Abstract
Purpose of Review
In this review, we provide a brief history of intraocular robotic surgical systems and review the latest technological advancements. The goals are to (a) provide readers with a clear understanding of the important work that has been done in this field; (b) illuminate existing challenges towards full clinical adoption; and (c) speculate on future directions.
Recent Findings
The majority of work on intraocular robotic surgical systems has been done in university research settings, although two systems have been evaluated in human clinical trials and one system is commercially available for use in human patients.
Summary
The future of robotic systems in intraocular surgical procedures will depend on the results of ongoing clinical trials and the success of recent start-up companies. Many challenges remain before such systems can become safe and effective treatment options. However, the future of intraocular robotic surgical systems is bright and full of promise.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The incorporation of robotic surgical systems has become increasingly common throughout many surgical fields over the past few decades. The increased adoption rates can be attributed to a number of improvements offered by robotic systems over unassisted surgeons such as increased precision, accuracy, and stability; improved maneuverability and dexterity; and improved visualization and sensing. As an example, the success of the da Vinci Surgical System (Intuitive Surgical, Inc.) has become widely recognized and perhaps the most prevalent robotic surgical system in the world. In the past 2 decades, its clinical installation base has grown 7% year-over-year with nearly 5,000 units sold worldwide [1].
However, despite the success of the da Vinci Surgical System, the incorporation of surgical robotics into ophthalmic surgery has not enjoyed the same degree of rapid success or growth. This delayed transition from laboratory research into clinical practice can be ascribed to the distinct advantages of ophthalmic surgery, which decrease the apparent benefits of using robotic assistance. These advantages include the unobstructed physical access to the surgical workspace; the clear, high-magnification views of the surgical field and tool-to-tissue interaction; and the minimally invasive nature of intraocular tools.
However, despite these advantages, ophthalmic surgery continues to be challenging to perform for an unassisted surgeon. The manipulation of intraocular tissue, sometimes on the scale of a few micrometers, must be performed with high accuracy, without tactile feedback, and within a workspace environment less than 2.5 cm in diameter. Especially in the case of vitreoretinal procedures, the magnitude of motion is so small that intrinsic physiological conditions such as hand tremor and fatigue represent substantial limitations on human performance. For example, it is infeasible for a surgeon to accurately target a specific intraocular location to within a few micrometers and certainly impossible for him or her to hold the surgical instrument completely stationary for minutes at a time. Furthermore, the depth perception of a surgeon is limited in resolution, making it impossible to sense the exact depth of a surgical instrument or the thickness of a thin membrane [2].
In contrast, a robotic system is not inhibited by the aforementioned limitations to the same degree and—for these reasons—may offer promising solutions to improving the safety and efficacy of surgical procedures. For example, a robotic system can enable precise, tremor-free motion of a surgical instrument; positional stability for an indefinite period of time; and increased resolution of depth sensing through an integrated imaging technology such as digital microscopy or optical coherence tomography (OCT). By augmenting a surgeon’s limited capabilities with robotic assistance, the safety, efficacy, and efficiency of ophthalmic surgical procedures can be increased. Additional benefits may include the potential for collaboration between the robot and surgeon during specific procedures; the ability to autonomously perform routine, well-defined tasks; and the incorporation of augmented sensing such as visual overlays or tactile feedback.
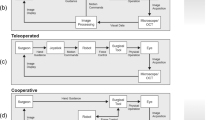
While it can be argued that the integration of robotic systems into ophthalmic surgery has suffered from a delayed start in comparison to other surgical fields, momentum is building in research laboratories and start-up companies across the world. The amount of research continues to increase and is performed across a variety of topics including robotic manipulator design; novel sensing modalities such as OCT and integrated tool sensors; and image processing and segmentation of visual feedback of the intraoperative surgical environment. Figure 1 provides an impression of these emerging trends in the context of published peer-reviewed research articles.
Number of publications per year for keyword searches of titles and abstracts of peer-reviewed publications, collected using Dimensions [3]. Note the predominance of vitreoretinal surgical systems (blue) compared to systems geared towards cataract surgery (orange) as well as the steady increase of intraocular-specific image segmentation work
A Brief History and the Current State-of-the Art
One of the first intraocular robotic systems presented in the literature was the “Stereotaxical Micro-telemanipulator” developed in 1989 [4]. This mechanism was comprised of a 3-DOF spherical manipulator mounted to a 3-DOF translational stage and was used to perform teleoperated vitrectomy and radial keratotomy with improved accuracy, but slower speed, over the unassisted surgeon [5]. For nearly a decade afterwards, little work was done until after the turn of the century when several universities began developing robotic systems specific to the intraocular workspace, the most important of which are described next.
The MICRON is a handheld manipulator developed in 2010 by a collaboration between Johns Hopkins University and the Robotics Institute at Carnegie Mellon University [6•]. The device has been shown to be capable of enforcing smooth motion during emulated surgical procedures with large (~ 50%) reduction in instrument tip positional errors. This device was later improved by the integration of a 6-DOF platform to constrain the remote center of motion (the surgical “pivot point”) near the instrument tip. It was evaluated and shown to demonstrate reduced trajectory following errors of ≤ 20 μm and reductions in hand tremor up to 90% [7]. More recent work includes using the device to detect the puncture of an artificial membrane using a force-sensing needle as well as the ability to maintain tool-to-surface distance despite tool motion and tremor [8].
Another notable system is RAM!S, from the Department of Robotics and Embedded Systems at the Technical University of Munich [9•]. This device is composed of a hybrid parallel-serial mechanism actuated by piezoelectric motors, resulting in a compact system (94 ± 28 × 33.5 × 18.5 mm) capable of 6-DOF of instrument motion [10]. Additional cited metrics include an instrument tip positional precision of 5 μm and a total weight of only 306 g. The device has been evaluated in ex vivo pig eye models using software-imposed virtual fixtures to constrain the RCM location and operate the device using teleoperated control [11, 12]. Most recently, subretinal instrument localization and tracking has been demonstrated in a step towards improved robotic-assisted subretinal cannulation and injection [13].
While robotic surgical systems have nearly unanimously been developed towards vitreoretinal procedures, significant progress has been made by a research team at UCLA towards performing cataract surgical procedures using a partially automated intraocular robotic surgical system. This system, the Intraocular Robotic Interventional Surgical System (IRISS), was originally presented in 2013 where it was used to perform a wide range of teleoperated anterior and posterior intraocular surgical procedures [14]. The mechanical design of this system is unique in that it features a quick tool-exchange mechanism capable of mounting many commercially available intraocular surgical instruments as well as a dual-arm configuration allowing for simultaneous use of two instruments [15]. The original system was teleoperated with motion scaling and tremor reduction and used to demonstrate anterior lens capsulorhexis, viscoelastic injection, hydrodissection, lens aspiration, retinal vein cannulation, and vitrectomy on ex vivo pig eyes in a standard operating theater [14, 16]. The IRISS was also the first (and to date, only) robotic system to mechanically perform a complete, curvilinear capsulorhexis and an entire cataract surgery from corneal incision to lens implantation.
Since this initial success, the UCLA team has since expanded the IRISS into performing partially automated, OCT-guided cataract-extraction procedures. To do so, a commercially available OCT system was integrated with the IRISS and its acquired data used to model the eye anatomy and generate a lens-extraction trajectory [17••, 18•]. To demonstrate the feasibility of this system, 30 ex vivo pig eyes had their lenses autonomously extracted without iatrogenic damage and without surgeon intervention or guidance. Furthermore, intraoperative OCT scans tracked the tip of the surgical instrument during the lens-extraction procedure, allowing for real-time feedback of surgical progress. The UCLA team continues to pursue a wide array of research including an immersive, augmented-reality surgical “cockpit”; a next-generation, high-precision robotic system (Fig. 2); and intraocular-specific real-time image segmentation techniques based on artificial intelligence for use in autonomous robotic guidance [19].
One of the first clinical studies performed on human patients was done with the Preceyes Surgical System in 2018 [20••]. The design of the Preceyes system is based around a parallelogram linkage that offers a mechanically enforced remote center of motion and an instrument tip positional precision of 5 μm. Prior to in-human studies, the system was validated by creating venous occlusions in live, anesthetized pigs, where the use of the system was deemed unobstructive to the established surgical workflow and shown to be effective and safe [21]. Following that initial success, the Preceyes Surgical System was used to perform membrane dissection in human patients and demonstrated that the results with robotic assistance were equally successful to those without. Furthermore, to demonstrate the ability to supersede the limitations of human tactile control, the system was also used to inject recombinant tissue plasminogen activator into subretinal spaces. The success of this system proved to be a clear demonstration of the degree of attainable accuracy, stability, and safety of using robotic assistance in otherwise challenging intraocular surgical procedures.
Since the in-human studies of 2018, the Preceyes Surgical System has become the only commercially available intraocular robotic surgical system [22]. The available system offers a high degree of instrument tip positional precision (< 10 μm) and the ability to impose virtual boundaries to prevent undesirable motion and eliminate iatrogenic retinal trauma. The Preceyes group has continued to publish results of ongoing research, and at least three systems have currently been purchased by hospitals in Europe [23].
A second noteworthy clinical success on human patients was also demonstrated in 2018, in this case by the University of Leuven, Belgium, in the context of a phase I clinical trial [24••].
Their robotic system was first presented in 2014 as a co-manipulated system developed by the Micro- and Precision Engineering Group [25]. This device consists of a parallel arm mechanism with a mechanically constrained remote center of motion and is operated by the surgeon simultaneously holding the surgical instrument that is mounted to the robotic device. In this way, the system offers improved motion scaling, tremor cancellation, and scalable force feedback to increase stability and precision and facilitate maintaining a fixed position for a prolonged duration of time. Original work demonstrated the system being used to perform retinal vein cannulation on in vivo pig eye models with “complete success” being defined as stable intravenous position of the needle tip for more than three minutes; this was confirmed in 15 out of 18 eyes [26].
Later the same year, the same group reported on the clinical use of their device in robot-assisted retinal vein cannulation on live human patients [24••]. In that study, four patients presenting with retinal vein occlusion were treated by the robot-assisted surgeon in the context of a phase I clinical trial with injection durations of up to 10 min. This widely hailed success made clear the technical feasibility of robotic assistance in the ophthalmic surgical field. The robotic system is now being developed by Mynutia (a spinoff company of the university), and the company continues to pursue commercialization of their system.
Finally, a few start-up companies which aim to bring an intraocular robotic surgical system to market have recently received funding and are pursuing clinical validation. These companies have reported progress towards CE-mark certification and clinical trials and include Cambridge Consultants (https://www.cambridgeconsultants.com/us/case-studies/axsis-medical-robotic-concept), AcuSurgical (https://acusurgical.com/), and Ophthorobotics (https://www.ophthorobotics.com/home-en.html). Unlike the major systems presented in this paper (Table 1), no publications or evaluations of their systems are known to exist.
Future of Robotic Systems in Ophthalmology
The future of robotic systems in ophthalmic surgical practice promises increased accuracy, positional stability, and improved sensing of the surgical environment. The possibility of new (as of yet unperformed) surgical procedures also exists, because procedures that are infeasible for a surgeon to perform may fall well within the capabilities of a robotic system. Current procedures that use lasers (for example, femtosecond laser-assisted cataract surgery) already demonstrate levels of accuracy and safety outside the limits of human abilities, which have been shown to lower iatrogenic damage and postoperative complication rates. Microscope-integrated OCT technologies have also introduced the ability to provide visual cross-sectional images of the surgical field through the display of real-time OCT image data alongside the standard microscope view. However, despite the aforementioned technological advancements, surgical complications remain a reality because the physical manipulation of intraocular anatomy continues to depend on human capabilities.
In the realm of cataract surgery, the possibility exists to integrate a robotic manipulator and improved sensing with existing femtosecond laser systems to perform a complete cataract surgery. The critical step of cataract surgery involves removing the cataract from the capsular bag without iatrogenic damage, which relies on both accurate instrument tool control and real-time, accurate knowledge of the surgical environment (namely the posterior capsule location). By incorporating real-time OCT or other imaging modalities to sense the capsule location in response to robot-guided tool motion, the cataract can be completely removed from both pupillary and equatorial regions of the capsular bag while decreasing the risk of complications.
In the realm of vitreoretinal surgery, potential future applications of intraocular robotic surgical systems include gene and stem-cell therapeutic treatments, which are currently experiencing significant progress in treating severe retinal disorders [27]. However, to be effective, these procedures would require micrometer-level accuracy and prolonged tool stability in the presence of eye motion to deliver therapeutics between specific layers within the subretinal space. While subretinal injection has been associated with an increase in complications such as vitreous hemorrhage, retinal detachment, and postoperative development of choroidal neovascularization when performed by a surgeon, a surgical robotic system possesses the accuracy and stability critical for proper therapeutic delivery and can superseded current surgeon performance.
In the near future, the incorporation of intraocular surgical robotic systems may be focused on improving visualization of the intraocular workspace and developing robotic assistance methods for specific and routine tasks. These specific procedures could be fully automated and performed independently from those of a surgeon. However, such possibilities can only be achieved through improving the acquisition quality, speed, and interpretation of OCT or other imaging data; developing robust and accurate segmentation techniques to make sense of the acquired data; and designing mechanical systems that are tightly integrated with the visual acquisition technology such that closed-loop, real-time control can be enabled.
Another potential direction is a surgical system with an immersive, augmented-reality environment equipped with a wide range multi-sensory feedback provided to a surgeon. Physically, such a system may resemble a surgical “cockpit” where the surgeon comfortably sits in an ergonomically optimized position while operating a pair of joysticks or command interfaces (Fig. 3). For feedback, a range of auditory, visual, and haptic inputs can be integrated to provide the surgeon with the most relevant and accurate information possible for each step of a surgical procedure. For example, in the case of complex retinal surgical cases such as dissecting severe epiretinal tissue under the requirements of accurate, bimanual operation, this type of system could be especially beneficial. By augmenting the surgeon’s sensing capabilities through visual overlays to highlight membrane dissection planes atop a highly magnified visualization of the retinal environment, the surgeon could benefit from clear, accurate information that cannot otherwise be provided in a standard operating room setup.
Shown is a photograph of a prototype of an immersive, augmented-reality surgical “cockpit” created at UCLA for the purpose of testing the feasibility of using such a system in robot-assisted retinal surgery. (Reprinted by permission from Springer Nature from: Gerber MJ, et al. Eye (Lond). 2020 Sep; 34(9): 1554–1562. https://doi.org/10.1038/s41433-020-0837-9) [28]
Finally, in the distant future, we envision intraocular robotic surgical systems guided by artificial intelligence capable of making surgical decisions without any supervision from the surgeon. While such a system is perhaps decades away from being realized into actual clinical practice, the underlying technology that will make this vision into reality continues to be developed and improved in research laboratories across the world.
Conclusion
The future of intraocular robotic surgical systems will largely depend on the results of clinical trials currently being performed by commercial companies such as Preceyes and Mynutia, in addition to the successes of a handful of recent start-up companies. As discussed in this article, the systems employed by these groups have already been used to demonstrate promising results, but many challenges remain. Undoubtedly, engineers and surgeons will continue to develop novel technologies and advance current capabilities to ensure that future systems will improve surgical outcomes, extend surgical capabilities into the area of feasible treatment options, and enhance the safety and efficacy of surgical procedures.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Annual Report 2020, Intuitive Surgical, Inc. [https://isrg.intuitive.com/static-files/80b10bf5-c1da-4ad3-bb0e-8c595e2c712c] Accessed date 3 Jan 2022
Hibbard PB, Haines AE, Hornsey RL. Magnitude, precision, and realism of depth perception in stereoscopic vision. Cognitive Research: Principles and Implications. 2017;2(1):25.
Dimensions. Digital science, https://app.dimensions.ai Accessed date 3 Jan 2022
Guerrouad A, Jolly D. Automatic analysis of weariness during a micromanipulation task by SMOS. IEEE Conference Proceeding. 1989;3:906–7.
Guerrouad A, Vidal P. SMOS: stereotaxical microtelemanipulator for ocular surgery. IEEE Conference Proceeding. 1989;3:879–80.
• MacLachlan RA, Becker BC, Tabarés JC, Podnar GW, Lobes Jr LA, Riviere CN. Micron: an actively stabilized handheld tool for microsurgery. IEEE Transactions on Robotics. 2011;28(1):195–212. This is the main work for this research group on intraocular robotic surgical systems.
Yang S, MacLachlan RA, Riviere CN. Manipulator design and operation of a six-degree-of-freedom handheld tremor-canceling microsurgical instrument. IEEE/ASME Trans Mechatron. 2014;20(2):761–72.
Gonenc B, Chae J, Gehlbach P, Taylor RH, Iordachita I. Towards robot-assisted retinal vein cannulation: a motorized force-sensing microneedle integrated with a handheld micromanipulator. Sensors. 2017;17(10):2195.
• Nasseri MA, Eder M, Nair S, Dean EC, Maier M, Zapp D, Lohmann CP, Knoll A. The introduction of a new robot for assistance in ophthalmic surgery. In2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC) 2013 Jul 3 (pp. 5682–5685). IEEE. This is the main work for this research group on intraocular robotic surgical systems.
Nasseri MA, Eder M, Eberts D, Nair S, Maier M, Zapp D, Lohmann CP, Knoll A. Kinematics and dynamics analysis of a hybrid parallel-serial micromanipulator designed for biomedical applications. In2013 IEEE/ASME International Conference on Advanced Intelligent Mechatronics 2013 Jul 9 (pp. 293–299). IEEE.
Nasseri MA, Gschirr P, Eder M, Nair S, Kobuch K, Maier M, Zapp D, Lohmann C, Knoll A. Virtual fixture control of a hybrid parallel-serial robot for assisting ophthalmic surgery: an experimental study. In5th IEEE RAS/EMBS International Conference on Biomedical Robotics and Biomechatronics 2014 Aug 12 (pp. 732–738). IEEE.
Barthel A, Trematerra D, Nasseri MA, Zapp D, Lohmann CP, Knoll A, Maier M. Haptic interface for robot-assisted ophthalmic surgery. In2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC) 2015 Aug 25 (pp. 4906–4909). IEEE.
Zhou M, Roodaki H, Eslami A, Chen G, Huang K, Maier M, Lohmann CP, Knoll A, Nasseri MA. Needle segmentation in volumetric optical coherence tomography images for ophthalmic microsurgery. Appl Sci. 2017;7(8):748.
Rahimy E, Wilson J, Tsao TC, Schwartz S, Hubschman JP. Robot-assisted intraocular surgery: development of the IRISS and feasibility studies in an animal model. Eye. 2013;27(8):972–8.
Wilson JT, Tsao TC, Hubschman JP, Schwartz S. Evaluating remote centers of motion for minimally invasive surgical robots by computer vision. In2010 IEEE/ASME International Conference on Advanced Intelligent Mechatronics 2010 Jul 6 (pp. 1413–1418). IEEE.
Wilson JT, Gerber MJ, Prince SW, Chen CW, Schwartz SD, Hubschman JP, Tsao TC. Intraocular robotic interventional surgical system (iriss): mechanical design, evaluation, and master–slave manipulation. The International Journal of Medical Robotics and Computer Assisted Surgery. 2018;14(1):e1842.
•• Chen CW, Lee YH, Gerber MJ, Cheng H, Yang YC, Govetto A, Francone AA, Soatto S, Grundfest WS, Hubschman JP, Tsao TC. Intraocular robotic interventional surgical system (IRISS): semi-automated OCT-guided cataract removal. The International Journal of Medical Robotics and Computer Assisted Surgery. 2018;14(6):e1949. This is the main work for this research group on intraocular robotic surgical systems.
• Chen CW, Francone AA, Gerber MJ, Lee YH, Govetto A, Tsao TC, Hubschman JP. Semiautomated optical coherence tomography-guided robotic surgery for porcine lens removal. Journal of Cataract & Refractive Surgery. 2019;45(11):1665–9. This is the main work for this research group on intraocular robotic surgical systems.
Shin C, et al. Semi-automated extraction of lens fragments via a surgical robot using semantic segmentation of OCT images with deep learning-experimental results in ex vivo animal model. IEEE Robotics and Automation Letters. 2021;6(3):5261–8.
•• Edwards TL, Xue K, Meenink HCM, Beelen MJ, Naus GJL, Simunovic MP, et al. First-in-human study of the safety and viability of intraocular robotic surgery. Nat Biomed Eng. 2018;2:649–56. This reports on in-human trials using a robotic system.
de Smet MD, Stassen JM, Meenink TC, Janssens T, Vanheukelom V, Naus GJ, Beelen MJ, Jonckx B. Release of experimental retinal vein occlusions by direct intraluminal injection of ocriplasmin. Br J Ophthalmol. 2016;100(12):1742–6.
Preceyes Inc. https://www.preceyes.nl/ Accessed date 3 Jan 2022
Ladha, Reza, et al. "Advantages of robotic assistance over a manual approach in simulated subretinal injections and its relevance for gene therapy." Gene Therapy (2021): 1–7.
•• Gijbels A, Smits J, Schoevaerdts L, Willekens K, Vander Poorten EB, Stalmans P, Reynaerts D. In-human robot-assisted retinal vein cannulation, a world first. Annals of Biomedical Engineering. 2018;46(10):1676–85. This reports on in-human trials using a robotic system.
Gijbels A, Vander Poorten EB, Stalmans P, Van Brussel H, Reynaerts D. Design of a teleoperated robotic system for retinal surgery. In2014 IEEE International Conference on Robotics and Automation (ICRA) 2014 May 31 (pp. 2357–2363). IEEE.
Willekens K, Gijbels A, Schoevaerdts L, Esteveny L, Janssens T, Jonckx B, Feyen JH, Meers C, Reynaerts D, Vander Poorten E, Stalmans P. Robot-assisted retinal vein cannulation in an in vivo porcine retinal vein occlusion model. Acta Ophthalmol. 2017;95(3):270–5.
Schwartz SD, Regillo CD, Lam BL, Eliott D, Rosenfeld PJ, Gregori NZ, Hubschman JP, Davis JL, Heilwell G, Spirn M, Maguire J. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: follow-up of two open-label phase 1/2 studies. The Lancet. 2015;385(9967):509–16.
Gerber MJ, Pettenkofer M, Hubschman J-P. Advanced robotic surgical systems in ophthalmology. Eye (Lond). 2020;34(9):1554–62. https://doi.org/10.1038/s41433-020-0837-9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Gerber and Dr. Hubschman report they have the following patents: a patent 62/671,928 | PCT/US2019/032236 issued to The Regents of the University of California, a patent PCT/US2018/019760 issued to The Regents of the University of California, a patent PCT/US2019/023193 issued to The Regents of the University of California, a patent PCT/US2019/022986 issued to The Regents of the University of California, a patent PCT/US2019/028937 issued to The Regents of the University of California, a patent 63/210,256 (Provisional) pending to The Regents of the University of California, a patent 62/985,143 (Provisional) pending to The Regents of the University of California, and a patent 62/934,694 (Provisional) pending to The Regents of the University of California.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Medical and Surgical Robotics
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gerber, M.J., Hubschman, J.P. Intraocular Robotic Surgical Systems. Curr Robot Rep 3, 1–7 (2022). https://doi.org/10.1007/s43154-021-00071-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43154-021-00071-4