Abstract
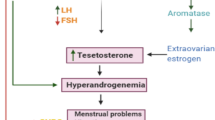
The present study aimed to determine the clinical predictive significance of HIF-1α in follicular development and assisted reproductive technology (ART). We collected follicular fluid (FF) and granulosa cells (GCs) from PCOS (polycystic ovary syndrome) patients (experimental group) and other patients who were infertile due to tubal factors or male factors (control group) with IVF/ICSI-ET. The localization and expression of HIF-1α in GCs were determined by immunofluorescence staining. HIF-1α protein and mRNA expression were detected by enzyme-linked immunosorbent assay and quantitative real-time PCR, respectively. To clarify the regulation of HIF-1α by TGF-β1, we added the HIF-1α-specific blocker YC-1 to GCs. The serum AMH, LH, LH/FSH, testosterone, BMI and the number of oocytes retrieved in the PCOS group were significantly higher, while the cleavage rate was significantly lower, than those in the control group. HIF-1α protein was expressed in the cytoplasm of GCs. The expression of HIF-1α protein in the FF of the PCOS group was significantly lower than that in the control group. However, the expression of HIF-1α protein in GCs between the two groups was not significantly different. HIF-1α protein was highly expressed in large FF (follicular diameter ≥ 14 mm). Compared with the control group, the expression of HIF-1α mRNA in GCs of the PCOS group was significantly lower. The results showed a significant positive correlation between HIF-1α and TGF-β1 expression. We found that both HIF-1α and TGF-β1 were involved in the development of PCOS follicular development. The mutual regulation of HIF-1α and TGF-β1 may be one of the important mechanisms of the occurrence and development of PCOS.







Similar content being viewed by others
Data Availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
References
Kumariya S, Ubba V, Jha RK, et al. Autophagy in ovary and polycystic ovary syndrome: role, dispute and future perspective. Autophagy. 2021;17(10):2706–33.
Wang L, Zhou J, Gober HJ, Leung WT, Huang Z, Pan X, Li C, Zhang N, Wang L. Alterations in the intestinal microbiome associated with PCOS affect the clinical phenotype. Biomed Pharmacother. 2021;133:110958.
Endocrinology group and guideline expert group of Obstetrics and Gynecology branch of Chinese Medical Association. Chinese guidelines for diagnosis and treatment of polycystic ovary syndrome. Chin J Obstet Gynecol. 2018;53(1):2–6.
Patel S. Polycystic ovary syndrome (PCOS), an inflammatory, systemic, lifestyle endocrinopathy. J Steroid Biochem Mol Biol. 2018;182:27–36.
Collée J, Mawet M, Tebache L, et al. Polycystic ovarian syndrome and infertility: overview and insights of the putative treatments. Gynecol Endocrinol. 2021;37(10):869–74.
Jarrett BY, Vanden Brink H, Oldfield AL, et al. Ultrasound characterization of disordered antral follicle development in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2020;105(11):e3847–61.
He H, Zhang H, Pan Y, et al. Low oxygen concentration improves yak oocyte maturation and inhibits apoptosis through HIF-1 and VEGF. Reprod Domest Anim. 2022;57(4):381–92.
Wu D, Su X, Lu J, et al. Bidirectional modulation of HIF-2 activity through chemical ligands. Nat Chem Biol. 2019;15(4):367–76.
Yadav AK, Yadav PK, Chaudhary GR, et al. Autophagy in hypoxic ovary[J]. Cell Mol Life Sci. 2019;76(17):3311–22.
Lim M, Thompson JG, Dunning KR. Hypoxia and reproductive health: hypoxia and ovarian function: follicle development, ovulation, oocyte maturation. Reproduction. 2021;161(1):F33–40.
Kim J, Bagchi IC, Bagchi MK. Signaling by hypoxia-inducible factors is critical for ovulation in mice. Endocrinology. 2009;150(7):3392–400.
Du XH, Cao R, Xiao P, et al. HIF-l in ovarian tissue of mice treated with different treatments α gene expression analysis and construction of eukaryotic expression vector. J Nanjing Agric Univ. 2014;37(6):137–42.
Fang L, Li Y, Wang S, et al. TGF-β1 induces VEGF expression in human granulosa-lutein cells: a potential mechanism for the pathogenesis of ovarian hyperstimulation syndrome. Exp Mol Med. 2020;52(3):450–60.
Du X, Zhang L, Li X, et al. TGF-β signaling controls FSHR signaling-reduced ovarian granulosa cell apoptosis through the SMAD4/miR-143 axis. Cell Death Dis. 2016;7(11):e2476.
Yang J, Zhang Y, Xu X, et al. Transforming growth factor-β is involved in maintaining oocyte meiotic arrest by promoting natriuretic peptide type C expression in mouse granulosa cells. Cell Death Dis. 2019;10(8):558.
Miao ZL, Wang ZN, Yang YD, et al. Expression and role of TGF-β1 and its receptor in ovary of PCOS rats. Guangdong Med. 2008;29(12):1966–8.
Chen W. Insulin like growth factor-1 and transforming growth factor-β expression and significance of 1 in letrozole induced polycystic ovary syndrome model rats. Chin J Gerontol. 2016;36(14):3395–7.
The Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81(1):19–25.
Cena H, Chiovato L, Nappi RE. Obesity, polycystic ovary syndrome, and infertility: a new avenue for GLP-1 receptor agonists. J Clin Endocrinol Metab. 2020;105(8):e2695–709.
Tamer Erel C, Senturk LM. The impact of body mass index on assisted reproduction. Curr Opin Obstet Gynecol. 2009;21(3):228–35.
Bruni V, Capozzi A, Lello S. The role of genetics, epigenetics and lifestyle in polycystic ovary syndrome development: the state of the art. Reprod Sci. 2022;29(3):668–79.
Tanbo T, Mellembakken J, Bjercke S, et al. Ovulation induction in polycystic ovary syndrome. Acta Obstet Gynecol Scand. 2018;97(10):1162–7.
Pan JX, Zhang JY, Ke ZH, et al. Androgens are double-edged swords: induction and suppression of follicular development. Hormones (Athens). 2015;14:190–200.
Wawrzkiewicz-Jałowiecka A, Kowalczyk K, Trybek P, et al. In search of new therapeutics-molecular aspects of the PCOS pathophysiology: genetics, hormones, metabolism and beyond. Int J Mol Sci. 2020;21(19):7054.
Wang J, Wu X. The effects of mitochondrial dysfunction on energy metabolism switch by HIF-1α signalling in granulosa cells of polycystic ovary syndrome. Endokrynol Pol. 2020;71(2):134–45.
Patil K, Hinduja I, Mukherjee S. Alteration in angiogenic potential of granulosa-lutein cells and FF contributes to luteal defects in polycystic ovary syndrome. Hum Reprod. 2021;36(4):1052–64.
Tal R, Seifer DB, Arici A. The emerging role of angiogenic factor dys-regulation in the pathogenesis of polycystic ovarian syndrome. Semin Reprod Med. 2015;33:195–207.
Raja-Khan N, Urbanek M, Rodgers RJ, et al. The role of TGF-β in polycystic ovary syndrome. Reprod Sci. 2014;21(1):20–31.
Shen H, Wang Y. Activation of TGF-β1/Smad3 signaling pathway inhibits the development of ovarian follicle in polycystic ovary syndrome by promoting apoptosis of granulosa cells. J Cell Physiol. 2019;234(7):11976–85.
Fuse S, Suzuki K, Kuchimaru T, et al. Design, synthesis, and evaluation of indeno [2,1-c] pyrazolones for use as inhibitors against hypoxia-inducible factor (HIF)-1 transcriptional activity. Bioorg Med Chem. 2020;28(1):115207.
Wang Y. Expression of TGF-β1, HIF-1α and TSLP in nasal polyps and its predictive value for short-term prognosis of nasal polyps. Henan: Zhengzhou University; 2019.
Miao ZL, Guo L, Wang YX, et al. The intervention effect of rosiglitozone in ovarian fibrosis of PCOS rats. Biomed Environ Sci. 2012;25(1):46–52.
Lam YT, Lecce L, Yuen SC, et al. Androgens ameliorate impaired ischemia-induced neovascularization due to aging in male mice. Endocrinology. 2019;160(5):1137–49.
Xu JW. Estrogen affects blood lipid and insulin sensitivity by improving visceral fat function. Guangdong: Sun Yat-sen University; 2010.
Ouellette Y, Price CA, Carriere PD. FF concentration of trans-forming growth factor-beta1 is negatively correlated with estradiol and follicle size at the early stage of development of the first-wave cohort of bovine ovarian follicles. Domest Anim Endocrinol. 2005;29(4):623–33.
Bai L, Chu G, Wang W, et al. BAMBI promotes porcine granulosa cell steroidogenesis involving TGF-β signaling. Theriogenology. 2017;100:24–31.
Fang WL, Lee MT, Wu LS, et al. CREB coactivator CRTC2/TORC2 and its regulator calcineurin crucially mediate follicle-stimulating hormone and transforming growth factor β1 upregulation of steroidogenesis. J Cell Physiol. 2012;227(6):2430–40.
Zhou F, Shi LB, Zhang SY. Ovarian fibrosis: a phenomenon of concern. Chin Med J (Engl). 2017;130(3):365–71.
Acknowledgements
We would like to thank the whole ART laboratory staffs for their patient assistance in the sample collection.
Funding
This study was supported by grants from Shanghai Municipal Health Commission Project (ZYKC2019036), the Shanghai Municipal Science and Technology Major Project (2021SHZDZX0100) and the Fundamental Research Funds for the Central Universities.
Author information
Authors and Affiliations
Contributions
Fu Xiayan wrote the manuscript; Lihong Shi, Ping Liu and Yufan Jiao collected the data; Yanqiu Wang, Qihchang Zheng and Xiangyun Chen designed the current study; and Shana Guo and Qizhen Chen participated in the revision of the manuscript. All the authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
This study was approved by the Ethics Committee of Tongji Hospital affiliated to Tongji University (approve ID K-W-2019–007) and informed consent was obtained from all the participants. All the authors have given approval to the final version of the manuscript.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xiayan Fu, Lihong Shi, and Ping Liu are Co-First authors.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fu, X., Shi, L., Liu, P. et al. Expression and Clinical Significance of HIF-1α in Follicular Fluid and Granulosa Cells in Infertile PCOS Patients. Reprod. Sci. 30, 2263–2274 (2023). https://doi.org/10.1007/s43032-022-01135-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-022-01135-2



