Abstract
Purpose
Cryopreservation of ovarian tissue is paramount for fertility preservation, with important clinical applications, especially for women suffering from an oncological condition. Several cryopreservation methodologies have been tried in search of better outcomes, especially in terms of primor-dial and primary follicles integrity post-cryopreservation. Vitrification has successfully been applied to ovarian tissue using different carriers for tissue exposure to the liquid nitrogen (LN2).
Methods
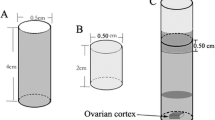
We developed an enclosed metal vessel, which has the advantage of a faster heat transfer, when in contact with LN2 avoiding at the same time, the direct contact with tissue. Additionally, we assessed the effect of different times and temperatures of transport between the collection of mouse ovaries and the beginning of cryopreservation, on follicular morphology after vitrification.
Results
Our results suggest that 37 °C and R.T. help to maintain normal primordial and primary follicle morphology for up to 4 hrs after collection and beginning of vitrification in a metal container.
Conclusion
These data show that the metal container is an appropriate carrier for mouse ovary vitrification. The rate of morphologically normal primordial follicles up to 4 hrs.


Similar content being viewed by others
References
Almodin CG, Minguetti-Câmara VC, Meister H, Ferreira JO, Franco RL, Cavalcante AA, Radaelli MR, Bahls AS, Moron AF, Murta CG. Recovery of fertility after grafting of cryopreserved germinative tissue in female rabbits following radiotherapy. Hum Reprod. 2004;19:1287–93.
Almodin CG, Minguetti-Camara VC, Meister H, Ceschin AP, Kriger E, Ferreira JO. Recovery of natural fertility after grafting of cryopreserved germinative tissue in ewes subjected to radiotherapy. Fertil Steril. 2004;81:160–4.
Andersen CY, Rosendahl M, Byskov AG, Loft A, Ottosen C, Dueholm M, Schmidt KL, Andersen AN, Ernst E. Two successful pregnancies following autotransplantation of frozen/thawed ovarian tissue. Hum Reprod. 2008;23:2266–72.
Chen SU, Chien CL, Wu MY, Chen TH, Lai SM, Lin CW, et al. Novel direct cover vitrification for cryopreservation of ovarian tissues increases follicle viability and pregnancy capability in mice. Hum Reprod. 2006;21:2794–800.
Choi JY, Lee J-Y, Lee EY, Yoon B-K, Boe DS, Choi DS. Cryopreservation of the mouse ovary inhibits the onset of primordial follicle development. Cryobiology. 2007;54:55–62.
Donnez J, Dolmans MM, Demylle D, Jadoul P, Pirard C, Squifflet J, Martinez-Madrid B, van Langendonckt A. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;364:1405–10.
Ferreira M, Bos-Mikich A, Frantz N, Rodrigues JL, Brunetto AL, Schwartsmann G. The effects of sample size on the outcome of ovarian tissue cryopreservation. Reprod Dom Anim. 2009;45:99–102.
Gandolfi F, Paffoni A, Papasso Brambilla E, Bonetti S, Brevini TA, Ragni G. Efficiency of equilibrium cooling and vitrification procedures for the cryopreservation of ovarian tissue: comparative analysis between human and animal models. Fertil Steril. 2006;85:1150–6.
Henry L, Fransolet M, Labied S, Blacher S, Munaut C, Colige A, Kirschvink N, Noël A, Nisolle M, Foidart J-M. Does isoforms 111 and 165 of vascular endothelial growth factor (VEGF111 and 165) improve transplanted ovarian tissue survival? J Assist Reprod Genet. 2011;28:1007–24.
Isachenko V, Lapidus I, Isachenko E, Krivokharchenko A, Kreienberg R, Woriedh M, Bader M, Weiss JM. Human ovarian tissue vitrification versus conventional freezing: morphological, endocrinological, and molecular biological evaluation. Reproduction. 2009;138:319–27.
Jeremias E, Bedaiwy MA, Nelson D, Biscotti CV, Falcone T. Assessment of tissue injury in cryopreserved ovarian tissue. Fertil Steril. 2003;79:651–3.
Jin SY, Lei L, Shea LD, Zelinski MB, Stouffer RL, Woodruff TK. Markers of growth and development in primate primordial follicles are preserved after slow cryopreservation. Fertil Steril. 2010;93:2627–32.
Keros V, Xella S, Hultenby K, Pettersson K, Sheikhi M, Volpe A, Hreinsson J, Hovatta O. Vitrification versus controlled-rate freezing in cryopreservation of human ovarian tissue. Hum Reprod. 2009;24:1670–83.
Labied S, Delforges Y, Blacher S, Munaut C, Signole N, Colige A, Janssen L, Henry L, d’Hauterive SP, Jouan C, Kirschvink N, Noël A, Nisolle M, Foidart J-M. Isoform 111 of vascular endothelial growth factor (VEGF111) improves angiogenesis of ovarian tissue xenotransplantation. J Assist Reprod Genet. 2011;28:1007–24.
Meirow D, Levron J, Eldar-Geva T, Hardan I, Fridman E, Zalel Y, Schiff E, Dor J. Pregnancy after transplantation of cryopreserved ovarian tissue in a patient with ovarian failure after chemotherapy. N Engl J Med. 2005;353:318–21.
Migishima F, Suzuki-Migishima R, Song SY, Kuramochi T, Azuma S, Nishijimi M. Successful cryopreservation of mouse ovaries by vitrification. Biol Reprod. 2003;68:881–7.
Sheikhi M, Hultenby K, Niklasson B, Lundqvist M, Hovatta O. Clinical grade vitrification of human ovarian tissue: an ultrastructural analysis of follicles and stroma in vitrified tissue. Hum Reprod. 2011;26:594–603.
Shikanov A, Zhang Z, Xu M, Smith RM, Rajan A, Woodruff TK, Shea LD. Fibrin encapsulation and vascular endothelial growth factor delivery promotes ovarian graft survival in mice. Tissue Eng Part A. 2011;17:3095–104.
Sugimoto M, Maeda S, Manabe N, Miyamoto H. Development of infantile rat ovaries autotransplanted after cryopreservation by vitrification. Theriogeneology. 2000;53:1093–103.
Ting AY, Yeoman RR, Lawson MS, Zelinski MB. In vitro development of secondary follicles from cryopreserved rhesus macaque ovarian tissue after slow-rate freeze or vitrification. Hum Reprod. 2011;26:2461–72.
Tokieda Y, Ishiwata I, Segino M, Ishikawa H, Sato K. Establishment of a novel method for cryopreservation and thawing of the mouse ovary. Hum Cell. 2002;15:230–7.
von Wolff M, Donnez J, Hovatta O, Keros V, Maltaris T, Montag M, Salle B, Sonmezer M, Andersen CY. Cryopreservation and autotransplantation of human ovarian tissue prior to cytotoxic therapy- a technique in its infancy but already successful in fertility treatment. Eur J Cancer. 2009;45:1547–53.
Acknowledgements
The authors are most thankful to Dr. Elsa Cristina de Mundstock from NAE-Federal University of Rio Grande do Sul, for the critical statistics help and supervision.
Author information
Authors and Affiliations
Corresponding author
Additional information
Capsule Vitrification of ovarian tissue in a metal container results in high rates of morphologically normal primordial follicles up to 4 hrs between tissue collection and cryopreservation.
Rights and permissions
About this article
Cite this article
Bos-Mikich, A., Marques, L., Rodrigues, J.L. et al. The use of a metal container for vitrification of mouse ovaries, as a clinical grade model for human ovarian tissue cryopreservation, after different times and temperatures of transport. J Assist Reprod Genet 29, 1267–1271 (2012). https://doi.org/10.1007/s10815-012-9867-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-012-9867-y