introduced predators, or promoting contact between humans and birds (Dolman and Sutherland 1995; Bradbury et al. 2000; Owens and Bennett 2000; Zuberogoitia et al. 2008). Empirical evidence showed that conversion into crop fields and human settlements reduces species richness of bird communities within mangrove forests (Mohd-Taib et al. 2020). Besides, habitat loss is driving a serious threat to bird diversity and their role in the trophic network and ecosystem processes within coastal lagoons and their mangrove forests (CONABIO 2008; Polidoro et al. 2010; Li et al. 2013; Branoff 2017; Mohd-Azlan et al. 2015; Bryan-Brown et al. 2020).
Because of the role of birds in the trophic network and their ecological function within mangrove forests, tracking changes in bird functional diversity is important as an indicator of biodiversity’s response to anthropic disturbance (Dobrowolski et al. 1993; Ligeza and Smal 2003; Şekercioğlu 2006). However, most studies analyzing the effects of anthropization on mangrove bird communities are focused on a species abundance-based ecological approach. In addition, it’s worth noting that many works analyze bird communities in Asia and Oceania (Li et al. 2013; Mohd-Azlan et al. 2015; Tran and Fischer 2017; Lee et al. 2020; Mohd-Taib et al. 2020; Stiepani et al. 2021), where the most extensive and diverse mangroves are found (Luther and Greenberg 2009). Notably, even though the study of anthropic influence on the relationship between birds and ecological function is a key conservation issue (Şekercioğlu 2006), the application of trait-based ecology to study mangrove bird communities is a barely explored topic (but see De Arruda-Almeida et al. 2018, 2019). Trait-based ecology is focused on assessing the changes in the representation of physiological, morphological, and behavioral characters associated with the success of organisms facing ecological pressure, as well as their effect on ecosystem regulation processes. So, the ecology approach based on functional traits improves our comprehension of biodiversity’s response to human activity, as well as the ecological consequences that result from it (Violle et al. 2007; Díaz and Cabido 2001; Suding et al. 2008; Mouillot et al. 2013; Salgado-Negret and Paz 2016).
Here, using a functional trait-based approach to evaluate the effect of mangrove loss and habitat fragmentation on waterbird communities, we studied bird communities in 23 coastal lagoons of the Mexican South Pacific coast, a territory that is severely threatened by human activity. In Mexico, papers addressing mangrove birds are mainly assessments of species richness without focusing on the effects of human disturbance on functional aspects (e.g., Bojorges-Baños 2011; Serrano et al. 2013; Ruiz Bruce Taylor et al. 2017), despite the severe fragmentation that this ecosystem has suffered as a consequence of human activities (CONABIO 2009). We use observational data to assess the hypothesis that lagoons with smaller natural habitat area, larger anthropic area (urban coverage, agriculture, and no vegetation), and higher mangrove fragmentation would have bird communities that are less taxonomically and functionally diverse.
Methods
Study area
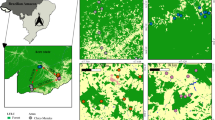
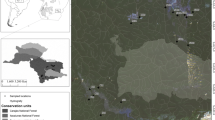
This study included 23 coastal lagoons along the southern Mexican Pacific coast, from the extreme coordinates 20° 40’ 18.33” N to the North and 16° 0’ 11.23” N to the South. The study area includes the south of Jalisco, the coasts of Colima and Guerrero, and the middle point of Oaxaca (Fig. 1). This zone has a warm sub-humid climate, with summer rainfall and precipitation that ranges from 600 to 1500 mm yearly (García 1998; INEGI 2016). The original plant cover of coastal lagoons is mainly mangrove, predominantly Rhizophora mangle, Laguncularia racemosa¸ Avicennia germinans, and Conocarpus erectus (Rodríguez-Zúñiga et al. 2013).
Bird community composition
Bird records were performed between June 2010 and June 2011, utilizing transects traveled for two to four hours after sunrise, considering birds’ peak of diurnal activity, and depending on lagoon size. Transects were traveled at slow speed on a boat, approaching the borders of the lagoon and the central areas. When possible, foot transects of up to 1 km were surveyed, and all-terrain vehicles were used for longer distances. For the analyses, we considered only records of waterbirds (i.e., species that require the aquatic habitat to complete their biological cycle, sensu Ramírez-Bastida 2008). Given that the lagoons were visited only twice, it is not feasible to analyze the total of the species’ catalogs using conventional methods, such as species accumulation curves or rarefaction analyses. Thus, in order to gauge the effectiveness of the censuses, we followed the proposal of Gómez de Silva and Medellín (2001), which suggests the absence of “omnipresent” taxa as an indicator of incomplete catalogs due to lack of sampling. Therefore, in this study, indicator species were considered common birds on the Mexican Pacific coast and had a frequency record greater than 75%: Actitis macularius, Fregata magnificens, Nannopterum brasilianum, Ardea herodias, A. alba, Butorides virescens, Egretta thula, E. caerulea and E. tricolor. Supplementary material 1 shows the species list, according to the AOS taxonomic criteria (Chesser et al. 2021), records by site, and record frequency of waterbird communities in the study.
Bird functional traits
We used specialized databases (Wilman et al. 2014; de Magalhães 2021; Myers et al. 2021; Billerman et al. 2022) to construct a matrix of functional traits for all birds in the study. We considered three continuous life-history traits: body mass, clutch size, and life span. Furthermore, we considered categorical traits related to fulfillment of ecological requirements composed of eight categories related to diet type and eight categories for foraging habitat (Table 1). Finally, for cases in which we lacked specific data, we considered the species with the closest phylogenetic relationship for which information was available. The used trait values are available in Supplementary Material 2.
Habitat characterization
To generate a proxy of the relative cover per habitat type, we traced polygons of the 23 coastal lagoons in kml language using Google Earth Pro (www.earth.google.com), which were then translated into kmz format. The resolution of the satellite images used did not allow the use of elevation over sea level data to define the area of each lagoon. Thus, we included the adjacent habitat to each lagoon based on the available satellite imaging, aiming to encompass the terrain directly associated with the wetland area. Considering the 23 sites, we included the adjacent habitat up to 693.12 m (+/- 841.75 standard deviation) from the edge of the water cover. Due to the required spatial analyses, we converted the polygons into spatial data archives compatible with ArcGIS (Environmental Systems Research Institute 2015). The resulting polygons were intersected with the land use and vegetation cover in Mexico (Series VI: land use and vegetation, INEGI 2016) using Lambert’s conformal conic projection. After the intersection mentioned above, land use categories were simplified into the following categories: (1) water, (2) urban area, (3) agriculture, (4) mangrove, (5) deciduous forest, (6) semideciduous forest, (7) aquatic vegetation, and (8) no vegetation. This step was necessary to simplify data management by reducing vegetation groups, which can be redundant in this kind of analysis. We quantified the area of each lagoon and the area of each type of cover, both in km2. Using those data, we calculated the area representing natural habitat (mangrove + deciduous forest + semideciduous forest + aquatic vegetation) and the area of anthropized habitat (urban area + agriculture + no vegetation). We used the Shannon-Wiener diversity index (Shannon 1948) as a proxy of habitat fragmentation, which we defined in accordance with Franklin et al. (2002), as a discontinuity in the spatial distribution of resources in such way that it affects species’ survival, reproduction, and habitat occupancy. This index reflects the heterogeneity of a data set depending on the number of current categories (here, land use types) and their relative abundance (area in km2). When the abundance differs greatly among the categories within the data set, the index generates low diversity values. Meanwhile, similar relative abundance among categories results in high diversity values (Shannon 1948). Lagoons with higher habitat fragmentation will have more cover types, with similar areas among them, such that higher habitat fragmentation will result in high Shannon diversity values. As support for this premise, a simple linear regression showed that the number of polygons in each lagoon showed a positive correlation with the estimated Shannon index value (r = 0.69, P < 0.001). To calculate the index, we did not include the water cover since it represents an area that birds do not utilize for perching, resting, or nesting.
Estimation of biodiversity indicators
We used the recorded species richness value as an approximation of the taxonomic dimension of the bird diversity. To evaluate the representation of the functional dimension of biodiversity, we calculated the functional richness index, Fric (Villéger et al. 2007). This index measures the functional space occupied by the traits represented in a community (Mason et al. 2005; Villéger et al. 2007). High Fric values imply more ecologically resilient and complex communities (Lozanovska et al. 2018; Feng et al. 2020). There is evidence that anthropization causes functional diversity linked to life-history traits to decrease, while functional diversity linked to ecological requirement traits increases (Vázquez-Reyes et al. 2022). Hence, in this study, we calculated the Fric index independently, first considering each trait set (life-history traits and ecological requirement traits) and then using the complete trait set. In order to obtain the Fric value, we calculated the distance between every possible pair of species within the data matrix utilizing Gower distance. With the resulting distance matrix, we calculated a principal coordinate analysis (PCoA). We used the first axes of the PCoA to calculate Fric (Si et al. 2016). These estimates were made with the FD package (Laliberté and Legendre 2010) in RStudio (RStudio Team 2021).
To determine the relationship between bird diversity in coastal lagoons and habitat characteristics, we calculated generalized linear models. The predictive variables for the models were: area of the land use cover categories (water, urban area, agriculture, mangrove, deciduous forest, semideciduous forest, aquatic vegetation, and surface with no vegetation; determined using GIS), total polygon area (in km2), and forest habitat fragmentation (Shannon diversity). As response variables, we considered: sampled taxonomic richness and the three Fric values mentioned above. Data from all the variables were transformed (log10 + 1), and we utilized the lowest Akaike Information Criterion, AIC (Akaike 1974) value to determine which model best explained the behavior of the response variable (Burnham and Anderson 1998). We used JMP Pro 14 software (www.jpm.com) to construct the generalized linear models.
Results
Evaluation of species and functional richness
Taxonomic richness values ranged from 15 to 64 species, with a median of 31 species. The lagoons with the highest taxonomic richness were Cuyutlán (64 species), Tres Palos (47 species), and Boca de Apiza (47 species). Fric values varied from 3.236 to 14.082, with a median of 10.022. Cuyutlán (14.082) and Boca de Apiza (13.307) had the highest Fric values. The lagoons with the largest total area were Cuyutlán (148.7 km2) and Tres Palos (138.6 km2) were the lagoons with the largest total area. In contrast, Potrero Grande, with one of the smallest areas (5.77 km2), had the lowest values for both taxonomic (15 species) and functional richness (3.236) (Table 2).
Generalized linear models
Models including every habitat cover as predictive variables were all significant, except for the one using life-history traits Fric as the response variable. In all four models, the predictive variables total polygon area and habitat fragmentation, or either, were significant. After readjusting the generalized linear models and contemplating a more reduced model using only total polygon area and habitat fragmentation as environmental variables, we obtained significant models with AIC values expressing better fitness (Table 3). In each of these, the effect of total polygon area was positive and significant. In contrast, the effect of habitat fragmentation on the response variables was negative (Table 4).
Discussion
Consistent with our hypothesis, our results suggest that waterbird taxonomic and functional diversities increased with total polygon area but diminished in response to forest habitat fragmentation.
Generalized linear model results indicated that bird communities’ taxonomic and functional diversities increase in larger coastal lagoons. Several studies within Neotropical and European wetlands have documented that available habitat area represents a dominant factor in taxonomic richness increases for waterbirds (de Arruda-Almeida et al. 2018; Sebastián-González and Green 2013). This trend is consistent with the theoretical relationship between area size and taxonomic richness (MacArthur and Wilson 1963). Given that a larger size involves a larger quantity and diversity of niches and resources, a greater diversity of species could potentially exploit them (Weisberg et al. 2014; Karadimou et al. 2016; Oliveira et al. 2020). The resource abundance resulting from a larger-sized area likewise explains the positive effect of area size on Fric (Weisberg et al. 2014; Karadimou et al. 2016; Lee and Carroll 2018; Oliveira et al. 2020). Nevertheless, although they respond similarly to area size, the taxonomic and functional aspects of diversity are not mutually related in the same way, particularly in disturbed environments (Mayfield et al. 2010; Biswas and Mallik 2011).
It is worth mentioning that the generalized linear models did not completely explain the variation in the data. This could be because, on a local scale, the specific characteristics of each coastal lagoon determine response patterns that decrease the efficiency of the model. For example, Boca de Apiza and Chacahua had the highest taxonomic and functional diversity values, respectively, yet they are not the lagoons with the highest mangrove cover. This may be because of elements that locally draw higher diversity in bird communities, such as better food availability (Ramírez-Bastida et al. 2018). For example, the structural complexity of mangroves benefits aquatic organisms such as fish and crustaceans, which constitute part of the diet of several waterbirds (Robertson and Duke 1987; Nagelkerken et al. 2008; Buelow and Sheaves 2015). However, these elements were not evaluated in the current study.
In addition, model results showed that, in accordance with our hypothesis, fragmentation of the forest habitat surrounding coastal lagoons negatively affected taxonomic and functional species richness. Because fragmentation negatively affects habitat in terms of continuity and structural and floristic diversity, birds that take advantage of the mangrove structure and associated aquatic vegetation will be negatively affected (Haddad et al. 2015). The loss of mangrove structural complexity will therefore affect several species, including birds that nest in the arboreal stratum of mangroves, such as frigatebirds, cormorants, and herons, in addition to harming the crustacean and fish populations on which they feed (Robertson and Duke 1987; Pfister et al. 2006; Nagelkerken 2008; McFadden et al. 2016; Tran and Fischer 2017). The mangrove forests surrounding the lagoons are associated to the adjacent land vegetation, which consists mostly of deciduous forest. This represents a continuous habitat with resources that waterbird communities can exploit. In fragmentation scenarios, habitat patches are progressively further isolated, which favors the invasion of foreign species which inhabit the matrix surrounding mangroves (Fahrig 2003; Mohd-Azlan and Lawes 2011). Species movement among fragments is also limited, restricting birds’ benefits to habitat connectivity (Buelow and Sheaves 2015; Haddad et al. 2015). On the other hand, the diversity of birds linked to emergent aquatic vegetation associated with mangroves, such as ducks and rails, is directly related to the composition and diversity of plant species (Mohd-Azlan et al. 2015; Bannor and Kiviat 2020; Eitniear et al. 2020; Gauthier 2020; West and Hess 2020). Corralero, Chacahua, Tecomate, Tres Palos, and El Potosí are the five lagoons with the highest habitat fragmentation index in this study, and excluding Tres Palos, they all have intermediate taxonomic richness and Fric values (Table 2). Bird communities in these sites could therefore face a decline which would increase in response to the degree of fragmentation.
The erosion of taxonomic richness and Fric of bird communities in coastal lagoons have potential consequences on the trophic network (Cardinale et al. 2006). The families Charadriidae and Scolopacidae contribute to population control of mollusks and crustaceans (Robert and McNeil 1989), while herons (Ardeidae) feed on many species of invertebrates and fish (Miranda and Collazo 1997). These groups are well represented in sites with high fragmentation (Chacahua, Tecomate, Tres Palos) and low fragmentation (Ventanilla, La Escobilla), indicating they were not affected by it. On the other hand, ducks (Anatidae) and rails (Rallidae) feed on aquatic plants and contribute to seed dispersal (Şekercioğlu 2006; Green et al. 2016). In this study, these groups had a larger representation in sites with intermediate to low levels of fragmentation (Mitla, Chautengo, La Escobilla) and thus more extensive aquatic vegetation cover. The decline of these taxa could have consequences for the renewal processes of the natural cover of emergent aquatic vegetation (Green et al. 2016). Additionally, birds’ role in mineral transport will be lost, leading to impoverishment of water quality and reduced soil nutrients for plants (Vanni 2002; Ligeza and Smal 2003; Şekercioğlu 2006). Given the importance of wetlands as links between land and aquatic habitats and as a hotspot for bird species, their disappearance would represent a severe loss of biodiversity in the region (Li et al. 2021). It is notable that life history traits’ functional richness increases with total habitat area but diminishes in response to habitat fragmentation. This pattern could suggest that, besides the loss of several ecological interactions, fragmentation could drive a selective pressure against several life history traits, as has been well documented (Mckinney and Lockwood 1999; Cooke et al. 2019), or even, acting as a filter against birds with specific ecological strategies (Vázquez-Reyes et al. 2022). To answer these issues, it will be necessary to develop detailed assessments of functional traits covariation shifts in response to habitat loss and fragmentation.
Conclusions
Our results suggest that preserving a larger extension of mangrove cover favors the increase of taxonomic and functional diversity of waterbird communities. We also show that stopping and even reversing mangrove fragmentation processes would favor waterbird taxonomical and functional diversity. The conservation of mangrove biodiversity could in turn benefit ecosystem functioning and regulation processes in coastal lagoons. Unfortunately, human activities are involved in the erosion of diversity, compromising ecosystem functioning in coastal lagoons (Thébault and Loreau 2006; Rodríguez-Zúñiga et al. 2013; Mohd-Taib et al. 2020). This problem has been approached from the environmental legal framework since the protection of mangrove coverage in Mexico (CONABIO 2009); however, negative human impact surpasses the protection of mangrove cover, posing a challenge for the conservation of mangroves, their biodiversity and ecosystem functioning (Velázquez-Salazar et al. 2021).
References
Akaike H (1974) A new look at the statistical model identification. IEEE Trans Automatic Control 19:716–723. https://doi.org/10.1109/TAC.1974.1100705
ArcGIS 10.2.2 (2015) Environmental Systems Research Institute. https://enterprise.arcgis.com
Bannor BK, Kiviat E (2020) Common gallinule (Gallinula galeata). In: Poole AF, Gill FB(eds) Birds of the World. Cornell Lab of Ornithology. https://doi.org/10.2173/bow.comgal1.01
Billerman SM, Keeney BK, Rodewald PG, Schulenberg TS (2022) Birds of the World. Cornell Lab of Ornithology, Ithaca, NY, USA
Biswas SR, Mallik AU (2011) Species diversity and functional diversity relationship varies with disturbance intensity. Ecosphere 2:1–10. https://doi.org/10.1890/ES10-00206.1
Bojorges-Baños JC (2011) Riqueza Y Diversidad De especies de aves asociadas al manglar en tres sistemas lagunares en la región costera de Oaxaca, México. Rev Mex Biodivers 82:202–215. https://doi.org/10.22201/ib.20078706e.2011.1.445
Bouillon S (2011) Storage beneath mangroves. Nat Geosc 4:282–283. https://doi.org/10.1038/ngeo1130
Bradbury RB, Kyrkos A, Morris AJ, Calark SC, Perkins AJ, Wilson JD (2000) Habitat associations and breeding success of yellowhammers on lowland farmland. J Appl Ecol 37:789–805. https://doi.org/10.1046/j.1365-2664.2000.00552.x
Branoff BL (2017) Quantifying the influence of urban land use on mangrove biology and ecology: a meta-analysis. Glob Ecol Biogeogr 26:1339–1356. https://doi.org/10.1111/geb.12638
Bryan-Brown DN, Connoly RM, Richards DR, Adame F, Friess DA, Brown CJ (2020) Global trends in mangrove forest fragmentation. Sci Rep 10:7117. https://doi.org/10.1038/s41598-020-63880-1
Buelow C, Sheaves M (2015) Mangrove forests: a birds-eye view of connectivity in coastal ecosystem mosaics. Est Coast Shelf Sci 152:33–43. https://doi.org/10.1016/j.ecss.2014.10.014
Burnham KP, Anderson DR (1998) Model selection and inference: a practical information-theoretic approach. Springer-, New York
Calderón C, Aburto O, Ezcurra E (2009) El valor de Los manglares. Biodiversitas 82:1–6
Cardinale BJ, Srivastava DS, Emmett Duffy J, Wright JP, Downing AL, Sankaran M, Jouseau (2006) Effects of biodiversity on the functioning of trophic groups and ecosystems. Nature 443:989–992. https://doi.org/10.1038/nature05202
Chesser RT, Billerman SM, Burns KJ, Cicero C, Dunn JL, Hernández-Baños BE, Kratter AW, Lovette IJ, Mason NA, Rasmussen PC, Remsen JV, Stotz DF, Winker K (2021) Checklist of North American Birds (online). American Ornithological Society. http://checklist.aou.org/taxa
CONABIO (2008) Manglares De México. Comisión Nacional para el Conocimiento y Uso de la Biodiversidad, México
CONABIO (2009) Manglares De México: extensión y distribución. Comisión Nacional para el Conocimiento y Uso de la Biodiversidad, México
Cooke RSC, Eigenbrod F, Bates AE (2019) Projected losses of mammal and bird ecological strategies. Nat Commun 10:2279. https://doi.org/10.1038/s41467-019-10284-z
De Arruda-Almeida B, de Green AJ, Sebastián-González E, dos Anjos L (2018) Comparing species richness, functional diversity, and functional composition of waterbird communities along environmental gradients in the neotropics. PLoS ONE 13:e0200959. https://doi.org/10.1371/journal.pone.0200959
De Arruda-Almeida B, Sebastián-González E, dos Anjos L, Green AJ, Botella F (2019) A functional perspective for breeding and wintering waterbird communities: temporal trends in species and trait diversity. Oikos 128:1103–1115. https://doi.org/10.1111/oik.05903
de Magalhães JP (2021) Human Ageing Genomic Resources. https://genomics.senescence.info/about.html. Accessed 8 July 2023
Díaz S, Cabido M (2001) Vive La différence: plant functional diversity matters to ecosystem processes. Trends Ecol Evol 16:646–655. https://doi.org/10.1016/S0169-5347(01)02283-2
Dobrowolski KA, Kozakiewicz A, Léznicka B (1993) The roles of small mammals and birds in transport of matter through the shore zone of lakes. Hydrobiologia 251:81–93. https://doi.org/10.1007/BF00007168
Dobson A, Lodge D, Jackie A, Cumming GS, Keymer J, McGlade J, Mooney H, Rusak JA, Sala O, Wolters V, Wall D, Winfree R, Xenopoulos MA (2006) Habitat loss, trophic collapse, and the decline of ecosystem services. Ecology 87:1915–1924. https://doi.org/10.1890/0012-9658(2006)87[1915:hltcat]2.0.co;2
Dolman PM, Sutherland WJ (1995) The response of bird populations to habitat loss. Ibis 137:S38–S46. https://doi.org/10.1111/j.1474-919X.1995.tb08456.x
Eitniear JC, Bribiesca-Formisano R, Rodríguez-Flores CI, Soberanes-González CA, Arizmendi M del (2020) C Muscovy duck (Cairina moschata). In: Schulenberg TS (ed), Birds of the World. Cornell Lab of Ornithology. https://doi.org/10.2173/bow.musduc.01
Fahrig L (2003) Effects of habitat fragmentation on biodiversity. Annu Rev Ecol Evol Syst 34:487–515. https://doi.org/10.1146/annurev.ecolsys.34.011802.132419
Feller IC, Lovelock CE, Berger U, McKee KL, Joye SB, Ball MC (2010) Biocomplexity in mangrove ecosystems. Annu Rev Mar Sci 2:395–417. https://doi.org/10.1146/annurev.marine.010908.163809
Feng G, Zhang J, Girardello M, Pellissier V, Svenning JC (2020) Forest canopy co-determines taxonomic and functional richness, but not functional dispersion of mammals and birds globally. Glob Ecol Biogeogr 29:1350–1359. https://doi.org/10.1111/geb.13110
Franklin AB, Noon BR, George TL (2002) What is habitat fragmentation? Stud Avian Biol 25:20–29
Fujita M, Kameda KO (2016) Nutrient dynamics and nutrient cycling by birds. In: Şekercioğlu CH, Wenny D, Whelan CJ (eds) Why birds Matter. The University of Chicago, pp 271–297
García E (1998) Precipitación total anuscala 1:1000000. Comisión Nacional para el Conocimiento y Uso de la Biodiversidad. http://geoportal.conabio.gob.mx/metadatos/doc/html/clima1mgw.html. Accessed 10 July 2023
Gauthier G (2020) Bufflehead (Bucephala albeola). In: Poole AF (ed) Birds of the World. Cornell Lab of Ornithology. https://doi.org/10.2173/bow.buffle.01
Goldberg L, Lagomasino D, Thomas N, Fatoyinbo T (2020) Global declines in human-driven mangrove loss. Global Chang Biol 26:5844–5855. https://doi.org/10.1111/gcb.15275
Gómez de Silva H, Medellín RA (2001) Evaluating completeness of species lists for conservation and macroecology: a case study of Mexican land birds. Conserv Biol 15:1384–1395. https://doi.org/10.1111/j.1523-1739.2001.00177.x
Green AJ, Soons M, Brochet AL, Kleyheeg E (2016) Dispersal of plants by waterBirds. In: Şekercioğlu CH, Wenny D, Whelan CJ (eds) Why birds Matter. The University of Chicago, pp 147–195
Haddad NM, Brudvig LA, Clobert J, Davies KF, Gonzalez A, Holt RH, Lovejoy TE, Sexton JO, Austin MP, Collins CD, Cook WM, Damschen EI, Ewers RM, Foster BL, Jenkins CN, King AJ, Laurance WF, Levey DJ, Margules CR, Melbourne BA, Nicholls AO, Orrock JL, Song DX, Townshend JR (2015) Habitat fragmentation and its lasting impact on Earth’s ecosystems. Sci Adv 1:e1500052. https://doi.org/10.1126/sciadv.1500052
Hooper DU, Chapin FS III, Ewel JJ, Hector A, Inchausti P, Lavorel S, Lawton JH, Lodge DM, Loreau M, Naeem S, Schmid B, Setälä H, Symstad AJ, Vandermeer J, Wardle DA (2005) Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecol Monogr 75:3–35. https://doi.org/10.1890/04-0922
INEGI (2016) Conjunto De Datos Vectoriales De Uso De Suelo Y Vegetación. Escala 1:250 000. Serie VI. Instituto Nacional de Estadística y Geografía
Karadimou EK, Kallimanis AS, Tsiripidis I, Dimopoulos P (2016) Functional diversity exhibits a diverse relationship with area, even a decreasing one. Sci Rep 6:35420. https://doi.org/10.1038/srep35420
Kjerfve B (1994) Coastal lagoon processes. Elsevier Science
Kutt A (2007) Bird assemblage in a dune-mangrove mosaic, Cairns, Queensland. Aust Zool 34:158–164. https://doi.org/10.7882/AZ.2007.013
Laliberté E, Legendre P (2010) A distance-based framework for measuring functional diversity from multiple traits. Ecology 91:299–305. https://doi.org/10.1890/08-2244.1
Lee MB, Carroll JP (2018) Effects of patch size and basal area on avian taxonomic and functional diversity in pine forests: implication for the influence of habitat quality on the species–area relationship. Ecol Evol 8:6909–6920. https://doi.org/10.1002/ece3.4208
Lee E, Sagong J, Lee Y (2020) Influence of land use change on the waterbird community of Sihwa Lake, Republic of Korea. Avian Res 11:36. https://doi.org/10.1186/s40657-020-00221-w
Li MS, Mao LJ, Shen WJ, Liu SQ, Wei AS (2013) Change and fragmentation trends of Zhanjiang mangrove forests in southern China using multi-temporal landsat imagery (1977–2010). Estuar Coast Shelf Sci 130:111–120. https://doi.org/10.1016/j.ecss.2013.03.023
Li Y, Chen Z, Peng C, Huang G, Niu H, Zhang H (2021) Assessment of habitat change on bird diversity and bird-habitat network of a Coral Island, South China Sea. BMC Ecol Evol 21:137. https://doi.org/10.1186/s12862-021-01865-y
Ligeza S, Smal H (2003) Accumulation of nutrients in soils affected by perennial colonies of piscivorous birds with reference to biochemical cycles of elements. Chemosphere 52:595–602. https://doi.org/10.1016/S0045-6535(03)00241-8
Lozanovska I, Ferreira MT, Segurado P, Aguiar FC (2018) Limited resilience in hotspots of functional richness: the Mediterranean riparian shrublands. Aquat Sci 80:25. https://doi.org/10.1007/s00027-018-0576-1
Luther DA, Greenberg R (2009) Mangroves: a global perspective on the evolution and conservation of their terrestrial vertebrates. Bioscience 59:602–612. https://doi.org/10.1525/bio.2009.59.7.11
MacArthur RH, Wilson EO (1963) An equilibrium theory of Insular Zoogeography. Evolution 17:373–387. https://doi.org/10.1111/j.1558-5646.1963.tb03295.x
Mason NWH, Mouillot D, Lee WG, Wilson JB (2005) Functional richness, functional evenness and functional divergence: the primary components of functional diversity. Oikos 111:112–118. https://doi.org/10.1111/j.0030-1299.2005.13886.x
Mayfield MM, Bonser SP, Morgan JW, Aubin I, McNamara S, Vesk PA (2010) What does species richness tell us about functional trait diversity? Predictions and evidence for responses of species and functional trait diversity to land-use change. Glob Ecol Biogeogr 19:423–431. https://doi.org/10.1111/j.1466-8238.2010.00532.x
McFadden TN, Kauffman JB, Bhomia RK (2016) Effects of nesting birds on nutrient levels in mangroves, Gulf of Fonseca, Honduras. Wetl Ecol Manag 24:217–229. https://doi.org/10.1007/s11273-016-9480-4
McKinney ML, Lockwood JL (1999) Biotic homogenization: a few winners replacing many losers in the next mass extinction. Trends Ecol Evol 14:450–453. https://doi.org/10.1016/S0169-5347(99)01679-1
Miranda L, Collazo JA (1997) Food habits of 4 species of wading birds (Ardeidae) in a Tropical Mangrove Swamp. Colon Waterbirds 20:413–418. https://doi.org/10.2307/1521591
Mohd-Azlan J, Lawes MJ (2011) The effect of the surrounding landscape matrix on mangrove bird community assembly in north Australia. Biol Conserv 144:2134–2141. https://doi.org/10.1016/j.biocon.2011.04.003
Mohd-Azlan J, Noske RA, Lawes MJ (2015) The role of habitat heterogeneity in structuring mangrove bird assemblages. Diversity 7:118–136. https://doi.org/10.3390/d7020118
Mohd-Taib FS, Mohd-Saleh W, Asyikha R, Mansor MS, Ahmad-Mustapha M, Mustafa-Bakray NA, Mod-Husin S, Md-Shukor A, Amat-Darbis ND, Sulaiman N (2020) Effects of anthropogenic disturbance on the species assemblages of birds in the back mangrove forests. Wetl Ecol Manag 28:479–494. https://doi.org/10.1007/s11273-020-09726-z
Mouillot D, Graham NAJ, Villéger S, Mason NWH, Bellwood DR (2013) A functional approach reveals community responses to disturbances. Trends Ecol Evol 28:167–177. https://doi.org/10.1016/j.tree.2012.10.004
Myers P, Espinosa R, Parr CS, Jones T, Hammond GS, Dewey TA (2021) The Animal Diversity Web. https://animaldiversity.org. Accessed 8 July 2023
Nagelkerken I, Blaber SJM, Bouillon S, Green P, Haywood M, Kirton LG, Meynecke JO, Pawlik J, Penrose HM, Sasekumar A, Somerfield PJ (2008) The habitat function of mangroves for terrestrial and marine fauna: a review. Aquat Bot 89:155–185. https://doi.org/10.1016/j.aquabot.2007.12.007
Nagelkerken I, Sheaves M, Baker R, Connolly RM (2013) The seascape nursery: a novel spatial approach to identify and manage nurseries for coastal marine fauna. Fish Fisher 16:362–371. https://doi.org/10.1111/faf.12057
Oliveira SH, Gouveia SF, Esparz JR, Ferrari SF (2020) Fragment size and the disassembling of local bird communities in the Atlantic Forest: a taxonomic and functional approach. Perspec Ecol Conserv 18:304–312. https://doi.org/10.1016/j.pecon.2020.09.003
Owens IPF, Bennett PM (2000) Ecological basis of extinction risk in birds: habitat loss versus human persecution and introduced predators. Proc Nat Acad Sci 97:12144–12148. https://doi.org/10.1073/pnas.200223397
Pfister T, Pfister B, Jiménez-Serranía V, De-Pedro-Díaz D, Suárez-Gracida G, Duberstein JN (2006) Assessment of the nesting population of double-crested cormorants Phalacrocorax auritus albociliatus on Isla Alcatraz, Gulf of California, México. Mar Ornithol 33:195–197
Polidoro BA, Carpenter KE, Collins L, Duke NC, Ellison AM, Ellison JC, Farnsworth EJ, Fernando ES, Kathiresan K, Koedam NE, Livingstone SR, Miyagi T, Moore GE, Ngoc NV, Ong JE, Primavera JH, Salmo SG, Sanciangco JC, Sukardjo S, Wang Y, Yong JWH, Hansen DM (2010) The loss of species: mangrove extinction risk and geographic areas of global concern. PLoS ONE 5:e10095. https://doi.org/10.1371/journal.pone.0010095
Polidoro BA, Carpenter KE, Dahdouh-Guebas F, Ellison JC, Koedam NE, Yong JWH (2014) Global patterns of mangrove extinction risk: implications for ecosystem services and biodiversity loss. In: Maslo B, Lockwood JL (eds) Coastal Conservation. Cambridge University Press, pp 15–36
Pool DJ, Snedaker SC, Lugo AE (1977) Structure of mangrove forests in Florida, Puerto Rico, Mexico, and Costa Rica. Biotropica 9:195–212. https://doi.org/10.2307/2387881
RStudio: Integrated Development Environment for R. R Studio Team, RStudio (2021) PBC, Boston, MA. http://www.rstudio.com. Accessed 15 January 2023
Ramírez-Bastida P (2008) Análisis ecológico y biogeográfico de la avifauna lacustre de México. Doctoral thesis. Universidad Nacional Autónoma de México
Ramírez-Bastida P, Meléndez-Herrada A, López-Saut EG, Saldaña-Martínez S, Ruiz-Rodríguez A, Vargas-Gómez M, Cruz-Nava AR, Dávalos-Fong MI, García-Valencia UD, Sánchez-Sánchez C, Gómez-Rosas A (2018) Importancia De Los ambientes acuáticos urbanos para las aves nativas: El caso de la zona metropolitana de la ciudad de México. In: Ramírez-Bautista A, Pineda-López R (eds) Ecología y Conservación de fauna en Ambientes Antropizados. REFAMA-CONACyT-UAQ, pp 5–28
Robert M, McNeil R (1989) Comparative day and night feeding strategies of shorebirds species in a tropical environment. Ibis 131:69–79. https://doi.org/10.1111/j.1474-919X.1989.tb02745.x
Robertson AI, Duke NC (1987) Mangroves as nursery sites: comparisons of the abundance and species compositions of fish and crustaceans in mangroves and other nearshore habitats in tropical Australia. Mar Biol 96:193–205. https://doi.org/10.1007/BF00427019
Rodríguez-Zúñiga MT, Troche-Souza C, Vázquez-Lule AD, Márquez-Mendoza JD, Vázquez- Balderas B, Valderrama-Landeros L, Velázquez-Salazar S, Cruz-López MI, Ressl R, Uribe-Martínez A, Cerdeira-Estrada S, Acosta-Velázquez J, Díaz-Gallegos J, Jiménez-Rosenberg R, Fueyo-Mac Donald L, Galindo-Leal C (2013) Manglares De México/ Extensión, distribución y monitoreo. Comisión Nacional para el Conocimiento y Uso de la Biodiversidad
Ruiz Bruce Taylor MDM, Salazar JLR, Enríquez PL, León-Cortés JL, García-Estrada C (2017) Variation in hierarchical guild structure between two bird assemblages of a wetland in the Mexican Pacific. Rev Biol Trop 65:1540–1553. https://doi.org/10.15517/rbt.v65i4.26266
Salgado-Negret B, Paz H (2016) Escalando De Los rasgos funcionales a procesos poblacionales, comunitarios y ecosistémicos. In: Salgado-Negret B (ed) La Ecología Funcional como aproximación Al Estudio, manejo y conservación de la biodiversidad: protocolos y aplicaciones. Instituto de Investigación de Recursos Biológicos Alexander von Humboldt, pp 12–35
Schaffelke B, Mellors J, Duke NC (2005) Water quality in the Great Barrier Reef region: responses of mangrove, seagrass and macroalgal communities. Mar Pollut Bull 51:279–296. https://doi.org/10.1016/j.marpolbul.2004.10.025
Sebastián-González E, Green AJ (2013) Habitat use by waterbirds in relation to pond size, water depth, and isolation: lessons from a restoration in Southern Spain. Restor Ecol 22:311–318. https://doi.org/10.1111/rec.12078
Şekercioğlu CH (2006) Increasing awareness of avian ecological function. Trends Ecol Evol 21:464–471. https://doi.org/10.1016/j.tree.2006.05.007
Serrano A, Vázquez-Castán L, Ramos-Ramos M, Basáñez-Muñoz AdeJ, Naval-Ávila C (2013) Diversidad Y abundancia de aves en un humedal del norte de Veracruz, México. Acta Zool Mex 29:473–485
Shannon CE (1948) A Mathematical Theory of Communication. Bell Syst Tech J 27:379–423. https://doi.org/10.1002/j.1538-7305.1948.tb01338.x
Sheaves M (2009) Consequences of ecological connectivity: the coastal ecosystem mosaic. Mar Ecol Prog Ser 391:107–115. https://doi.org/10.3354/MEPS08121
Si X, Baselga A, Leprieur S, Song X, Ding P (2016) Selective extinction drives taxonomic and functional alpha and beta diversities in island bird assemblages. J Anim Ecol 85:409–418. https://doi.org/10.1111/1365-2656.12478
Smith VH, Tilman GD, Nekola JC (1999) Eutrophication impacts of excess nutrient inputs on freshwater, marine, and terrestrial ecosystems. Environ Pollut 100:179–196. https://doi.org/10.1016/S0269-7491(99)00091-3
Stiepani J, Gillis LG, Chee SY, Pfeiffer M, Nordhaus I (2021) Impacts of urbanization on mangrove forests and brachyuran crabs in Penang, Malaysia. Reg Environ Change 21:17–25. https://doi.org/10.1007/s10113-021-01800-3
Suding KN, Lavorel S, Chapin FS, Cornelissen JHC, Díaz S, Garnier E, Goldberg D, Hooper DU, Jackson ST, Navass ML (2008) Scaling environmental change through the community level: a trait base response-and-effect framework for plants. Glob Change Biol 14:1125–1140. https://doi.org/10.1111/j.1365-2486.2008.01557.x
Thébault E, Loreau M (2006) The relationship between biodiversity and ecosystem functioning in food webs. Ecol Res 21:17–25. https://doi.org/10.1007/s11284-005-0127-9
Tran LX, Fischer A (2017) Spatiotemporal changes and fragmentation of mangroves and its effects on fish diversity in Ca Mau Province (Vietnam). J Coast Conserv 21:355–368. https://doi.org/10.1007/s11852-017-0513-9
Vanni MJ (2002) Nutrient cycling by animals in freshwater ecosystems. Annu Rev Ecol Syst 33:341–370. https://doi.org/10.1146/annurev.ecolsys.33.010802.150519
Vázquez-Reyes LD, Paz-Hernández H, Godínez-Álvarez HO, Arizmendi M, del Navarro-Sigüenza C AG (2022) Trait shifts in bird communities from primary forest to human settlements in Mexican seasonal forests. Are there ruderal birds? Perspect Ecol Conserv 20:117–125. https://doi.org/10.1016/j.pecon.2021.11.005
Velázquez-Salazar S, Rodríguez-Zúñiga MT, Alcántara-Maya JA, Villeda-Chávez E, Valderrama-Landeros L, Troche-Souza C, Vázquez-Balderas B, Pérez-Espinosa I, Cruz-López MI, Ressl R, De la Borbolla DVG, Paz O, Aguilar-Sierra V, Hruby F, Muñoa-Coutiño JH (2021) Manglares De México. Actualización Y análisis De Los datos 2020. Comisión Nacional para el Conocimiento y Uso de la Biodiversidad
Villéger S, Mason NWH, Mouillot D (2007) New multidimensional functional diversity indices for a multifaceted framework in functional ecology. Ecology 89:2290–2301. https://doi.org/10.1890/07-1206.1
Violle C, Navas ML, Vile D, Kazakou E, Fortunel C, Hummel I, Garnier E (2007) Let the concept of trait be functional! Oikos 116:882–892. https://doi.org/10.1111/j.0030-1299.2007.15559.x
Weisberg PJ, Dilts TE, Becker ME, Young JS, Wong-Kone DC, Newton WE, Ammon EM (2014) Guild-specific responses of avian species to LiDAR-derived habitat heterogeneity. Acta Oecol 59:72–83. https://doi.org/10.1016/j.actao.2014.06.002
West RL, Hess GK (2020) Purple gallinule (Porphyrio martinica). In: Poole AF, Gill FB (eds) Birds of the World. Cornell Lab of Ornithology. https://doi.org/10.2173/bow.purgal2.01
Whelan CJ, Şekercioğlu CH, Wenny D (2016) Bird ecosystem services: economic ornithology of the 21st century. In: Şekercioğlu CH, Wenny D, Whelan CJ (eds) Why birds Matter. The University of Chicago, pp 1–26
Wilman H, Belmaker J, Simpson J, de la Rosa C, Rivadeneira MM, Jetz W (2014) Elton traits 1.0: species-level foraging attributes of the world’s birds and mammals. Ecology 95:2027–2027. https://doi.org/10.1890/13-1917.1
Wootton JT (1995) Effects of birds on sea urchins and algae: a lower-intertidal trophic cascade. Écoscience 2:321–328. https://doi.org/10.1080/11956860.1995.11682299
Zuberogoitia I, Zabala J, Martínez JA, Martínez JE, Akzona A (2008) Effect of human activities on Egyptian vulture breeding success. Anim Conserv 11:313–320. https://doi.org/10.1111/j.1469-1795.2008.00184.x
Acknowledgements
We thank Comisión Nacional de Áreas Naturales Protegidas (CONANP) for their valuable support for fieldwork development. The Museo de Zoología (Facultad de Ciencias, UNAM) provided logistic support in this project. Martha García Hernández, Mauricio Montaño Rendón, Diego Roldán Piña, Jaime Castro Navarro and Enrique Arbeláez Cortés provided help for fieldwork. We thank Katherine Renton, Rodolfo Rioja and Irma Trejo for their comments, and Lynna Kiere for her valuable comments and assistance reviewing the English.
Funding
This paper is part of the fullfilments of AS-T for his Master’s degree studies in the Posgrado en Ciencias Biológicas UNAM, and received a scholarship from the Consejo Nacional de Ciencia y Tecnología (CONACyT 000289). Fieldwork was funded by project HJ006, CONABIO: Aves Acuáticas y Marinas de las Costas de Colima, Guerrero y Oaxaca (PR-B). Raw data are available for public access at the following GBIF url: https://www.gbif.org/dataset/ebc70f17-2ae6-4f59-ad23-cba07d77b2a4. The project DGAPA-UNAM PAPIIT IA210820 (LDV-R) provided facilities for manuscript development.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that there is no conflict of interest.
Additional information
Communicated by Sandra Maria Hartz.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Santos-Tovar, A., Ramírez-Bastida, P., Navarro-Sigüenza, A.G. et al. Habitat fragmentation erodes taxonomic and functional diversity of waterbird communities of the South Pacific coast of Mexico. Ornithol. Res. (2024). https://doi.org/10.1007/s43388-024-00172-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43388-024-00172-6