Abstract
Purpose
Genotoxic chemotherapy and radiotherapy can cause DNA double stranded breaks (DSBs) in primordial follicle (PMF) oocytes, which then undergo apoptosis. The development of effective new fertility preservation agents has been hampered, in part, by a limited understanding of DNA repair in PMF oocytes. This study investigated the induction of classical DSB repair pathways in the follicles of wild type (WT) and apoptosis-deficient Puma-/- mice in response to DSBs caused by the chemotherapy agent cisplatin.
Methods
Adult C57BL/6 WT and Puma-/- mice were injected i.p. with saline or cisplatin (5 mg/kg); ovaries were harvested at 8 or 24 h. Follicles were counted, and H2A histone family member (γH2AX) immunofluorescence used to demonstrate DSBs. DNA repair protein RAD51 homolog 1 (RAD51) and DNA-dependent protein kinase, catalytic subunit (DNA-PKcs) immunofluorescence were used to identify DNA repair pathways utilised.
Results
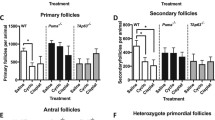
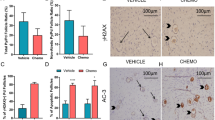
Puma-/- mice retained 100% of follicles 24 h after cisplatin treatment. Eight hours post-treatment, γH2AX immunofluorescence showed DSBs across follicular stages in Puma-/- mice; staining returned to control levels in PMFs within 5 days, suggesting repair of PMF oocytes in this window. RAD51 immunofluorescence eight hours post-cisplatin was positive in damaged cell types in both WT and Puma-/- mice, demonstrating induction of the homologous recombination pathway. In contrast, DNA-PKcs staining were rarely observed in PMFs, indicating non-homologous end joining plays an insignificant role.
Conclusion
PMF oocytes are able to conduct high-fidelity repair of DNA damage accumulated during chemotherapy. Therefore, apoptosis inhibition presents a viable strategy for fertility preservation in women undergoing treatment.






Similar content being viewed by others
Data Availability
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
References
Bath LE, Wallace WH, Critchley HO. Late effects of the treatment of childhood cancer on the female reproductive system and the potential for fertility preservation. BJOG. 2002;109(2):107–14. https://doi.org/10.1111/j.1471-0528.2002.t01-1-01007.x.
Hudson MM. Reproductive outcomes for survivors of childhood cancer. Obstetrics and gynecology. 2010;116(5):1171–83. https://doi.org/10.1097/AOG.0b013e3181f87c4b.
Faubion SS, Kuhle CL, Shuster LT, Rocca WA. Long-term health consequences of premature or early menopause and considerations for management. Climacteric. 2015;18(4):483–91. https://doi.org/10.3109/13697137.2015.1020484.
Meirow D, Biederman H, Anderson RA, Wallace WH. Toxicity of chemotherapy and radiation on female reproduction. Clinical obstetrics and gynecology. 2010;53(4):727–39. https://doi.org/10.1097/GRF.0b013e3181f96b54.
Byrne J, Fears TR, Gail MH, Pee D, Connelly RR, Austin DF, et al. Early menopause in long-term survivors of cancer during adolescence. American journal of obstetrics and gynecology. 1992;166(3):788–93.
Beaumont HM. The radiosensitivity of germ-cells at various stages of ovarian development. International journal of radiation biology and related studies in physics, chemistry, and medicine. 1962;4:581–90.
Bristol-Gould SK, Kreeger PK, Selkirk CG, Kilen SM, Mayo KE, Shea LD, et al. Fate of the initial follicle pool: empirical and mathematical evidence supporting its sufficiency for adult fertility. Developmental biology. 2006;298(1):149–54. https://doi.org/10.1016/j.ydbio.2006.06.023.
de Bruin JPV, E.R. Female reproductive aging: concepts and consequences. Preservation of Fertility. London: Taylor and Francis; 2004.
Findlay JK, Hutt KJ, Hickey M, Anderson RA. How Is the Number of Primordial Follicles in the Ovarian Reserve Established? Biology of reproduction. 2015;93(5):111. https://doi.org/10.1095/biolreprod.115.133652.
Hanoux V, Pairault C, Bakalska M, Habert R, Livera G. Caspase-2 involvement during ionizing radiation-induced oocyte death in the mouse ovary. Cell death and differentiation. 2007;14(4):671–81. https://doi.org/10.1038/sj.cdd.4402052.
Polo SE, Jackson SP. Dynamics of DNA damage response proteins at DNA breaks: a focus on protein modifications. Genes Dev. 2011;25(5):409–33. https://doi.org/10.1101/gad.2021311.
Lim AS, Tsakok MF. Age-related decline in fertility: a link to degenerative oocytes? Fertility and sterility. 1997;68(2):265–71. https://doi.org/10.1016/s0015-0282(97)81513-0.
Titus S, Li F, Stobezki R, Akula K, Unsal E, Jeong K, et al. Impairment of BRCA1-related DNA double-strand break repair leads to ovarian aging in mice and humans. Science translational medicine. 2013;5(172):172ra21. https://doi.org/10.1126/scitranslmed.3004925.
Kerr JB, Brogan L, Myers M, Hutt KJ, Mladenovska T, Ricardo S, et al. The primordial follicle reserve is not renewed after chemical or gamma-irradiation mediated depletion. Reproduction. 2012;143(4):469–76. https://doi.org/10.1530/REP-11-0430.
Tatone C, Amicarelli F, Carbone MC, Monteleone P, Caserta D, Marci R, et al. Cellular and molecular aspects of ovarian follicle ageing. Human reproduction update. 2008;14(2):131–42. https://doi.org/10.1093/humupd/dmm048.
Kerr JB, Hutt KJ, Cook M, Speed TP, Strasser A, Findlay JK, et al. Cisplatin-induced primordial follicle oocyte killing and loss of fertility are not prevented by imatinib. Nature medicine. 2012;18(8):1170–2; author reply 2-4. https://doi.org/10.1038/nm.2889.
Ravel C, Berthaut I, Bresson JL, Siffroi JP. Genetics Commission of the French Federation of C. Prevalence of chromosomal abnormalities in phenotypically normal and fertile adult males: large-scale survey of over 10,000 sperm donor karyotypes. Hum Reprod. 2006;21(6):1484–9. https://doi.org/10.1093/humrep/del024.
van den Berg MM, van Maarle MC, van Wely M, Goddijn M. Genetics of early miscarriage. Biochimica et biophysica acta. 2012;1822(12):1951–9. https://doi.org/10.1016/j.bbadis.2012.07.001.
McFadden DE, Friedman JM. Chromosome abnormalities in human beings. Mutation research. 1997;396(1-2):129–40. https://doi.org/10.1016/s0027-5107(97)00179-6.
Pellicer A, Rubio C, Vidal F, Minguez Y, Gimenez C, Egozcue J, et al. In vitro fertilization plus preimplantation genetic diagnosis in patients with recurrent miscarriage: an analysis of chromosome abnormalities in human preimplantation embryos. Fertility and sterility. 1999;71(6):1033–9. https://doi.org/10.1016/s0015-0282(99)00143-0.
Zhang YX, Zhang YP, Gu Y, Guan FJ, Li SL, Xie JS, et al. Genetic analysis of first-trimester miscarriages with a combination of cytogenetic karyotyping, microsatellite genotyping and arrayCGH. Clin Genet. 2009;75(2):133–40. https://doi.org/10.1111/j.1399-0004.2008.01131.x.
Khanna KK, Jackson SP. DNA double-strand breaks: signaling, repair and the cancer connection. Nat Genet. 2001;27(3):247–54. https://doi.org/10.1038/85798.
Baumann P, West SC. Role of the human RAD51 protein in homologous recombination and double-stranded-break repair. Trends Biochem Sci. 1998;23(7):247–51. https://doi.org/10.1016/s0968-0004(98)01232-8.
Mahaney BL, Meek K, Lees-Miller SP. Repair of ionizing radiation-induced DNA double-strand breaks by non-homologous end-joining. Biochem J. 2009;417(3):639–50. https://doi.org/10.1042/BJ20080413.
Winship AL, Stringer JM, Liew SH, Hutt KJ. The importance of DNA repair for maintaining oocyte quality in response to anti-cancer treatments, environmental toxins and maternal ageing. Human reproduction update. 2018;24:119–34. https://doi.org/10.1093/humupd/dmy002.
Kujjo LL, Laine T, Pereira RJ, Kagawa W, Kurumizaka H, Yokoyama S, et al. Enhancing survival of mouse oocytes following chemotherapy or aging by targeting Bax and Rad51. PloS one. 2010;5(2):e9204. https://doi.org/10.1371/journal.pone.0009204.
Suh EK, Yang A, Kettenbach A, Bamberger C, Michaelis AH, Zhu Z, et al. p63 protects the female germ line during meiotic arrest. Nature. 2006;444(7119):624–8. https://doi.org/10.1038/nature05337.
Gonfloni S, Di Tella L, Caldarola S, Cannata SM, Klinger FG, Di Bartolomeo C, et al. Inhibition of the c-Abl-TAp63 pathway protects mouse oocytes from chemotherapy-induced death. Nature medicine. 2009;15(10):1179–85. https://doi.org/10.1038/nm.2033.
Kerr JB, Hutt KJ, Michalak EM, Cook M, Vandenberg CJ, Liew SH, et al. DNA damage-induced primordial follicle oocyte apoptosis and loss of fertility require TAp63-mediated induction of Puma and Noxa. Molecular cell. 2012;48(3):343–52. https://doi.org/10.1016/j.molcel.2012.08.017.
Nguyen QN, Zerafa N, Liew SH, Morgan FH, Strasser A, Scott CL, et al. Loss of PUMA protects the ovarian reserve during DNA-damaging chemotherapy and preserves fertility. Cell Death Dis. 2018;9(6):618. https://doi.org/10.1038/s41419-018-0633-7.
Livera G, Petre-Lazar B, Guerquin MJ, Trautmann E, Coffigny H, Habert R. p63 null mutation protects mouse oocytes from radio-induced apoptosis. Reproduction. 2008;135(1):3–12. https://doi.org/10.1530/REP-07-0054.
Nguyen QN, Zerafa N, Liew SH, Findlay JK, Hickey M, Hutt KJ. Cisplatin- and cyclophosphamide-induced primordial follicle depletion is caused by direct damage to oocytes. Molecular human reproduction. 2019;25(8):433–44. https://doi.org/10.1093/molehr/gaz020.
Stringer JM, Winship A, Zerafa N, Wakefield M, Hutt K. Oocytes can efficiently repair DNA double-strand breaks to restore genetic integrity and protect offspring health. Proceedings of the National Academy of Sciences of the United States of America. 2020;117(21):11513–22. https://doi.org/10.1073/pnas.2001124117.
Villunger A, Michalak EM, Coultas L, Mullauer F, Bock G, Ausserlechner MJ, et al. p53- and drug-induced apoptotic responses mediated by BH3-only proteins puma and noxa. Science. 2003;302(5647):1036–8. https://doi.org/10.1126/science.1090072.
Mabuchi S, Ohmichi M, Nishio Y, Hayasaka T, Kimura A, Ohta T, et al. Inhibition of NFkappaB increases the efficacy of cisplatin in in vitro and in vivo ovarian cancer models. J Biol Chem. 2004;279(22):23477–85. https://doi.org/10.1074/jbc.M313709200.
Spanos WC, Nowicki P, Lee DW, Hoover A, Hostager B, Gupta A, et al. Immune response during therapy with cisplatin or radiation for human papillomavirus-related head and neck cancer. Arch Otolaryngol Head Neck Surg. 2009;135(11):1137–46. https://doi.org/10.1001/archoto.2009.159.
Myers M, Britt KL, Wreford NG, Ebling FJ, Kerr JB. Methods for quantifying follicular numbers within the mouse ovary. Reproduction. 2004;127(5):569–80. https://doi.org/10.1530/rep.1.00095.
Bolcun-Filas E, Rinaldi VD, White ME, Schimenti JC. Reversal of female infertility by Chk2 ablation reveals the oocyte DNA damage checkpoint pathway. Science. 2014;343(6170):533–6. https://doi.org/10.1126/science.1247671.
Hancke K, Strauch O, Kissel C, Gobel H, Schafer W, Denschlag D. Sphingosine 1-phosphate protects ovaries from chemotherapy-induced damage in vivo. Fertility and sterility. 2007;87(1):172–7. https://doi.org/10.1016/j.fertnstert.2006.06.020.
Hancke K, Walker E, Strauch O, Gobel H, Hanjalic-Beck A, Denschlag D. Ovarian transplantation for fertility preservation in a sheep model: can follicle loss be prevented by antiapoptotic sphingosine-1-phosphate administration? Gynecological endocrinology : the official journal of the International Society of Gynecological Endocrinology. 2009;25(12):839–43. https://doi.org/10.3109/09513590903159524.
Kaya H, Desdicioglu R, Sezik M, Ulukaya E, Ozkaya O, Yilmaztepe A, et al. Does sphingosine-1-phosphate have a protective effect on cyclophosphamide- and irradiation-induced ovarian damage in the rat model? Fertility and sterility. 2008;89(3):732–5. https://doi.org/10.1016/j.fertnstert.2007.03.065.
Kim SY, Cordeiro MH, Serna VA, Ebbert K, Butler LM, Sinha S, et al. Rescue of platinum-damaged oocytes from programmed cell death through inactivation of the p53 family signaling network. Cell death and differentiation. 2013;20(8):987–97. https://doi.org/10.1038/cdd.2013.31.
Kim SY, Nair DM, Romero M, Serna VA, Koleske AJ, Woodruff TK, et al. Transient inhibition of p53 homologs protects ovarian function from two distinct apoptotic pathways triggered by anticancer therapies. Cell death and differentiation. 2019;26(3):502–15. https://doi.org/10.1038/s41418-018-0151-2.
Luan Y, Edmonds ME, Woodruff TK, Kim SY. Inhibitors of apoptosis protect the ovarian reserve from cyclophosphamide. The Journal of endocrinology. 2019;240(2):243–56. https://doi.org/10.1530/JOE-18-0370.
Meng Y, Xu Z, Wu F, Chen W, Xie S, Liu J, et al. Sphingosine-1-phosphate suppresses cyclophosphamide induced follicle apoptosis in human fetal ovarian xenografts in nude mice. Fertility and sterility. 2014;102(3):871–7 e3. https://doi.org/10.1016/j.fertnstert.2014.05.040.
Paris F, Perez GI, Fuks Z, Haimovitz-Friedman A, Nguyen H, Bose M, et al. Sphingosine 1-phosphate preserves fertility in irradiated female mice without propagating genomic damage in offspring. Nature medicine. 2002;8(9):901–2. https://doi.org/10.1038/nm0902-901.
Pascuali N, Scotti L, Di Pietro M, Oubina G, Bas D, May M, et al. Ceramide-1-phosphate has protective properties against cyclophosphamide-induced ovarian damage in a mice model of premature ovarian failure. Hum Reprod. 2018;33(5):844–59. https://doi.org/10.1093/humrep/dey045.
Rinaldi VD, Bolcun-Filas E, Kogo H, Kurahashi H, Schimenti JC. The DNA Damage Checkpoint Eliminates Mouse Oocytes with Chromosome Synapsis Failure. Molecular cell. 2017;67(6):1026–36 e2. https://doi.org/10.1016/j.molcel.2017.07.027.
Spears N, Lopes F, Stefansdottir A, Rossi V, De Felici M, Anderson RA, et al. Ovarian damage from chemotherapy and current approaches to its protection. Human reproduction update. 2019;25(6):673–93. https://doi.org/10.1093/humupd/dmz027.
Tuppi M, Kehrloesser S, Coutandin DW, Rossi V, Luh LM, Strubel A, et al. Oocyte DNA damage quality control requires consecutive interplay of CHK2 and CK1 to activate p63. Nat Struct Mol Biol. 2018;25(3):261–9. https://doi.org/10.1038/s41594-018-0035-7.
Stringer J, Groenewegen E, Liew SH, Hutt KJ. Nicotinamide mononucleotide does not protect the ovarian reserve from cancer treatments. Reproduction. 2019. https://doi.org/10.1530/REP-19-0337.
Rinaldi VD, Hsieh K, Munroe R, Bolcun-Filas E, Schimenti JC. Pharmacological Inhibition of the DNA Damage Checkpoint Prevents Radiation-Induced Oocyte Death. Genetics. 2017;206(4):1823–8. https://doi.org/10.1534/genetics.117.203455.
McDougall A, Elliott DJ, Hunter N. Pairing, connecting, exchanging, pausing and pulling chromosomes. EMBO Rep. 2005;6(2):120–5. https://doi.org/10.1038/sj.embor.7400331.
Carroll J, Marangos P. The DNA damage response in mammalian oocytes. Front Genet. 2013;4:117. https://doi.org/10.3389/fgene.2013.00117.
Levine AJ, Tomasini R, McKeon FD, Mak TW, Melino G. The p53 family: guardians of maternal reproduction. Nature reviews Molecular cell biology. 2011;12(4):259–65. https://doi.org/10.1038/nrm3086.
Goedecke W, Vielmetter W, Pfeiffer P. Activation of a system for the joining of nonhomologous DNA ends during Xenopus egg maturation. Mol Cell Biol. 1992;12(2):811–6. https://doi.org/10.1128/mcb.12.2.811.
Hagmann M, Adlkofer K, Pfeiffer P, Bruggmann R, Georgiev O, Rungger D, et al. Dramatic changes in the ratio of homologous recombination to nonhomologous DNA-end joining in oocytes and early embryos of Xenopus laevis. Biol Chem Hoppe Seyler. 1996;377(4):239–50. https://doi.org/10.1515/bchm3.1996.377.4.239.
Her J, Bunting SF. How cells ensure correct repair of DNA double-strand breaks. J Biol Chem. 2018;293(27):10502–11. https://doi.org/10.1074/jbc.TM118.000371.
Lieber MR. The mechanism of double-strand DNA break repair by the nonhomologous DNA end-joining pathway. Annual review of biochemistry. 2010;79:181–211. https://doi.org/10.1146/annurev.biochem.052308.093131.
Martin JH, Bromfield EG, Aitken RJ, Lord T, Nixon B. Double Strand Break DNA Repair occurs via Non-Homologous End-Joining in Mouse MII Oocytes. Sci Rep. 2018;8(1):9685. https://doi.org/10.1038/s41598-018-27892-2.
Acknowledgements
The authors acknowledge the facilities, scientific and technical assistance of Monash Micro Imaging, the Monash Animal Research Platform, and the Monash Histology Platform, Monash University, Victoria, Australia.
Funding
This work was supported by fellowships and grants from the National Health and Medical Research Council Australia (Project Grant #1007027, Program Grant #1016701, and Fellowship KJH #1050130); National Breast Cancer Foundation (#NC14-001). This work was made possible through Victorian State Government Operational Infrastructure Support and Australian Government NHMRC IRIISS.
Author information
Authors and Affiliations
Contributions
QNN planned experiments, obtained ethics approvals, conducted experiments, analysed data, and prepared the manuscript. NZ provided laboratory support and assisted with experiments. MH and JKF provided supervision, assisted in planning experiments, and revised the manuscript. KJH provided supervision, gave assistance with ethics applications, project funding, laboratory resources and infrastructure, planned experiments, and prepared the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest/competing interests
None to declare.
Ethics approval
All animal experiments and procedures were carried out in accordance with the NHMRC Australian Code of Practice for the Care and Use of Animals and approved by the Monash University School of Biomedical Sciences (MARP/2017/077) and the Monash Medical Centre Animal Ethics (MMCB/2014/13) Committees.
Consent to participate
Not applicable
Consent for publication
Not applicable
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nguyen, QN., Zerafa, N., Findlay, J.K. et al. DNA repair in primordial follicle oocytes following cisplatin treatment. J Assist Reprod Genet 38, 1405–1417 (2021). https://doi.org/10.1007/s10815-021-02184-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-021-02184-3