Abstract
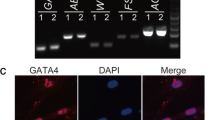
The self-renewal of spermatogonial cells (SCs) provides the foundation for life-long spermatogenesis. To date, only a few growth factors have been used for the culture of SCs in vitro, and how to enhance proliferation capacity of SCs in vitro needs further research. This study aimed to explore the effects of periostin (POSTN) on the proliferation of human SCs. GC-1 spg cells were cultured in a medium with POSTN, cell proliferation was evaluated by MTS analysis and EdU assay, and the Wnt/β-catenin signaling pathway was examined. Thereafter, the proliferations of human SC were detected using immunofluorescence and RT-PCR. In this study, we found that CM secreted by human amniotic mesenchymal stem cells (hAMSCs) could enhance the proliferation capacity of mouse GC-1 spg cells. Label-free mass spectrometry and ELISA analysis demonstrated that high level of POSTN was secreted by hAMSCs. MTS and EdU staining showed that POSTN increased GC-1 spg cell proliferation, whereas CM from POSTN-silenced hAMSCs suppressed cell proliferation capacity. Then POSTN was found to activate the Wnt/β-catenin signaling pathway to regulate the proliferation of GC-1 spg cells. XAV-939, a Wnt/β-catenin inhibitor, partially reversed the effects of POSTN on GC-1 spg cell proliferation. We then analyzed human SCs and found that POSTN promoted human SC proliferation in vitro. These findings provide insights regarding the role of POSTN in regulating SC proliferation via the Wnt/β-catenin signaling pathway and suggest that POSTN may serve as a cytokine for male infertility therapy.





Similar content being viewed by others
Abbreviations
- SC:
-
spermatogonial cell
- hAMSCs:
-
human amniotic mesenchymal stem cells
- CM:
-
condition medium
- hAECs:
-
human amniotic epithelial cells
- ESCs:
-
human embryonic stem cells
References
Toolee H, Rastegar T, Solhjoo S, Mortezaee K, Mohammadipour M, Kashani IR, et al. Roles for Kisspeptin in proliferation and differentiation of spermatogonial cells isolated from mice offspring when the cells are cocultured with somatic cells. J Cell Biochem. 2019;120(4):5042–54. https://doi.org/10.1002/jcb.27780.
Sahare MG. Suyatno, Imai H. Recent advances of in vitro culture systems for spermatogonial stem cells in mammals. Reprod Med Biol. 2018;17(2):134–42. https://doi.org/10.1002/rmb2.12087.
Liu T, Guo L, Liu Z, Cheng W. Human amniotic epithelial cells maintain mouse spermatogonial stem cells in an undifferentiated state due to high leukemia inhibitor factor (LIF) expression. In Vitro Cell Dev Biol Anim. 2011;47(4):318–26. https://doi.org/10.1007/s11626-011-9396-5.
Koruji M, Shahverdi A, Janan A, Piryaei A, Lakpour MR, Gilani SM. Proliferation of small number of human spermatogonial stem cells obtained from azoospermic patients. J Assist Reprod Genet. 2012;29(9):957–67. https://doi.org/10.1007/s10815-012-9817-8.
He Y, Chen X, Zhu H, Wang D. Developments in techniques for the isolation, enrichment, main culture conditions and identification of spermatogonial stem cells. CYTOTECHNOLOGY. 2015;67(6):921–30. https://doi.org/10.1007/s10616-015-9850-4.
Meirelles LS, Fontes AM, Covas DT, Caplan AI. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev. 2009;20(5-6):419–27. https://doi.org/10.1016/j.cytogfr.2009.10.002.
Saheli M, Bayat M, Ganji R, Hendudari F, Kheirjou R, Pakzad M, et al. Human mesenchymal stem cells-conditioned medium improves diabetic wound healing mainly through modulating fibroblast behaviors. Arch Dermatol Res. 2020;312(5):325–36. https://doi.org/10.1007/s00403-019-02016-6.
Ge L, Jiang M, Duan D, Wang Z, Qi L, Teng X, et al. Secretome of olfactory mucosa mesenchymal stem cell, a multiple potential stem cell. Stem Cells Int. 2016;2016:1243659–16. https://doi.org/10.1155/2016/1243659.
Pischiutta F, Brunelli L, Romele P, Silini A, Sammali E, Paracchini L, et al. Protection of brain injury by amniotic mesenchymal stromal cell-secreted metabolites. Crit Care Med. 2016;44(11):e1118–31. https://doi.org/10.1097/CCM.0000000000001864.
He D, Zhao F, Jiang H, Kang Y, Song Y, Lin X, et al. LOXL2 from human amniotic mesenchymal stem cells accelerates wound epithelialization by promoting differentiation and migration of keratinocytes. Aging (Albany NY). 2020;12(13):12960–86. https://doi.org/10.18632/aging.103384.
Idolazzi L, Ridolo E, Fassio A, Gatti D, Montagni M, Caminati M, et al. Periostin: the bone and beyond. EUR J INTERN MED. 2017;38:12–6. https://doi.org/10.1016/j.ejim.2016.11.015.
Lambert AW, Wong CK, Ozturk S, Papageorgis P, Raghunathan R, Alekseyev Y, et al. Tumor cell-derived periostin regulates cytokines that maintain breast cancer stem cells. Mol Cancer Res. 2016;14(1):103–13. https://doi.org/10.1158/1541-7786.MCR-15-0079.
Kormann R, Kavvadas P, Placier S, Vandermeersch S, Dorison A, Dussaule JC, et al. Periostin promotes cell proliferation and macrophage polarization to drive repair after AKI. J Am Soc Nephrol. 2020;31(1):85–100. https://doi.org/10.1681/ASN.2019020113.
Venning FA, Wullkopf L, Erler JT. Targeting ECM disrupts cancer progression. Front Oncol. 2015;5:224. https://doi.org/10.3389/fonc.2015.00224.
Morra L, Moch H. Periostin expression and epithelial-mesenchymal transition in cancer: a review and an update. Virchows Arch. 2011;459(5):465–75. https://doi.org/10.1007/s00428-011-1151-5.
Wu Q, Fang T, Lang H, Chen M, Shi P, Pang X, et al. Comparison of the proliferation, migration and angiogenic properties of human amniotic epithelial and mesenchymal stem cells and their effects on endothelial cells. Int J Mol Med. 2017;39(4):918–26. https://doi.org/10.3892/ijmm.2017.2897.
Chassot AA, Le Rolle M, Jourden M, Taketo MM, Ghyselinck NB, Chaboissier MC. Constitutive WNT/CTNNB1 activation triggers spermatogonial stem cell proliferation and germ cell depletion. Dev Biol. 2017;426(1):17–27. https://doi.org/10.1016/j.ydbio.2017.04.010.
Dann CT, Alvarado AL, Molyneux LA, Denard BS, Garbers DL, Porteus MH. Spermatogonial stem cell self-renewal requires OCT4, a factor downregulated during retinoic acid-induced differentiation. Stem Cells. 2008;26(11):2928–37. https://doi.org/10.1634/stemcells.2008-0134.
Zhaleh H, Bidmeshki PA, Azadbakht M. Mesenchymal stem cell condition medium enhanced cell viability in morphine-treated cells. Bratisl Lek Listy. 2020;121(4):263–70. https://doi.org/10.4149/BLL_2020_040.
Ohinata Y, Ohta H, Shigeta M, Yamanaka K, Wakayama T, Saitou M. A signaling principle for the specification of the germ cell lineage in mice. CELL. 2009;137(3):571–84. https://doi.org/10.1016/j.cell.2009.03.014.
Zhao B, Liu JQ, Zheng Z, Zhang J, Wang SY, Han SC, et al. Human amniotic epithelial stem cells promote wound healing by facilitating migration and proliferation of keratinocytes via ERK, JNK and AKT signaling pathways. Cell Tissue Res. 2016;365(1):85–99. https://doi.org/10.1007/s00441-016-2366-1.
Gonzalez-Gonzalez L, Alonso J. Periostin: a matricellular protein with multiple functions in cancer development and progression. Front Oncol. 2018;8:225. https://doi.org/10.3389/fonc.2018.00225.
Murphy-Ullrich JE, Suto MJ. Thrombospondin-1 regulation of latent TGF-beta activation: a therapeutic target for fibrotic disease. Matrix Biol. 2018;68-69:28–43. https://doi.org/10.1016/j.matbio.2017.12.009.
Wu X, Schmidt JA, Avarbock MR, Tobias JW, Carlson CA, Kolon TF, et al. Prepubertal human spermatogonia and mouse gonocytes share conserved gene expression of germline stem cell regulatory molecules. Proc Natl Acad Sci U S A. 2009;106(51):21672–7. https://doi.org/10.1073/pnas.0912432106.
Abofoul-Azab M, AbuMadighem A, Lunenfeld E, Kapelushnik J, Shi Q, Pinkas H, et al. Development of postmeiotic cells in vitro from spermatogonial cells of prepubertal cancer patients. Stem Cells Dev. 2018;27(15):1007–20. https://doi.org/10.1089/scd.2017.0301.
Wang S, Wang X, Wu Y, Han C. IGF-1R signaling is essential for the proliferation of cultured mouse spermatogonial stem cells by promoting the G2/M progression of the cell cycle. Stem Cells Dev. 2015;24(4):471–83. https://doi.org/10.1089/scd.2014.0376.
Sharma M, Braun RE. Cyclical expression of GDNF is required for spermatogonial stem cell homeostasis. DEVELOPMENT. 2018;145(5). https://doi.org/10.1242/dev.151555.
Golestaneh N, Beauchamp E, Fallen S, Kokkinaki M, Uren A, Dym M. Wnt signaling promotes proliferation and stemness regulation of spermatogonial stem/progenitor cells. REPRODUCTION. 2009;138(1):151–62. https://doi.org/10.1530/REP-08-0510.
Takase HM, Nusse R. Paracrine Wnt/beta-catenin signaling mediates proliferation of undifferentiated spermatogonia in the adult mouse testis. Proc Natl Acad Sci U S A. 2016;113(11):E1489–97. https://doi.org/10.1073/pnas.1601461113.
Malanchi I, Santamaria-Martinez A, Susanto E, Peng H, Lehr HA, Delaloye JF, et al. Interactions between cancer stem cells and their niche govern metastatic colonization. NATURE. 2011;481(7379):85–9. https://doi.org/10.1038/nature10694.
Funding
This research was funded by Shenyang Science and Technology Plan, grant number 20-204-4-31, and National Key Research and Development Program of China, grant number 2016YFC1000600, and The APC was funded by 20-204-4-31.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
About this article
Cite this article
Li, C., Cheng, D., Xu, P. et al. POSTN Promotes the Proliferation of Spermatogonial Cells by Activating the Wnt/β-Catenin Signaling Pathway. Reprod. Sci. 28, 2906–2915 (2021). https://doi.org/10.1007/s43032-021-00596-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-021-00596-1