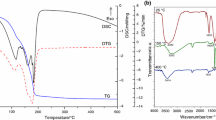
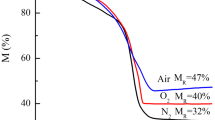
Abstract
Magnetite nanoparticles were synthesized by two methods: reverse coprecipitation and partial oxidation of ferrous ions. The particles were characterized by scanning electron microscopy (SEM), X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), Raman spectroscopy, vibrating sample magnetometry (VSM), and thermogravimetric analysis (TGA). The prepared nanoparticles exhibited superparamagnetic properties at room temperature. The mean size of the particles was in the range of 10-29 nm. TGA curves showed different thermal decomposition behaviors depending on the synthesis method. Distinct magnetic responsiveness was observed for the TGA residue samples. Thermal decomposition kinetics was evaluated by the model-based approach using TGA curves measured at three distinct heating rates in the temperature range of 298-1173 K under a synthetic air atmosphere. The model fit resulted in a correlation coefficient of 0.998 and the order of magnitude for the estimated kinetic parameters agreed with the reported data in the literature.












Similar content being viewed by others
Abbreviations
- A :
-
Pre-exponential factor, \(\mathrm {s^{-1}}\)
- B :
-
Half-maximum height of diffraction peak, Radians
- D :
-
Crystallite size, nm
- E :
-
Activation energy, J \(\mathrm {mol^{-1}}\)
- F :
-
Shape factor in Scherrer equation
- m :
-
Sample mass (mg)
- R :
-
Ideal gas constant, J \(\mathrm {mol^{-1} K^{-1}}\)
- T :
-
Temperature, K
- w :
-
Mass loss ratio, \(\%\)
- \(\alpha \) :
-
Degree of conversion
- \(\beta \) :
-
Heating rate, K \(\mathrm {min^{-1}}\)
- \(\theta \) :
-
Braggs angle, Radians
- \(\lambda \) :
-
X-ray radiation wavelength, nm
References
Alibeigi S, Vaezi MR (2008) Phase transformation of iron oxide nanoparticles by varying the molar ratio of fe2+: Fe3+. Chem Eng Technol Ind Chem Plant Equip Process Eng Biotechnol 31(11):1591–1596. https://doi.org/10.1002/ceat.200800093
Banerjee S, Moskowitz B (1985) Ferrimagnetic properties of magnetite In: Kirschvink JL, Jones DS, Macfadden BJ (eds). Magnetite biomineralization and magnetoreception in organisms. https://doi.org/10.1007/978-1-4613-0313-8_2
Batista MA, Costa ACSd, Bigham JM et al (2013) Structural and magnetic characterization of maghemites prepared from al-substituted magnetites. Rev Bras Ciênc Solo 37(6):1569–1575. https://doi.org/10.1590/S0100-06832013000600013
Budrugeac P (2002) Differential non-linear isoconversional procedure for evaluating the activation energy of non-isothermal reactions. J Therm Anal Calorim 68(1):131–139. https://doi.org/10.1023/A:1014932903582
Byrd RH, Hribar ME, Nocedal J (1999) An interior point algorithm for large-scale nonlinear programming. SIAM J Optim 9(4):877–900. https://doi.org/10.1137/S1052623497325107
Cornell RM, Schwertmann U (2003) Transformations. John Wiley & Sons Ltd, chap 14:365–407. https://doi.org/10.1002/3527602097.ch14
Dar MI, Shivashankar S (2014) Single crystalline magnetite, maghemite, and hematite nanoparticles with rich coercivity. RSC Adv 4(8):4105–4113. https://doi.org/10.1039/C3RA45457F
De Faria DL, Venâncio Silva S, De Oliveira M (1997) Raman microspectroscopy of some iron oxides and oxyhydroxides. J Raman Spectrosc 28(11):873–878. https://doi.org/10.1002/(SICI)1097-4555(199711)28:11<873::AID-JRS177>3.0.CO;2-B
Dinçer CA, Erdek AM, Karakeçili A et al (2019) Preparation of chitosan and glycol chitosan coated magnetic nanoparticles loaded with carboplatin as anticancer drug. J Politechnic 22(4):1017–1022. https://doi.org/10.2339/politeknik.501694
Faiyas A, Vinod E, Joseph J et al (2010) Dependence of ph and surfactant effect in the synthesis of magnetite (fe3o4) nanoparticles and its properties. J Magn Magn Mater 322(4):400–404. https://doi.org/10.1016/j.jmmm.2009.09.064
Feltin N, Pileni M (1997) New technique for synthesizing iron ferrite magnetic nanosized particles. Langmuir 13(15):3927–3933. https://doi.org/10.1021/la960854q
Flynn JH, Wall LA (1966) General treatment of the thermogravimetry of polymers. J Res Natl Bur Stand A Phys Chem 70(6):487. https://doi.org/10.6028/jres.070A.043
Forsmo S, Forsmo SE, Samskog PO et al (2008) Mechanisms in oxidation and sintering of magnetite iron ore green pellets. Powder Technol 183(2):247–259. https://doi.org/10.1016/j.powtec.2007.07.032
Friedman HL (1964) Kinetics of thermal degradation of char-forming plastics from thermogravimetry. application to a phenolic plastic. In: Journal of polymer science part C: polymer symposia, Wiley Online Library, pp 183–195, https://doi.org/10.1002/POLC.5070060121
Gao M, Li W, Dong J et al (2011) Synthesis and characterization of superparamagnetic fe3o4@ sio2 core-shell composite nanoparticles. World J Condens Matter Phys 1(2):49–54. https://doi.org/10.4236/wjcmp.2011.12008
Gemeay AH, Keshta BE, El-Sharkawy RG et al (2020) Chemical insight into the adsorption of reactive wool dyes onto amine-functionalized magnetite/silica core-shell from industrial wastewaters. Environ Sci Pollut Res 27(26):32341–32358. https://doi.org/10.1007/s11356-019-06530-y
Génin JMR, Ruby C, Géhin A et al (2006) Synthesis of green rusts by oxidation of fe (oh) 2, their products of oxidation and reduction of ferric oxyhydroxides; eh-ph pourbaix diagrams. Comptes Rendus Geosci 338(6–7):433–446. https://doi.org/10.1016/j.crte.2006.04.004
Ghosh Chaudhuri R, Paria S (2012) Core/shell nanoparticles: classes, properties, synthesis mechanisms, characterization, and applications. Chem Rev 112(4):2373–2433. https://doi.org/10.1021/cr100449n
Hong NH (2019) Introduction to nanomaterials: basic properties, synthesis, and characterization. Nano-Sized Multifunctional Materials. Elsevier, Amsterdam, pp 1–19. https://doi.org/10.1016/B978-0-12-813934-9.00001-3
Ishii M, Nakahira M, Yamanaka T (1972) Infrared absorption spectra and cation distributions in (mn, fe) 3o4. Solid State Commun 11(1):209–212. https://doi.org/10.1016/0038-1098(72)91162-3
Jubb AM, Allen HC (2010) Vibrational spectroscopic characterization of hematite, maghemite, and magnetite thin films produced by vapor deposition. ACS Appl Mater Interfaces 2(10):2804–2812. https://doi.org/10.1021/am1004943
Kissinger HE (1957) Reaction kinetics in differential thermal analysis. Anal Chem 29(11):1702–1706. https://doi.org/10.1021/ac60131a045
Li B, Qiao Y, An J et al (2018) Synthesis, characterisation, and evaluation of core-shell fe3o4/sio2/polypyrrole composite nanoparticles. Micro Nano Lett 13(7):902–906. https://doi.org/10.1049/mnl.2017.0907
Mahmed N, Heczko O, Lancok A et al (2014) The magnetic and oxidation behavior of bare and silica-coated iron oxide nanoparticles synthesized by reverse co-precipitation of ferrous ion (fe2+) in ambient atmosphere. J Magn Magn Mater 353:15–22. https://doi.org/10.1016/j.jmmm.2013.10.012
Maity D, Agrawal D (2007) Synthesis of iron oxide nanoparticles under oxidizing environment and their stabilization in aqueous and non-aqueous media. J Magn Magn Mater 308(1):46–55. https://doi.org/10.1016/j.jmmm.2006.05.001
Malek TJ, Chaki S, Tailor J et al (2018) Nonisothermal decomposition kinetics of pure and mn-doped fe3o4 nanoparticles. J Therm Anal Calorim 132(2):895–905. https://doi.org/10.1007/s10973-018-7013-5
Mascolo MC, Pei Y, Ring TA (2013) Room temperature co-precipitation synthesis of magnetite nanoparticles in a large ph window with different bases. Materials 6(12):5549–5567. https://doi.org/10.3390/ma6125549
McCarty KF, Monti M, Nie S et al (2014) Oxidation of magnetite (100) to hematite observed by in situ spectroscopy and microscopy. J Phys Chem C. https://doi.org/10.1021/jp5037603
Meng J, Yang G, Yan L et al (2005) Synthesis and characterization of magnetic nanometer pigment fe3o4. Dyes Pigm 66(2):109–113. https://doi.org/10.1016/j.dyepig.2004.08.016
Mohammed HB, Rayyif SMI, Curutiu C et al (2021) Eugenol-functionalized magnetite nanoparticles modulate virulence and persistence in pseudomonas aeruginosa clinical strains. Molecules 26(8):2189. https://doi.org/10.3390/molecules26082189
Mürbe J, Rechtenbach A, Töpfer J (2008) Synthesis and physical characterization of magnetite nanoparticles for biomedical applications. Mater Chem Phys 110(2–3):426–433. https://doi.org/10.1016/j.matchemphys.2008.02.037
Opfermann J (2000) Kinetic analysis using multivariate non-linear regression .i. basic concepts. J Therm Anal Calorim 60(2):641–658. https://doi.org/10.1023/A:1010167626551
Ozawa T (1965) A new method of analyzing thermogravimetric data. Bull Chem Soc Jpn 38(11):1881–1886. https://doi.org/10.1246/bcsj.38.1881
Peternele WS, Monge Fuentes V, Fascineli ML et al (2014) Experimental investigation of the coprecipitation method: an approach to obtain magnetite and maghemite nanoparticles with improved properties. J Nanomater. https://doi.org/10.1155/2014/682985
Pradhan P, Giri J, Samanta G et al (2007) Comparative evaluation of heating ability and biocompatibility of different ferrite-based magnetic fluids for hyperthermia application. J Biomed Mater Res B Appl Biomater 81(1):12–22. https://doi.org/10.1002/jbm.b.30630
Qu J, Liu G, Wang Y et al (2010) Preparation of fe3o4-chitosan nanoparticles used for hyperthermia. Adv Powder Technol 21(4):461–467. https://doi.org/10.1016/j.apt.2010.01.008
Sanders J, Gallagher P (2003) Kinetics of the oxidation of magnetite using simultaneous tg/dsc. J Therm Anal Calorim 72(3):777–789. https://doi.org/10.1023/A:1025053828639
Scherrer P (1918) Nachr ges wiss goettingen. Math Phys 2:98–100
Shampine LF, Reichelt MW (1997) The matlab ode suite. SIAM J Sci Comput 18(1):1–22. https://doi.org/10.1137/S1064827594276424
Sundar S, Mariappan R, Piraman S (2014) Synthesis and characterization of amine modified magnetite nanoparticles as carriers of curcumin-anticancer drug. Powder Technol 266:321–328. https://doi.org/10.1016/j.powtec.2014.06.033
Tahar LB, Oueslati MH, Abualreish MJA (2018) Synthesis of magnetite derivatives nanoparticles and their application for the removal of chromium (vi) from aqueous solutions. J Colloid Interface Sci 512:115–126. https://doi.org/10.1016/j.jcis.2017.10.044
Teja AS, Koh PY (2009) Synthesis, properties, and applications of magnetic iron oxide nanoparticles. Prog Cryst Growth Charact Mater 55(1–2):22–45. https://doi.org/10.1016/j.pcrysgrow.2008.08.003
Thomas LC (2001) Use of multiple heating rate dsc and modulated temperature dsc to detect and analyze temperature-time-dependent transitions in materials. Am Lab 33(1):26–31
Usman M, Byrne J, Chaudhary A et al (2018) Magnetite and green rust: synthesis, properties, and environmental applications of mixed-valent iron minerals. Chem Rev 118(7):3251–3304. https://doi.org/10.1021/acs.chemrev.7b00224
Varganici CD, Durdureanu-Angheluta A, Rosu D et al (2012) Thermal degradation of magnetite nanoparticles with hydrophilic shell. J Anal Appl Pyrol 96:63–68. https://doi.org/10.1016/j.jaap.2012.03.005
Vayssieres L, Chanéac C, Tronc E et al (1998) Size tailoring of magnetite particles formed by aqueous precipitation: an example of thermodynamic stability of nanometric oxide particles. J Colloid Interface Sci 205(2):205–212. https://doi.org/10.1006/jcis.1998.5614
Vyazovkin S (2018) Modern isoconversional kinetics from misconceptions to advances. Handbook of thermal analysis and calorimetry. Elsevier, Amsterdam, pp 131–172
Vyazovkin S, Burnham AK, Favergeon L et al (2020) Ictac kinetics committee recommendations for analysis of multi-step kinetics. Thermochim Acta 689(178):597. https://doi.org/10.1016/j.tca.2020.178597
Wulandari IO, Mardila VT, Santjojo D, et al (2018) Preparation and characterization of chitosan-coated fe3o4 nanoparticles using ex-situ co-precipitation method and tripolyphosphate/sulphate as dual crosslinkers. In: IOP Conf Ser Mater Sci Eng, p 012064, https://doi.org/10.1088/1757-899X/299/1/012064
Yamaura M, Fungaro DA (2013) Synthesis and characterization of magnetic adsorbent prepared by magnetite nanoparticles and zeolite from coal fly ash. J Mater Sci 48(14):5093–5101. https://doi.org/10.1007/s10853-013-7297-6
Yazdani F, Seddigh M (2016) Magnetite nanoparticles synthesized by co-precipitation method: the effects of various iron anions on specifications. Mater Chem Phys 184:318–323. https://doi.org/10.1016/j.matchemphys.2016.09.058
Acknowledgements
The authors thank the research funding agencies CAPES and CNPq for their financial support. In addition, the authors thank the analytical center of the institute of chemistry (UFRN) for the infrastructure provided during the development of the work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Silva, M.G.d., Santiago, L.E.P., Fernandes, R.d.S. et al. Analysis of thermal decomposition of magnetic nanoparticles synthesized by reverse coprecipitation and partial oxidation of ferrous ions. Braz. J. Chem. Eng. 41, 347–357 (2024). https://doi.org/10.1007/s43153-023-00336-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43153-023-00336-9