Abstract
Bird migration is a highly complex, regulated process, of which timing is an essential element. The timing of migration is influenced by moult, age, sex and food of the birds, as well as the distance between the breeding and wintering sites. In this study, we used data from a ringing station on the shores of Lake Baikal to investigate factors influencing migration timing for species with different migration and moulting strategies, wintering sites and feeding habits. In general, we found that the migration of Passerine across Lake Baikal is influenced by similar factors to those of other migratory species in other migratory flyways. For most species, adult birds migrated through the area earlier in both spring and autumn. In spring, protandrous migration was detected for most of the species, while in autumn, differences in migration timing were less common. Migratory birds migrate later in spring and earlier in autumn, the longer the distance between nesting and wintering sites. It is important to highlight, however, that in both seasons only moulting, sex and food type had an equal influence on the timing of migration, while migration distance and age regulated migration in only one season or the other. In both spring and autumn, we observed differences in the timing of the migration of different species. Studies on the migration of north Asian Passerines are important in the future as the populations of several once common species have declined dramatically in recent times.
Similar content being viewed by others
Introduction
The timing of bird migration, whether intra- or interspecific, is influenced by several factors. Among the factors that cause inter- and intraspecific differences, the timing of moult plays an important role. Songbirds moult once or less often twice a year (e.g. willow warbler Phylloscopus trochilus) (Svensson 1992; Jenni and Winkler 2020) during which they change not only their body feathers but also their tail and flight feathers. This is a time- and energy-consuming process (Lindström et al. 1993), which some long-distance migrating Palaearctic species perform after breeding (postnuptial moult) and others perform at wintering sites before spring migration (prenuptial moult) (Barta et al. 2008; Jenni and Winkler 2020). The moulting strategy therefore essentially determines the timing of migration not only between species (Pulido and Coppack 2004) but also within species between different age classes, as the moulting of age classes of a given species may differ. For example, adult birds have complete moult, while juvenile/immature birds have partial moult. In species where adult birds have complete postnuptial moult, juveniles that have only partial moult will leave for wintering grounds earlier, while in species where adult birds have complete prenuptial moult, they will precede juveniles in autumn (Kiat and Izhaki 2016).
Within species, the migration of the males and females may also differ. Differences in the timing of male and female migration have been explained by different wintering grounds of the sexes (males sometimes winters northern than females), earlier departure or faster migration (Morbey and Ydenberg 2001; Coppack and Pulido 2009; Schmaljohann et al. 2016; Woodworth et al. 2016; Briedis et al. 2019; Schmaljohann 2019). Both protandrous and protogynous migration can occur, but the former is more common during spring migration than the latter (Morbey and Ydenberg 2001). Protandrous migration in spring is better known in species where males and females can be separated by plumage coloration (Spina et al. 1994; Rubolini et al. 2004; Nam et al. 2011) than in species where separation of the sexes is only possible using different biometrics, e.g. willow warbler (Durman 1967; Lawn 1974), common chiffchaff Ph. collybita (Reynolds 1978; Harnos and Csörgő 2011), several species of warblers nesting in Siberia (Bozó and Heim 2016) and eastern kingbird Tyrannus tyrannus (Cooper et al. 2009). Protandrous migration in spring is due to the fact that males need to return early in spring to occupy a suitable territory, and is also caused by sexual selection pressures (Reynolds et al. 1986). Protogynous migration has been recorded primarily in polyandrous species such as Wilson’s phalaropes Phalaropus tricolor and red-necked phalaropes Phalaropus lobatus (Reynolds et al. 1986) and spotted sandpiper Actitis macularius (Oring and Lank 1982).
In autumn, songbirds are usually characterised by the fact that females leave breeding sites earlier than males (Mills 2005; Lehikoinen et al. 2017). This may be due to intraspecific competition (males staying longer in their territories, Logan and Hyatt 1991; Weggler 2000) and the search for new nesting sites (Forstmeier 2002; Mills 2005). It should also be noted that in several migratory species, females often winter further south than males (e.g. hermit thrush Catharus guttatus, Stouffer and Dwyer 2003), so they have to leave breeding sites earlier in autumn (Gauthreaux 1982). However, males of some species, such as the red-backed shrike Lanius collurio, leave the breeding sites earlier than females (Alerstam 1993; Csörgő and Parádi 1998), which may be due to the fact that this species also holds territory at the wintering site and that occupation of the best quality territories is only possible with an earlier departure.
The timing of migration is also age-dependent. Studies on Mediterranean islands have shown that in 18 out of 26 long-distance migratory species, adults returned earlier in spring than second-year birds (Spina et al. 1994). In the Carpathian Basin, this phenomenon has been observed in reed warblers (Acrocephalus spp.) and pied flycatcher (Ficedula hypoleuca, Harnos et al. 2015a, 2015b), among others. Studies with American species have also led to the same result (e.g. Parulinae, Francis and Cooke 1986, rose-breasted grosbeak Pheucticus ludovicianus, Francis and Cooke 1990, purple martin Progne subis, Morton and Derrickson 1990).
In autumn, most bird species are characterised by an earlier departure of adults from their nesting sites, but the opposite is true for species such as the willow warbler (Hedenström and Petterson 1987) and the European robin Erithacus rubecula (Polak and Szewczyk 2007). This is due to the timing of moult, but in the case of the willow warbler, for example, adult birds are ahead of juveniles during migration due to their faster migration and the earlier arrival at the wintering sites (Hedenström and Petterson 1987).
There are several reasons for age-dependent migration, one of which is to avoid competition at stopover sites and wintering grounds, as adult birds are more efficient at exploiting available resources (Gochfeld and Burger 1984; Ellegren 1991) and therefore migrate faster than juveniles (Rguibi-Idrissi et al. 2003; Crysler et al. 2016). Therefore, for younger birds, earlier migration is not beneficial.
Migration distance also affects migration phenology (Marchetti et al. 1995; Schmaljohann 2019; Bozó et al. 2020; Wobker et al. 2021). Typically, the greater the distance between nesting and wintering sites is, the sooner birds leave breeding sites and the later they return in spring (Jenni and Kéry 2003). This may also cause morphological differences between populations, as individuals from populations nesting further north have a longer distance to travel, for which a longer and more pointed wing has evolved (Gaston 1974; Tiainen and Hanski 1985; Lockwood et al. 1998; Peiró 2003).
In recent years, the migration of songbirds using the East Asian-Australasian migratory flyway has been studied more intensively, and factors influencing the timing of migration (Bozó et al. 2020; Wobker et al. 2021) and sex-specific migration (Bozó and Heim 2016) have been investigated based on data from a ringing station in the Muraviovka Park in the Russian far east. In this regard, Bozó et al. (2020) found for Phylloscopus warblers that spring migration phenology was explained by moult strategy and the preferred prey size, while autumn migration phenology was linked to the migration distance of the species. Wobker et al. (2021) in a multispecies analysis obtained similar results for age- and sex-dependent migration, as well as for moult strategy and migration distance, as for other migration routes, as did Bozó and Heim (2016) for the sex-dependent migration of warblers.
In the present study, we used data from a ringing station on the shores of Lake Baikal in southern Siberia to investigate factors influencing migration timing for species with different migration and moulting strategies, wintering sites and feeding habits. We assumed that the timing of migration of females and males, and adults and juveniles would differ between autumn and spring, but we also assumed that the results might differ from this pattern in case of different taxonomic groups. We also assumed that species with longer migration routes arrive later in spring and earlier in autumn to the study site, just as species with postnuptial moult arrive later in autumn than species with prenuptial moult. As food can also influence the timing of bird migration (Katti and Price 2003; Bozó et al. 2020), we consider that it is likely that insectivorous species return from wintering grounds later in spring than seed-eating species. This is due to the fact that insects are available later than seeds (Deppe et al. 2005).
Material and methods
The study site was located in the buffer zone of the Baikalsky State Nature Biosphere Reserve, which is situated on the southeast coast of Lake Baikal, southwest from the Mishikha River mouth on the Pribaikalskaya flatland (51°38′38.3994″N; 105°31′22.7994″E). The surrounding vegetation is dominated by cedar forests Cedrus spp., mixed with birch Betula spp., aspen Populus spp. and fir Abies spp., and alternating with small willow bushes and grass meadows. Birds were captured with Japanese-type mist-nets with a total length of 210 linear metres and ringed within the Baikal Bird Ringing Scheme. Fieldwork was conducted between 1 April and 20 June in spring and 25 July and 20 October in autumn during 2014–2018.
Data analysis was based on 8334 captured individuals of 11 species: common rosefinch Carpodacus erythrinus, hawfinch Coccothraustes coccothraustes, little bunting Emberiza pusilla, black-faced bunting E. spodocephala, Taiga flycatcher Ficedula albicilla, brown shrike Lanius cristatus, Siberian rubythroat Calliope calliope, Eurasian siskin Spinus spinus, red-flanked bluetail Tarsiger cyanurus, dusky thrush Turdus eunomus and Naumann’s thrush T. naumanni (Table 1). For the analysis, species with different migration strategies, migration distances, nesting and wintering areas were selected from the 81 species ringed at the station, with a minimum number of 100 individuals caught during the study period, and for which either age or sex determination (or both) was possible. Species names and baseline taxonomy follow the IOC World Bird List version 9.2 (Gill et al. 2021). The species, sex and age of the captured individuals were identified using Svensson (1992), Brazil (2009) and Demongin (2016); however, age and sex determination were not possible in many cases. Two out of the 11 study species (common rosefinch and brown shrike) exhibit postnuptial moult while the other nine species exhibit prenuptial moult (Svensson 1992).
The median date of migration was calculated for each species in the spring and autumn migration periods based on the first captures (recaptures excluded). These calculations were also performed for males and females, and adult and juvenile birds within species. The timing of migration based on the median migration day of different species was compared using the Kruskal–Wallis test. As the data were not normally distributed, the timing of migration of different sex and age groups was compared using the Mann–Whitney U test. Multiple regression was used to test the effect of different variables on migration timing. In the analysis, the following variables were examined: sex, age, the distance between the centre of wintering sites and the ringing site per species, moulting strategy, and type of food (insectivorous, seed-eating). Year was added as a random effect.
Results
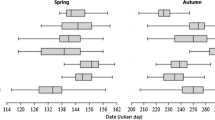
A total of 3672 individuals in spring and 4662 individuals in autumn of 11 species were used for the present analyses (Table 1). There was a significant difference between the timing of migration of the species studied in spring (H = 2406, P < 0.0001) and in autumn (H = 3841, P < 0.0001) (Fig. 1).
In spring, age (adults returned earlier), sex (males migrated earlier), food type (seed-eaters migrated later than insectivorous species) and timing of moult (species with prenuptial moult arrived later in spring) also affected migration periods (Table 2). There was no association between migration timing and migration distance (Fig. 2).
In autumn, the timing of migration was also influenced by the sex (males started later), migration distance (the shorter the migration distance, the later they arrive), food (seed-eaters migrate earlier than insectivorous species) and moult (species with postnuptial moult migrate later). In contrast, there was no association between the timing of migration and age (Table 2).
Age- and sex-dependent migration
In spring, males arrived significantly earlier than females in seven species (common rosefinch, little bunting, black-faced bunting, Taiga flycatcher, brown shrike, siberian rubythroat and red-flanked bluetail), while there was no significant difference in the timing of the arrival of the females in the other four species. When age was examined, adult birds arrived earlier in the spring than second-year birds for six species (little bunting, Taiga flycatcher, Siberian rubythroat, Eurasian siskin, Naumann’s thrush and dusky thrush), while there was no significant correlation for the other five species (Table 3).
In the autumn, males of four species (hawfinch, Taiga flycatcher, brown shrike and red-flanked bluetail) arrived later than females. In terms of age, adult birds migrated through the area earlier than young birds for four species (little bunting, Taiga flycatcher, brown shrike and red-flanked bluetail) (Table 3).
Discussion
There were significant intra- and interspecific differences in the timing of migration of the 11 songbird species studied in this paper, for complex reasons discussed as follows.
For the common rosefinch, Eurasian siskin, dusky and Naumann’s thrush, we could only carry out the analyses for the spring migration, because these four species were only caught in extremely small numbers in the autumn. The reason for this is not known, but the phenomenon of loop migration is one of the possible causes. During loop migration, birds do not use the same routes for spring and autumn migration, as has been shown in several different bird species using different methods (Gill et al. 2009; Klaassen et al. 2010; Szép et al. 2017; Tøttrup et al. 2017; Jónás et al. 2018). The exact cause of this is not known, but prevailing winds during migration and/or variation in food availability might play a role (Gauthreaux et al. 2006; Shaffer et al. 2006; Klaassen et al. 2011; Thorup et al. 2017; Tøttrup et al. 2017). In Lake Baikal and Muraviovka Park, loop migration has been investigated for six species based on biometric data and has been hypothesised for black-faced bunting and red-flanked bluetail (Bozó et al. 2019). However, these four species have not been studied, but in general, neither thrushes nor finches show loop migration, and such a difference in the number of individuals caught in the two seasons should not result from loop migration. The migration of siskins in Europe is highly complex, with a tendency to invasion, intra- and inter-seasonal wintering grounds changes and significant movements during winter, and no well-defined migration routes (Wernham et al. 2002), so the seasonal differences in catches at Lake Baikal may be due to the extreme migratory characteristics of the species. In the case of thrushes and common rosefinch, the area may be an important stopover site in spring, but not in autumn, when birds do not stop here. Indeed, the location of stopover sites may differ between spring and autumn migration periods due to the location of departure sites and barriers (Alerstam 1993).
In both spring and autumn, we observed differences in the timing of the migration of different species. Red-flanked bluetail was the earliest to migrate through the area in spring, while in autumn it was the latest to migrate there. This is interesting because it is an insectivorous species, and the median date of migration in both seasons was when the area typically experiences winter weather. This migration pattern is likely due to the fact that this species has the largest nesting range of any species studied, from the coast of east Asia to Scandinavia (BirdLife International 2021), and all populations winter in southeast Asia, which they probably reach by migrating along the coastal strip and lower elevation landscapes of China, as do most songbirds (Heim et al. 2020). For this reason, individuals from all populations may migrate through the area, birds that nest very far away have to migrate early in spring, while in autumn they can only leave later because of later breeding, which is reflected in the timing of the median of migration. The result is that they migrate even in winter weather conditions when the insect production is poor. A prolonged, less concentrated migration pattern is characteristic of the hawfinch, which is also typical of western breeding populations of the species, and is due to the fact that the birds’ movements are regulated by the current feeding conditions (Cramp and Brooks 1988). The brown shrike migrated very late in the spring, only in early June, and left the area in autumn, the earliest of all species, in August. A similar strategy is also used by European species (Cramp et al. 1993; Csörgő and Parádi 1998; Bártol 2009; Fuisz and Csörgő 2009), which may be related to the species’ moulting strategy. Brown shrikes have a very complex moulting strategy, but they are characterised by prenuptial moult in the wintering grounds (Svensson 1992), so that they can leave for wintering sites immediately after breeding, in contrast to the other species studied. And, as the species is also likely to hold territories on wintering grounds, it is also important that individuals start early to acquire territories of sufficient quality. Prenuptial moulting is still typical of common rosefinches, but we cannot compare their autumn migration with that of brown shrikes due to the low sample size.
By examining factors influencing migration timing, we obtained similar results to those obtained in other bird migration systems (Rubolini et al. 2004; Schmaljohann 2019) and those obtained by Bozó et al. (2020) and Wobker et al. (2021) in multispecies analyses in the Russian far east. This, as Wobker et al. (2021) concluded, implies that the factors influencing migration patterns are consistent across migration systems. It is important to highlight, however, that in both seasons, only moulting, sex and food type had an equal influence on the timing of migration, while migration distance and age regulated migration in only one season or the other.
Species with postnuptial moulting strategy typically migrated earlier in spring and later in autumn. As noted above for brown shrikes, spring migration might be delayed in species moulting in winter, as species replacing flight feathers during winter may delay departure compared to species that moult in summer (Rubolini et al. 2005), whereas species that complete postnuptial moult cannot depart earlier in autumn but return earlier in spring.
In general, migratory birds tend to migrate later in spring and earlier in autumn, the longer the distance between nesting and wintering sites (Jenni and Kéry 2003). In our study, we were able to demonstrate this relationship, although the result was not statistically significant for spring migration. Similar patterns are known from songbirds in other flyway systems (Alerstam 1993), as well as in the East Asian flyway system in leaf warblers (Bozó et al. 2020), in buntings (Heim et al. 2018) and a complex analysis of several species (Wobker et al. 2021).
Bozó et al. (2020) found a correlation between food size and the timing of spring migration in Siberian warblers, as did Katti and Price (2003) who attributed the distribution patterns of warblers at wintering sites to the abundance and size of different-sized food animals. In the present study, we obtained the result, which contradicts our null hypothesis, that the median migration date of insectivorous species was earlier in spring and later in autumn than that of seed-eaters. This could certainly be due to the fact that red-flanked bluetail migrate through the area weeks earlier (spring) or later (autumn) than other insectivorous species, which could significantly bias the results. It is also important to note that the migration of seed-eating species is much more prolonged and occurs over a wider interval than that of insectivores, which may also bias the results. This makes it impossible to realistically assess the resulting pattern.
In spring, protandrous migration was detected in eight out of the 11 species. The extent of protandrous migration varied between 1 and 9 days, which is similar to results obtained for the East Asian migration flyway (Nam et al. 2011; Bozó and Heim 2016; Wobker et al. 2021). In Korea, Nam et al. (2011) studied spring return times of buntings between 2006 and 2008, and male black-faced buntings preceded females by 7.6 days in spring, which is close to the 9-day value we obtained. Results from a multispecies analysis by Wobker et al. (2021) are also generally similar to our results, but in the case of some species (e.g. little bunting, Taiga flycatcher, red-flanked bluetail) there were also significant differences. These differences may be due to population differences, in that the extent of protandrous migration may vary not only at the species level but also between populations within species in different geographical regions (Wobker et al. 2021). However, this cannot be considered a general phenomenon, as males and females of some species, such as dusky thrush and brown shrike, migrated with similar time lags in spring in Muraviovka Park, located about 1500 km east of Lake Baikal.
In autumn, different timing of migration of the sexes was detected in only four species (hawfinch, Taiga flycatcher, brown shrike, red-flanked bluetail). Protogynous migration in autumn is typical of songbirds (Logan and Hyatt 1991; Weggler 2000; Mills 2005; Jakubas and Wojczulanis-Jakubas 2010; Lehikoinen et al. 2017) and has been detected in the East Asian migration flyway for warblers (Bozó and Heim 2016), as well as the brambling Fringilla montifringilla, Pallas’ reed bunting Emberiza pallasi and rustic bunting E. rustica (Wobker et al. 2021). Autumn protandrous migration is less common, as males often remain on breeding sites in search of nesting sites for the following year (Forstmeier 2002; Mills 2005), while females may benefit from an earlier departure, as they can arrive earlier at wintering sites where they can find suitable territory for themselves (Mills 2005). In contrast, autumn protandrous migration may occur in some species, probably those where the females invest more energy in the breeding (McKinnon et al. 2016). This pattern among species using the East Asian migratory flyway appears to be specific to buntings, while we have shown it in the case of little bunting in the present study. Wobker et al. (2021) recorded it in yellow-throated buntings E. elegans migrating through Muraviovka Park.
For most species, adult birds migrated through the area earlier in both spring and autumn. This is probably because adult birds are more experienced than second-year birds in spring (Sergio et al. 2014). In autumn, the situation is less clear, as, in a significant proportion of the species studied, adult individuals perform complete moult after breeding, while juveniles are characterised by only partial moult (Svensson 1992). For this reason, adult birds are able to leave later in the autumn. It is likely, however, that they are ahead of the juveniles during migration due to their faster migration (shorter stopovers, higher fat accumulation rate, Ellegren 1990; Restani 2000; Rguibi-Idrissi et al. 2003).
Overall, the migration of songbirds across Lake Baikal is influenced by similar factors to those of other migratory species in other migratory flyways. Nevertheless, as this is the least studied bird migration system to date (Yong et al. 2015), further research is needed to understand the migration and wintering patterns of these species, especially given that the populations of several species have declined dramatically in recent times (Kamp et al. 2015). In addition, effective conservation proposals can only be developed if basic research provides sufficient quantity and quality of data on these species.
References
Alerstam T (1993) Bird migration. Cambridge University Press
Barta Z, McNamara JM, Houston AI, Weber TP, Hedenström A, Fero O (2008) Optimal moult strategies in migratory birds. Philos Trans R Soc Lond B 363:211–229. https://doi.org/10.1098/rstb.2007.2136
Bártol I (2009) Lesser grey shrike. In: Halmos G, Magyar G, Gyurácz J, Szép T, Bankovics A, Schmidt A, Schmidt E (eds) Csörgő T, Karcza Zs. Hungarian bird migration atlas. Kossuth Kiadó Zrt, Budapest, pp 568–569
BirdLife International (2021) Species factsheet: Tarsiger cyanurus. http://www.birdlife.org. Accessed 1 April 2021
Bozó L, Heim W (2016) Sex-specific migration of Phylloscopus warblers at a stopover site in far eastern Russia. Ringing Migr 31:41–46. https://doi.org/10.1080/03078698.2016.1195213
Bozó L, Heim W, Anisimov Y, Csörgő T (2019) Seasonal morphological differences indicate possible loop migration in two, but not in another four, Siberian passerines. Forktail 35:10–17
Bozó L, Csörgő T, Heim W (2020) Factors controlling the migration phenology of Siberian Phylloscopus species. J Ornithol 162:53–59. https://doi.org/10.1007/s10336-020-01805-5
Brazil M (2009) Birds of east Asia. Christopher Helm, London
Briedis M, Bauer S, Adamík P, Alves JA, Costa JS, Emmenegger T, Gustafsson L, Koleček J, Liechti F, Meier CM, Procházka P, Hahn S (2019) A full annual perspective on sex-biased migration timing in long-distance migratory birds. Proc R Soc Lond B, Biol Sci 286:20182821. https://doi.org/10.5061/dryad.t78400r
Cooper NW, Murphy MT, Redmond LJ (2009) Age- and sex-dependent spring arrival dates of eastern kingbirds. J Field Ornithol 80:35–41. https://doi.org/10.1111/j.1557-9263.2009.00203.x
Coppack T, Pulido F (2009) Proximate control and adaptive potential of protandrous migration in birds. Integr Comp Biol 49:493–506. https://doi.org/10.1093/icb/icp029
Cramp S, Brooks DJ (1988) Handbook of the birds of Europe, the Middle East and north Africa: the birds of the Western Palearctic. In: Vol. V: Tyrant flycatchers to thrushes. Oxford Univesity Press, Oxford
Cramp S, Perrins CM, Brooks DJ (1993) Handbook of the birds of Europe, the Middle East and north Africa: the birds of the Western Palearctic. In: Vol. VII: Flycatchers to shrikes. Oxford Univesity Press, Oxford
Crysler ZJ, Ronconi RA, Taylor PD (2016) Differential fall migratory routes of adult and juvenile ipswich sparrows (Passerculus sandwichensis princeps). Mov Ecol 4:3. https://doi.org/10.1186/s40462-016-0067-8
Csörgő T, Parádi I (1998) Migration of red-backed shrike (Lanius collurio) in the Carpathian basin. Proceedings of the 2nd International Shrike Symposium in IBCE Tech Publ 6:1–5
Demongin L (2016) Identification guide to birds in the hand. Beauregard-Vendon, France
Deppe JL, Rotenberry JT (2005) Temporal patterns in fall migrant communities in Yucatan, Mexico. Condor 107:228–243. https://doi.org/10.1093/condor/107.2.228
Durman RF (1967) Weights and wing lengths of willow warblers caught on Bardsey, 1961–65. Bird Study 14:120–122. https://doi.org/10.1080/00063656709476153
Ellegren H (1990) Timing of autumn migration in bluethroats Luscinia s. svecica depends on timing of breeding. Ornis Fenn 67:13–17
Ellegren H (1991) Stopover ecology of autumn migrating bluethroats Luscinia s. svecica in relation to age and sex. Ornis Scand 22:340–348. https://doi.org/10.2307/3676506
Forstmeier W (2002) Benefits of early arrival at breeding grounds vary between males. J Anim Ecol 71:1–9. https://doi.org/10.1046/j.0021-8790.2001.00569.x
Francis CM, Cooke F (1986) Differential timing of spring migration in wood warblers (Parulinae). Auk 103:548–556. https://doi.org/10.1093/auk/103.3.548
Francis CM, Cooke F (1990) Differential timing of spring migration in rose-breasted grosbeaks. J Field Ornithol 61:404–412
Fuisz TI, Csörgő T (2009) Red-backed shrike. In: Halmos G, Magyar G, Gyurácz J, Szép T, Bankovics A, Schmidt A, Schmidt E (eds) Csörgő T, Karcza Zs. Hungarian bird migration atlas. Kossuth Kiadó Zrt, Budapest, pp 566–568
Gaston AJ (1974) Adaptation in the genus Phylloscopus. Ibis 116:432–450. https://doi.org/10.1111/j.1474-919X.1974.tb07644.x
Gauthreaux SA (1982) The ecology and evolution of avian migration systems. In: Farner DS, King JR, Parkes KC (eds) Avian biology, Vol. VI. Elsevier Science, Burlington, pp 93–168
Gauthreaux SA, Belser CG, Welch CM (2006) Atmospheric trajectories and spring bird migration across the Gulf of Mexico. J Ornithol 147:317–325. https://doi.org/10.1007/s10336-006-0063-7
Gill RE Jr, Tibbitts TL, Douglas DC, Handel CM, Mulcahy DM, Gottschalck JC, Warnock N, McCaffery BJ, Battley PF, Piersma T (2009) Extreme endurance flights by landbirds crossing the Pacific Ocean: ecological corridor rather than barrier? Proc R Soc Lond B, Biol Sci 276:447–457. https://doi.org/10.1098/rspb.2008.1142
Gill F, Donsker D, Rasmussen P (eds) (2021) IOC world bird list (v 11.1). https://doi.org/10.14344/IOC.ML.11.1. Accessed 1 April 2021
Gochfeld M, Burger J (1984) Age differences in foraging behavior of the American robin (Turdus migratorius). Behaviour 88:227–239. https://doi.org/10.1163/156853984X00335
Harnos A, Csörgő T (2011) Sex determination based on biometric data - case study of the chiffchaff. Ornis Hung 19:40–52
Harnos A, Ágh N, Sz K, Zs L, Csörgő T (2015a) Increasing protandry in the spring migration of the pied flycather (Fycedula hypoleuca) in central Europe. J Ornithol 156:543–546. https://doi.org/10.1007/s10336-014-1148-3
Harnos A, Zs L, Fehérvári P, Csörgő T (2015b) Sex and age dependent migration phenology of the pied flycatcher in a stopover site in the Carpathian Basin. Ornis Hung 23:10–19. https://doi.org/10.1515/orhu-2015-0010
Hedenström A, Petterson J (1987) Migration routes and wintering areas of willow warblers Phylloscopus trochilus (L.) ringed in Fennoscandia. Ornis Fenn 64:137–143
Heim W, Eccard JA, Bairlein F (2018) Migration phenology determines niche use of east Asian buntings (Emberizidae) during stopover. Curr Zool 64:681–692. https://doi.org/10.1093/cz/zoy016
Heim W, Heim RJ, Beermann I, Burkovskiy OA, Gerasimov Y, Ktitorov P, Ozaki K, Panov I, Sander MM, Sjöberg S, Smirenski SM, Thomas A, Tøttrup AP, Tiunov IM, Willemoes M, Hölzel N, Thorup K, Kamp J (2020) Using geolocator tracking data and ringing archives to validate citizen-science based seasonal predictions of bird distribution in a data-poor region. Global Ecol Conserv 24:e01215. https://doi.org/10.1016/j.gecco.2020.e01215
Jakubas D, Wojczulanis-Jakubas K (2010) Sex-and age-related differences in the timing and body condition of migrating reed warblers Acrocephalus scirpaceus and sedge warblers Acrocephalus schoenobaenus. Naturwissenschaften 97:505–511. https://doi.org/10.1007/s00114-010-0666-y
Jenni L, Kéry M (2003) Timing of autumn bird migration under climate change: advances in long-distance migrants, delays in short-distance migrants. Proc R Soc Lond B, Biol Sci 270:1467–1471. https://doi.org/10.1098/rspb.2003.2394
Jenni L, Winkler R (2020) Moult and ageing of European passerines. Bloomsbury Publishing, London
Jónás B, Harnos A, Csörgő T (2018) Detection of passerines’ loop migration pattern using biometric data. Acta Zool Acad Sci Hung 64:383–397. https://doi.org/10.17109/AZH.64.4
Kamp J, Oppel S, Ananin AA, Durnev YA, Gashev SN, Hölzel N, Mishchenko AL, Pessa J, Smirenski SM, Strelnikov EG, Timonen S, Wolanska K, Chan S (2015) Global population collapse in a superabundant migratory bird and illegal trapping in China. Conserv Biol 29:1684–1694. https://doi.org/10.1111/cobi.12537
Katti M, Price TD (2003) Latitudinal trends in body size among over-wintering leaf warblers (genus Phylloscopus). Ecography 26:69–79. https://doi.org/10.1034/j.1600-0587.2003.03264.x
Kiat Y, Izhaki I (2016) Moult strategies affect age differences in autumn migration timing in east Mediterranean migratory passerines. PLoS One 11:e0147471. https://doi.org/10.1371/journal.pone.0147471
Klaassen RH, Strandberg R, Hake M, Olofsson P, Tøttrup AP, Alerstam T (2010) Loop migration in adult marsh harriers Circus aeruginosus, as revealed by satellite telemetry. J Avian Biol 41:200–207. https://doi.org/10.1111/j.1600-048X.2010.05058.x
Klaassen RH, Ens BJ, Shamoun-Baranes J, Exo KM, Bairlein F (2011) Migration strategy of a flight generalist, the lesser black-backed gull Larus fuscus. Behav Ecol 23:58–68. https://doi.org/10.1093/beheco/arr150
Lawn M (1974) The willow warbler migration at Sandwich Bay Observatory. Sandwich Bay Bird Observatory Report 47–52
Lehikoinen A, Santaharju J, Møller AP (2017) Sex-specific timing of autumn migration in birds: the role of sexual size dimorphism, migration distance and differences in breeding investment. Ornis Fenn 94:53–65
Lindström Å, Pearson DJ, Hasselquist D, Hedenström A, Bensch S, Åkesson S (1993) The moult of barred warblers Sylvia nisoria in Kenya - evidence for a split wing-moult pattern initiated during the birds’ first winter. Ibis 135:403–409. https://doi.org/10.1111/j.1474-919X.1993.tb02112.x
Lockwood R, Swaddle JP, Rayner JMV (1998) Avian wingtip shape reconsidered: wingtip shape indices and morphological adaptation to migration. J Avian Biol 29:273–292. https://doi.org/10.2307/3677110
Logan CA, Hyatt LE (1991) Mate attraction by autumnal song in the northern mockingbird (Mimus polyglottos). Auk 108:429–432
Marchetti K, Price T, Richman A (1995) Correlates of wing morphology with foraging behaviour and migration distance in the genus Phylloscopus. J Avian Biol 26:177–181. https://doi.org/10.2307/3677316
McKinnon EA, Macdonald CM, Gilchrist HG, Love OP (2016) Spring and fall migration phenology of an Arctic-breeding passerine. J Ornithol 157:681–693. https://doi.org/10.1007/s10336-016-1333-7
Mills AM (2005) Protogyny in autumn migration: do male birds “play chicken”? Auk 122:71–81. https://doi.org/10.1093/auk/122.1.71
Morbey YE, Ydenberg RC (2001) Protandrous arrival timing to breeding areas: a review. Ecol Lett 4:663–673. https://doi.org/10.1046/j.1461-0248.2001.00265.x
Morton ES, Derrickson KC (1990) The biological significance of age-specific return schedules in breeding purple martins. Condor 92:1040–1050. https://doi.org/10.2307/1368740
Nam HY, Choi CY, Park JG, Hong GP, Won IJ, Kim SJ, Bing GC, Chae HY (2011) Protandrous migration and variation in morphological characters in Emberiza buntings at an East Asian stopover site. Ibis 153:494–501. https://doi.org/10.1111/j.1474-919X.2011.01134.x
Oring LW, Lank DB (1982) Sexual selection, arrival times, philopatry and site fidelity in the polyandrous spotted sandpiper. Behav Ecol Sociobiol 10:185–191. https://doi.org/10.1007/BF00299684
Peiró IG (2003) Intraspecific variation in the wing shape of the long-distance migrant reed warbler Acrocephalus scirpaceus: effects of age and distance of migration. Ardeola 50:31–37
Polak M, Szewczyk P (2007) Relation between stopover length and time and body parameters of European robin Erithacus rubecula (L., 1758) during autumn migration (central Poland). Pol J Ecol 55:511–517
Pulido F, Coppack T (2004) Correlation between timing of juvenile moult and onset of migration in the blackcap, Sylvia atricapilla. Anim Behav 68:167–173. https://doi.org/10.1016/j.anbehav.2003.11.006
Restani M (2000) Age-specific stopover behaviour of migrant bald eagles. Wilson Bull 112:28–34
Reynolds A (1978) Chiffchaffs at rye meads. Ringing Migr 2:38–41. https://doi.org/10.1080/03078698.1978.9673733
Reynolds JD, Colwell MA, Cooke F (1986) Sexual selection and spring arrival times of red-necked and Wilson’s phalaropes. Behav Ecol Sociobiol 18:303–310. https://doi.org/10.1007/BF00300008
Rguibi-Idrissi H, Julliard R, Bairlein F (2003) Variation in the stopover duration of reed warblers Acrocephalus scirpaceus in Morocco: effects of season, age and site. Ibis 145:650–656. https://doi.org/10.1046/j.1474-919X.2003.00208.x
Rubolini D, Spina F, Saino N (2004) Protandry and sexual dimorphism in trans-Saharan migratory birds. Behav Ecol 15:592–601. https://doi.org/10.1093/beheco/arh048
Rubolini D, Spina F, Saino N (2005) Correlates of timing of spring migration in birds: a comparative study of trans-Saharan migrants. Biol J Linn Soc 85:199–210. https://doi.org/10.1111/j.1095-8312.2005.00484.x
Schmaljohann H (2019) The start of migration correlates with arrival timing, and the total speed of migration increases with migration distance in migratory songbirds: a cross-continental analysis. Mov Ecol 7:25. https://doi.org/10.1186/s40462-019-0169-1
Schmaljohann H, Meier C, Arlt D, Bairlein F, van Oosten H, Morbey YE, Åkesson S, Buchmann M, Chernetsov N, Desaever R, Elliott J, Hellström M, Liechti F, López A, Middleton J, Ottosson U, Pärt T, Spina F, Eikenaar C (2016) Proximate causes of avian protandry differ between subspecies with contrasting migration challenges. Behav Ecol 27:321–331. https://doi.org/10.1093/beheco/arv160
Sergio F, Tanferna A, de Stephanis R, Jiménez LL, Blas J, Tavecchia G, Preatoni D, Hiraldo F (2014) Individual improvements and selective mortality shape lifelong migratory performance. Nature 515:410–413. https://doi.org/10.1038/nature13696
Shaffer SA, Tremblay Y, Weimerskirch H, Scott D, Thompson DR, Sagar PM, Moller H, Taylor GA, Foley DG, Block BA, Costa DP (2006) Migratory shearwaters integrate oceanic resources across the Pacific Ocean in an endless summer. Proc Natl Acad Sci USA 103:12799–12802. https://doi.org/10.1073/pnas.0603715103
Spina F, Massi A, Montemaggiori A (1994) Back from Africa: who’s running ahead? Aspects of differential migration of sex and age classes in Palearctic-African spring migrants. Ostrich 65:137–150. https://doi.org/10.1080/00306525.1994.9639676
Stouffer PC, Dwyer GM (2003) Sex-biased winter distribution and timing of migration of hermit thrushes (Catharus guttatus) in eastern North America. Auk 120:836–847. https://doi.org/10.1093/auk/120.3.836
Svensson L (1992) Identification guide to European passerines. Svensson, Stockholm
Sz K, Harnos A, Fehérvári P, Csörgő T (2011) Changes in migration phenology and biometrical traits of reed, marsh and sedge warblers. Cent Eur J Biol 7:115–125. https://doi.org/10.2478/s11535-011-0101-1
Szép T, Liechti F, Nagy K, Zs N, Hahn S (2017) Discovering the migration and non-breeding areas of sand martins and house martins breeding in the Pannonian Basin (central-eastern Europe). J Avian Biol 48:114–122. https://doi.org/10.1111/jav.01339
Thorup K, Tøttrup AP, Willemoes M, Klaassen RHG, Strandberg R, Vega ML, Dasari HP, Araújo MB, Wikelsi M, Rahbek C (2017) Resource tracking within and across continents in long-distance bird migrants. Sci Adv 3:e1601360. https://doi.org/10.1126/sciadv.1601360
Tiainen J, Hanski IK (1985) Wing shape variation of Finnish and Central European willow warblers Phylloscopus trochilus and chiffchaffs P. collybita. Ibis 127:365–371. https://doi.org/10.1111/j.1474-919X.1985.tb05078.x
Tøttrup AP, Pedersen L, Onrubia A, Klaassen RH, Thorup K (2017) Migration of red-backed shrikes from the Iberian Peninsula: optimal or sub-optimal detour? J Avian Biol 48:149–154. https://doi.org/10.1111/jav.01352
Weggler M (2000) Reproductive consequences of autumnal singing in black redstarts (Phoenicurus ochruros). Auk 117:65–73. https://doi.org/10.1093/auk/117.1.65
Wernham CV, Toms MP, Marchant JH, Clark JA, Siriwardena GM, Baillie SR (2002) The migration atlas: movements of the birds of Britain and Ireland. Poyser, London
Wobker J, Heim W, Schmaljohann H (2021) Sex, age, molt strategy, and migration distance explain the phenology of songbirds at a stopover along the East Asian flyway. Behav Ecol Sociobiol 75:25. https://doi.org/10.1007/s00265-020-02957-3
Woodworth BK, Newman AEM, Turbek SP, Dossman BC, Hobson KA, Wassenaar LI, Mitchell GW, Wheelwright NT, Norris DR (2016) Differential migration and the link between winter latitude, timing of migration, and breeding in a songbird. Oecologia 181:413–422. https://doi.org/10.1007/s00442-015-3527-8
Yong DL, Liu Y, Low BW, Espanola CP, Choi CY, Kawakami K (2015) Migratory songbirds in the East Asian-Australasian Flyway: a review from a conservation perspective. Bird Conserv Int 25:1–37. https://doi.org/10.1017/S0959270914000276
Zs G, Gyurácz J, Bank L, Bánhidi P, Farkas R, Németh Á, Csörgő T (2011) Autumn migration of robins (Erithacus rubecula) in Hungary. Biologia 66:548–555. https://doi.org/10.2478/s11756-011-0039-9
Acknowledgements
The authors wish to thank the staff of Baikal Bird Ringing Station as well as all volunteers who participated in the bird ringing work at the station.
Funding
Open access funding provided by Eötvös Loránd University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Research involving human participants and/or animals
The authors confirm that all experiments were carried out under the current law for scientific bird ringing in Russia, and all necessary permissions were obtained.
Informed consent
Not applicable.
Conflict of interest
The authors declare no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bozó, L., Anisimov, Y. & Csörgő, T. Moult, sex and food are the most important factors regulated the timing of migration of north Asian Passerines. Ornithol. Res. 30, 262–270 (2022). https://doi.org/10.1007/s43388-022-00108-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43388-022-00108-y