Abstract
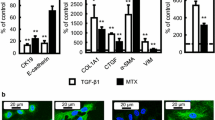
Epithelial-mesenchymal transition (EMT), a biological process through which epithelial cells transdifferentiate into mesenchymal cells, is involved in several pathological events, such as cancer progression and organ fibrosis. So far, we have found that methotrexate (MTX), an anticancer drug, induced EMT in the human A549 alveolar adenocarcinoma cell line. However, the relationship between EMT and the cytotoxicity induced by MTX remains unclear. In this study, we compared the processes of MTX-induced EMT and apoptosis in A549 cells. Q-VD-Oph, a caspase inhibitor, suppressed MTX-induced apoptosis, but not the increase in mRNA expression of α-smooth muscle actin (SMA), a representative EMT marker. In addition, SB431542, an EMT inhibitor, did not inhibit MTX-induced apoptosis. By using isolated clonal cells from wild-type A549 cells, the induction of EMT and apoptosis by MTX in each clone was analyzed, and no significant correlation was observed between the MTX-induced increase in α-SMA mRNA expression and the proportion of cells undergoing apoptosis. Furthermore, the increase in the mRNA expression of α-SMA was well correlated with cyclin-dependent kinase inhibitor 1A, a cell cycle arrest marker, but not with BCL-2 binding component 3 and Fas cell surface death receptor, which are both pro-apoptotic factors, indicating that the MTX-induced EMT may be related to cell cycle arrest, but not to apoptosis. These findings suggested that different mechanisms were involved in the MTX-induced EMT and apoptosis.





Similar content being viewed by others
References
Chen T, You Y, Jiang H, Wang ZZ (2017) Epithelial–mesenchymal transition (EMT): a biological process in the development, stem cell differentiation, and tumorigenesis. J Cell Physiol 232:3261–3272. https://doi.org/10.1002/jcp.25797
Thiery JP, Acloque H, Huang RYJ, Nieto MA (2009) Epithelial-mesenchymal transitions in development and disease. Cell 139:871–890. https://doi.org/10.1016/j.cell.2009.11.007
Salton F, Volpe MC, Confalonieri M (2019) Epithelial-mesenchymal transition in the pathogenesis of idiopathic pulmonary fibrosis. Meddicina 55:83. https://doi.org/10.3390/medicina55040083
Kalluri R, Neilson EG (2003) Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Investig 112:1776–1784. https://doi.org/10.1172/JCI20530
Dongre A, Weinberg RA (2019) New insights into the mechanisms of epithelial–mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol 20:69–84. https://doi.org/10.1038/s41580-018-0080-4
Connolly EC, Freimuth J, Akhurst RJ (2012) Complexities of TGF-β targeted cancer therapy. Int J Biol Sci 8:964–978. https://doi.org/10.7150/ijbs.4564
Chen KJ, Li Q, Wen CM, Duan ZX, Zhang JY, Xu C, Wang JM (2016) Bleomycin (BLM) induces epithelial-to-mesenchymal transition in cultured A549 cells via the TGF-β/Smad signaling pathway. J Cancer 7:1557–1564. https://doi.org/10.7150/jca.15566
Xu J, Liu D, Niu H, Zhu G, Xu Y, Ye D, Li J, Zhang Q (2017) Resveratrol reverses doxorubicin resistance by inhibiting epithelial-mesenchymal transition (EMT) through modulating PTEN/Akt signaling pathway in gastric cancer. J Exp Clin Cancer Res 36:19. https://doi.org/10.1186/s13046-016-0487-8
Kawami M, Harabayashi R, Miyamoto M, Harada R, Yumoto R, Takano M (2016) Methotrexate-induced epithelial–mesenchymal transition in the alveolar epithelial cell line A549. Lung 194:923–930. https://doi.org/10.1007/s00408-016-9935-7
Kubo K, Azuma A, Kanazawa M et al (2013) Japanese Respiratory Society Committee for formulation of consensus statement for the diagnosis and treatment of drug-induced lung injuries, consensus statement for the diagnosis and treatment of drug-induced lung injuries. Respir Investig 51:260–277. https://doi.org/10.1016/j.resinv.2013.09.001
Yamamoto A, Kawami M, Konaka T, Takenaka S, Yumoto R, Takano M (2019) Anticancer drug-induced epithelial-mesenchymal transition via p53/miR-34a axis in A549/ABCA3 cells. J Pharm Pharm Sci 22:516–524. https://doi.org/10.18433/jpps30660
Kawami M, Harada R, Ojima T, Yamagami Y, Yumoto R, Takano M (2019) Association of cell cycle arrest with anticancer drug-induced epithelial-mesenchymal transition in alveolar epithelial cells. Toxicology 424:152231. https://doi.org/10.1016/j.tox.2019.06.002
Sung JM, Cho HJ, Yi H et al (2008) Characterization of a stem cell population in lung cancer A549 cells. Biochem Biophys Res Commun 371:163–167. https://doi.org/10.1016/j.bbrc.2008.04.038
Kawami M, Harabayashi R, Harada R, Yamagami Y, Yumoto R, Takano M (2018) Folic acid prevents methotrexate-induced epithelial-mesenchymal transition via suppression of secreted factors from the human alveolar epithelial cell line A549. Biochem Biophys Res Commun 497:457–463. https://doi.org/10.1016/j.bbrc.2018.02.111
Takano M, Naka R, Sasaki Y, Nishimoto S, Yumoto R (2016) Effect of cigarette smoke extract on P-glycoprotein function in primary cultured and newly developed alveolar epithelial cells. Drug Metab Pharmacokinet 31:417–424. https://doi.org/10.1016/j.dmpk.2016.08.006
Morandi A, Taddei ML, Chiarugi P, Giannoni E (2017) Targeting the metabolic reprogramming that controls epithelial-to-mesenchymal transition in aggressive tumors. Front Oncol 7:40. https://doi.org/10.3389/fonc.2017.00040
Fischer KR, Durrans A, Lee S et al (2015) Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature 527:472–476. https://doi.org/10.1038/nature15748
Tang L (2019) Investigating heterogeneity in HeLa cells. Nat Methods 16:281. https://doi.org/10.1038/s41592-019-0375-1
Ben-David U, Siranosian B, Ha G et al (2018) Genetic and transcriptional evolution alters cancer cell line drug response. Nature 560:325–330. https://doi.org/10.1038/s41586-018-0409-3
Yang Y, Pan X, Lei W, Wang J, Song J (2006) Transforming growth factor-beta1 induces epithelial-to-mesenchymal transition and apoptosis via a cell cycle-dependent mechanism. Oncogene 25:7235–7244. https://doi.org/10.1038/sj.onc.1209712
El-Deiry WS (2016) p21 (WAF1) mediates cell-cycle inhibition, relevant to cancer suppression and therapy. Cancer Res 76:5189–5191. https://doi.org/10.1158/0008-5472.CAN-16-2055
Abbas T, Dutta A (2009) P21 in cancer: intricate networks and multiple activities. Nat Rev Cancer 9:400–414. https://doi.org/10.1038/nrc2657
Grassi ML, Palma Cde S, Thomé CH, Lanfredi GP, Poersch A, Faça VM (2017) Proteomic analysis of ovarian cancer cells during epithelial-mesenchymal transition (EMT) induced by epidermal growth factor (EGF) reveals mechanisms of cell cycle control. J Proteomics 151:2–11. https://doi.org/10.1016/j.jprot.2016.06.009
Li XL, Hara T, Choi Y et al (2014) A p21-ZEB1 complex inhibits epithelial-mesenchymal transition through the microRNA 183-96-182 cluster. Mol Cell Biol 34:533–550. https://doi.org/10.1128/mcb.01043-13
Bachman KE, Blair BG, Brenner K et al (2004) p21WAF1/CIP1 mediates the growth response to TGF-β in human epithelial cells. Cancer Biol Ther 3:221–225. https://doi.org/10.4161/cbt.3.2.666
Zhang Y, Yan W, Jung YS, Chen X (2016) PUMA cooperates with p21 to regulate mammary epithelial morphogenesis and epithelial-to-mesenchymal transition. PLoS ONE 8:e66464. https://doi.org/10.1371/journal.pone.0066464
Brosh R, Assia-Alroy Y, Molchadsky A et al (2013) p53 counteracts reprogramming by inhibiting mesenchymal-to-epithelial transition. Cell Death Differ 20:312–320. https://doi.org/10.1038/cdd.2012.125
Termén S, Tan EJ, Heldin CH, Moustakas A (2013) p53 regulates epithelial-mesenchymal transition induced by transforming growth factor β. J Cell Physiol 228:801–813. https://doi.org/10.1002/jcp.24229
Chang CJ, Chao CH, Xia W et al (2011) p53 regulates epithelial-mesenchymal transition and stem cell properties through modulating miRNAs. Nat Cell Biol 13:317–323. https://doi.org/10.1038/ncb2173
Wang Z, Jiang Y, Guan D et al (2013) Critical roles of p53 in epithelial-mesenchymal transition and metastasis of hepatocellular carcinoma cells. PLoS ONE 8:e72846. https://doi.org/10.1371/journal.pone.0072846
Rahmanian N, Hosseinimehr SJ, Khalaj A (2016) The paradox role of caspase cascade in ionizing radiation therapy. J Biomed Sci 23:88. https://doi.org/10.1186/s12929-016-0306-8
Hattangadi DK, DeMasters GA, Walker TD et al (2004) Influence of p53 and caspase 3 activity on cell death and senescence in response to methotrexate in the breast tumor cell. Biochem Pharmacol 68:1699–1708. https://doi.org/10.1016/j.bcp.2004.06.033
Kolesnick R (2002) The therapeutic potential of modulating the ceramide/sphingomyelin pathway. J Clin Investig 110:3–8. https://doi.org/10.1172/JCI16127
Fekry B, Esmaeilniakooshkghazi A, Krupenko SA, Krupenko NI (2016) Ceramide synthase 6 is a novel target of methotrexate mediating its antiproliferative effect in a p53-dependent manner. PLoS ONE 11:e0146618. https://doi.org/10.1371/journal.pone.0146618
Acknowledgements
This work was supported in part by the Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (JP18H02586, JP18K06749, and JP19K16447).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Ojima, T., Kawami, M., Yumoto, R. et al. Differential mechanisms underlying methotrexate-induced cell death and epithelial-mesenchymal transition in A549 cells. Toxicol Res. 37, 293–300 (2021). https://doi.org/10.1007/s43188-020-00067-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43188-020-00067-w