Abstract
Salinization and sodification are serious and worldwide growing threats to healthy soil functions. Although plants developed a plethora of traits to cope with high salinity, soil bacteria are also essential players of the adaptation process. However, there is still lack of knowledge on how other biotic and abiotic factors, such as land use or different soil properties, affect the bacterial community structure of these soils. Therefore, besides soil chemical and physical investigations, bacterial communities of differently managed salt-affected soils were analysed through 16S rRNA gene Illumina amplicon sequencing and compared. Results have shown that land use and soil texture were the main drivers in shaping the bacterial community structure of the Hungarian salt-affected soils. It was observed that at undisturbed pasture and meadow sites, soil texture and the ratio of vegetation cover were the determinative factors shaping the bacterial community structures, mainly at the level of phylum Acidobacteriota. Sandy soil texture promoted the high abundance of members of the class Blastocatellia, while at the slightly disturbed meadow soil showing high clay content was dominated by members of the class Acidobacteriia. The OTUs belonging to the class Ktedonobacteria, which were reported mostly in geothermal sediments, reached a relatively high abundance in the meadow soil.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to our current knowledge, salt-affected soils cover around 20% of agricultural lands globally (Wang et al. 2020), which is a threat to agriculture. These lands mostly occur in arid and semi-arid regions but can be also found in some humid to sub-humid climatic areas as well, particularly in the coastal regions where the inward movement of sea water through estuaries and rivers and through groundwater causes large-scale salinization (Abrol et al. 1988). High soil salinity is a major limiting factor of crop yield in these areas, since many crop species are very sensitive to soil salinity (e.g. glycophytes). Plants growing in salt-affected soils have to cope with multiple stress factors, including ionic and drought stress (Zhu 2002). The low water potential caused by high salt deposition in soil makes nutrient uptake increasingly different for plants. Besides, high concentration of sodium ions is toxic to plant cells, causing reduced photosynthesis, increased production of reactive oxygen species, growth inhibition or even plant death (Tuteja 2007).
Although plants have developed several traits to adapt to high soil salinity (e.g. the net exclusion of toxic ions from the shoot, or compartmentalization of toxic ions into specific tissues), rhizosphere microorganisms are also key players of the adaptation process by alleviating the abiotic stress effects (Khan et al. 2021; Negrão et al. 2017). Bacterial endophytes, which contain 1-aminocyclopropane-1-carboxylate (ACC) deaminase, were shown to possess several beneficial effects on plants affected by high salinity (e.g. higher fresh and dry biomass, higher chlorophyll contents and a greater number of flowers) (Ali et al. 2014). Similarly, rhizobacteria producing osmolytes, siderophores and antioxidant enzymes may also contribute to improved salt tolerance of plants (Negrão et al. 2017). Nevertheless, salinity is a dominant factor in shaping the soil microbial community structure (Lozupone and Knight 2007) and can negatively affect soil microbial activity (Borsodi et al. 2021). It was also suggested that high salinity can lower bacterial richness and increasing salinity–sodicity decreases the overall complexity of the bacterial network in soils (Guan et al. 2021). In general, it is observable that members of the phylum Pseudomonadota are usually the most dominant microbial community members in sodic soils (Borsodi et al. 2021; Wang et al. 2020; Zhang et al. 2015). Besides, members of the phyla Bacteroidota, Acidobacteriota, Gemmatimonadota and Bacillota are also often abundant in these environments (Borsodi et al. 2021; Guan et al. 2021; Wang et al. 2020). Further, a taxonomic analysis revealed that arbuscular mycorrhizal fungi and calcium treatment increased the abundance of Pseudomonadota and Bacillota at the phylum level in saline alkali soil (Ci et al. 2021). A major question regarding these microbial communities is that which environmental parameter is the dominant driving force that shapes the community structure. A strong correlation between microbial community composition with edaphic factors was observed by Wang et al. (2022a, b). Based on the microbial analyses of grassland sodic soils (Songnen Plain, China), Wang et al. (2020) suggested that electrical conductivity (EC) value of soil was the most important driving force for bacterial composition, followed by sodium ion content. Borsodi et al. (2021) investigated sodic soils covered with different kinds of alkali steppe vegetation (Danube-Tisza Interfluve, Hungary) and found that microbial catabolic profiles in the investigated soil samples were primarily driven by EC and soil water content. Besides, the only environmental variable which significantly influenced the bacterial community structure at taxonomic level was soil CaCO3.
Although significant new knowledge was gained in the past few years on microbial communities of salt-affected soils worldwide, still little is known on how different land usage affects bacterial assemblages in these environments. However, such studies are needed to reveal the possible negative effects of overgrazing or irrational utilization of alkali–saline lands to avoid, for example, the loss of bacterial diversity, which can severely affect ecosystem functions (Singh et al. 2014; Wagg et al. 2019). In the light of all the above, the aim of the present study was to investigate the possible effects of different land use types and soil properties on the bacterial community structure of salt-affected soils of Hungary.
Materials and methods
Site description, field sampling and sample preparation
Soil samples were collected from Nádudvar (Hajdú-Bihar County), Apaj (Pest County) and Szappanszék (Bács-Kiskun County) in Hungary, 2017. The climate of the sampling sites was typical European continental/Pannonian with warm, dry summers. Geographical characteristics of the sampling sites are given in Table 1.
At each site one soil profile was described and classified according to FAO (FAO 2006) and IUSS Working Group WRB (WRB 2015) to characterize the pedological conditions. The soil samples were collected with spade (surface sterilized with 70% v/v ethanol) from the upper surface layer (15 cm depth) during June 2017. For collection of soil samples, one plot of 100 m2 was selected from each site. Ten soil subsamples were randomly collected and combined to make a well-mixed composite sample from each plot. Although the plots were homogeneous in terms of soil type, composite samples were used for the average characterization of the entire plot. All the vegetation and litter from the soil surface was removed before sampling. Collected soil samples (1 kg) were placed in plastic bags. Samples for microbial analysis were transported to the laboratory in a cooling box, sieved through 2-mm sterile mesh to remove the plant residues and stones and get a well-homogenized sample. Then, samples were stored at − 80 °C in 50-ml plastic tubes until DNA isolation. For chemical analysis, the sieved soils were air-dried and stored at room temperature (22–24 °C).
Characteristics of the Nádudvar arable land
The site is characterized with two Soil Reference Groups. One is Mollic Solonetz (Cutanic, Endoclayic, Hypernatric, Episiltic, Endoprotovertic, Bathiprotocalcic, Bathigleyic, Bathisiltic) (NSnA) and the other is Endocalcic Chernozem (Aric, Pachic, Endosodic, Pantosiltic) (NChA). The cultivated arable sites (NSnA, NChA) were ploughed to a depth of 30 cm, and 400 kg ha−1 NPK (18:7:7) fertilizer was applied to the maize crop. In case of NSnA, bare soil surfaces could be observed among the maize rows.
Characteristics of the Nádudvar meadow
The soil is Katocalcic Katoprotosalic Solonetz (Epiclayic, Endosiltic, Cutanic, Differentic, Humic) (NSnM). The non-ploughed meadow site (NSnM) has not been cultivated for more than 30 years, covered with Festuca pseudovina, Agropyron repens, Convolvulus arvensis, Bolboschoenus maritimus, Taraxacum officinale and Limonium gmelinii, and the plant coverage was 100%.
Characteristics of the Apaj pasture land
The soil is Katofluvic Anocalcic Pantosodic Amphigleyic Solonchak (Alcalic, Carbonatic, Endosiltic, Bathyloamic) (AScP). The Apaj site (AScP) was grazed by sheep, and this site received grazed animal droppings. The plant coverage is 90% with the following dominant species Alopecurus pratensis, Tripolium pannonicum, Champhorosma annua, Puccinellia limosa and Festuca pseudovina.
Characteristics of the Szappanszék pasture land
The soil is Katofluvic Endocalcic Reductigleyic Gleysol (Alcalic, Katoarenic, Ochric, Pantosodic) (SGlP). The area is a drying saline lake occasionally flooded representatively during the early spring times, with continuous grassy vegetation (Agrostica stolonifera, Festuca pseudovina, Festuca vaginata, Cynodon dactylon, Aster tripolium and Juncus compressus) and extensive Hungarian grey ox grazing.
Soil pH and electrical conductivity (EC) were measured in soil-to-water suspension (1:2.5) (Buzás 1988). Soil organic carbon (%) was determined by wet oxidation (Walkley and Black 1934). Humic material (E4/E6) was determined by the method of Page et al. (1982). Plant available AL-(ammonium lactate) P2O5, AL-K2O and plant available nutrients (avNa+, avCa2+ and avMg2+) were extracted according to Egnér et al. (1960). Soil moisture content was determined using the gravimetric method (Buzás 1993). Particle size distribution was determined by pipetting (Klindworth et al. 2013).
Environmental DNA extraction and 16S rRNA gene-based amplicon sequencing
Illumina 16S rRNA gene amplicon sequencing was used to precisely assess the bacterial community composition of the soil samples. For this, community DNA was extracted from 0.5 g of the composite soil samples using the NucleoSpin Soil Mini Kit (Macherey–Nagel), according to the instructions of the manufacturer. NanoDrop spectrophotometer (Thermo Fisher Scientific, USA) was used to measure the concentration of the isolated DNA. Subsequently, for paired-end 16S rDNA amplicon sequencing, the variable V3 and V4 regions of the 16S rRNA gene were amplified using forward (5′-TCGT CGGCAGCGTCAGATGTG TATAAGAGACAGCCTA CGGGNGGCWGCAG-3′) and reverse (5′-GTCT CGTGGGCT CGGAGATGTGTATAAGAGAC AGGACTACHVGGGTATCTAATCC-3′) primers with Illumina adapter overhangs (Klindworth et al. 2013). PCR mixtures contained 12.5 ng of DNA, 0.2 μM of each primer and 12.5 μL of 2X KAPA HiFi HotStart Ready Mix (KAPA Biosystems, London, UK) supplemented with MQ water up to 25 μL final volume. The temperature profile was the following: initial denaturation for 5 min at 95 °C, 25 cycles of amplification (30 s at 95 °C, 30 s at 55 °C, 30 s at 72 °C). The last step was a final extension for 5 min at 72 °C. All amplifications were carried out in a ProFlex PCR System (Life Technologies, Carlsbad, USA). Amplicons were analysed by agarose gel electrophoresis. Paired-end fragment reads were generated on an Illumina MiSeq sequencer using MiSeq Reagent Kit v3 (600-cycle). Primary data analysis (base-calling) was carried out with Bbcl2fastq^ software (v2.17.1.14, Illumina).
Analysis of Illumina amplicon sequencing data
Reads were quality- and length-trimmed in CLC Genomics Workbench Tool 9.5.1 using an error probability of 0.05 (Q13) and a minimum length of 50 nucleotides as the threshold. Trimmed sequences were processed using mothur v 1.41.1. (Schloss et al. 2009) as recommended by the MiSeq SOP page (https://www.mothur.org/wiki/MiSeq_SOP) (Kozich et al. 2013). Paired-end sequence (contig) numbers ranged between 45,323 and 49,853. Based on the sequence alignment as determined by the SILVA 132 SSURef NR99 database (Quast et al. 2013), sequences were then assigned. The detection of chimeras was performed with Mothur’s uchime command, and the ‘split.abund’ command was also used to remove singleton reads (Edgar et al. 2011; Kunin et al. 2010). The standard 97% similarity threshold was used to determine operational taxonomic units (OTUs) as it was suggested for prokaryotic species delineation (Tindall et al. 2010). Raw sequence reads were deposited in NCBI SRA under BioProject ID PRJNA760983. The TOP20 OTUs, mostly showing > 2% relative abundance at least in one of the samples, were considered as major OTUs and were identified using the EzBioCloud 16S rDNA database (Yoon et al. 2017).
Statistical analysis
For comparative analysis of soil chemical and physical properties, the analyses of variance (ANOVA) of the data from different sites were computed using SPSS statistics vs 23.0. The mean of parameters of different sites was separated using Tukey HSD post hoc test at p < 0.05 level. Correlation between the TOP20 OTUs of soil samples (revealed by Illumina amplicon sequencing), environmental variables and sampling areas was calculated with canonical correspondence analysis (CCA) using PAST 4.05 software (Hammer et al. 2001).
Results
Physicochemical properties and structural characteristics of the investigated soils
Results of the soil characterization were partly published by Gangwar et al. (2022) and summarized in Table 2; furthermore, sand, silt and clay contents of the samples were determined (F(4,10) = 233.073 p < 0.05 for sand; F(4,10) = 151.259 p < 0.05 for silt and F(4,10) = 107.447 p < 0.05 for clay). Accordingly, the highest sand content was measured in case of soil SGlP (86.67%), which in turn contained the lowest levels of silt and clay. In the case of the other soils, sand content varied between 8 and 22%, silt content varied between 41 and 58%, while clay content varied between 29 and 38%.
Bacterial community structure of the soil samples
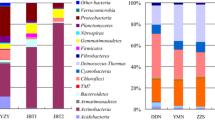
Bacterial communities of the soil samples were dominated by members of Pseudomonadota (former Proteobacteria) (23–37%), followed by Acidobacteriota (former Acidobacteria) (17–25%) and Actinobacteriota (former Actinobacteria) (7–20%).
Members of Chloroflexota (former Chloroflexi) were abundant in soils NSnM and SGlP (both 11%), while Gemmatimonadota (former Gemmatimonadetes) in soils NChA and NSnA (10% and 14%, respectively). Members of Verrucomicrobiota (former Verrucomicrobia) were abundant only in sample NSnM (12%), while bacteria belonging to the phylum Bacillota former Firmicutes) were detected in notable amount only in sample SGlP (9%).
Within the phylum Pseudomonadota, members of alpha- and gammaproteobacteria were dominant in all of the samples (Fig. 1). However, in sample NSnA most of the alphaproteobacterial sequences (~ 19%) belonged to a single OTU, which was most closely related to the Sphingomonas parvus/limnosediminicola lineages (98% 16S rRNA gene sequence homology). Not surprisingly, this sample had the lowest diversity index. The same OTU was also abundant in sample NChA, although at a much lower level (~ 6%). In general, it was observable that genus Sphingomonas-related OTUs were overrepresented and characteristic in these two soil samples. The most abundant gammaproteobacterial OTUs were detected mainly in sample AScP and were most closely related to Collimonas arenae/Glaciimonas singularis and Azoarcus olearius, respectively. In most of the soil samples, the phylum Acidobacteriota was represented by members of the class Blastocatellia, except sample NSnM, where this group was practically missing. Moreover, in samples SGlP and AScP the most abundant OTUs could be linked to the genus Brevitalea (9.4 and 7.7%, respectively). The closest relatives of these OTUs were B. aridisoli and B. deliciosa, although at a relatively low level of 16S rRNA gene similarity (~ 93–94.2%). These Brevitalea-related OTUs were detectable in all of the soil samples at minimum 4.9% abundance, except sample NSnM, in which these OTUs were not detectable. In the case of this latter sample, the most abundant Acidobacteriota-related OTU (with 4.6% abundance) could be linked to an Acetobacteraceae bacterium (Acidobacterium ailaaui) within the class Acidobacteriia. The most abundant OTU in this sample (4.8% abundance) was a Ktedonobacterales bacterium (distantly related to Dictyobacter aurantiacus) within the phylum Chloroflexota. Most importantly, these two later OTUs were characteristic only for sample NSnM and missing from other samples. The outlying nature of sample NSnM was clearly observable, since several other TOP20 OTUs were also exclusively abundant in this sample. These OTUs could be linked to an Actinoallomurus-related bacterium of the phylum Actinobacteriota (OTU13, 4%), a Candidatus Solibacter-related bacterium of the phylum Acidobacteriota (OTU14, 3.9%), a Chtoniobacteraceae-related bacterium of the phylum Verrucomicrobiota (OTU15, 3.2%), and to Bradyrhizobium macuxiense of the phylum Pseudomonadota (OTU11, 3%). Members of the class Bacilli within the phylum Bacillota were abundant only in sample SGlP. Not surprisingly, OTU5, which could be linked to Bacillus nealsonii (98.9% 16S rRNA gene homology), was characteristic for this sample (3.6% abundance). Members of the phylum Actinobacteriota were most abundant in samples NSnM and AScP (~ 19% abundance in both samples), although different lineages were detectable. While in sample NSnM members of the genera Actinoallomurus and Gaiella, together with a Solirubrobacterales bacterium were abundant, members of the family Iamiaceae were the most abundant Actinobacteriota-related bacteria in sample AScP. List of the 20 most abundant OTUs with their phylogenetical relationship is presented in Table 3.
Relative abundance of major classes in the soil bacterial communities revealed by Illumina paired-end 16S rDNA amplicon sequencing shown together with hierarchical clustering of the communities (based on calculation of the Bray–Curtis dissimilarity index). All taxa contributing more than 1% abundance were depicted. NSnM, Nádudvar Solonetz Meadow; NSnA, Nádudvar Solonetz Arable; NChA, Nádudvar Chernozem Arable; AScP, Apaj Solonchak, Pasture; SGlP, Szappanszék Gleysol Pasture
Regarding OTU-based diversity indices, it was observable that in the case of the Shannon index only small differences were detectable between the samples; thus, the Shannon index values ranged between 4.77 (soil NSnA) and 5.23 (soil SGlP) (Table 4). On the other hand, the Inverse Simpson value, which is influenced by dominance/abundance of OTUs, showed much larger variability. The lowest Inverse Simpson value was recorded in case of soil NSnA (23.97), followed by soil NSnM (64.18). The highest value was recorded in case of soil sample NChA (83.19). Unlike to α-diversity indices, which showed the lowest values in case of soil NSnA, the species richness estimators (Sobs, Chao and ACE) yielded the lowest value in case of soil NSnM, followed by soil NSnA.
To reveal relationships between the soil bacterial communities, an OTU-based UPGMA dendrogram was created by applying the Bray–Curtis similarity index. On the dendrogram it was clearly observable that bacterial community composition of soil NSnM distinctly differed from that of the other samples, which formed two subgroups according to their land use type. Consequently, one subgroup contained the pasture soils, and another one contained the arable soils (Fig. 1).
To better understand this grouping of the bacterial communities, Venn diagrams were generated revealing the distribution of OTUs among the samples (Fig. 2). The highest ratio of shared OTUs (20%) was observed between the arable soil samples NSnA and NChA, followed by the two pasture soils SGlP and AScP (19.2%). The lowest ratio of shared OTUs (6%) could be observed between the meadow soil NSnM and the pasture soil AScP.
Correlations between bacterial groups and soil chemical properties
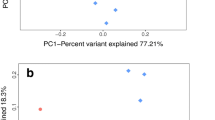
The canonical correspondence analysis based on the soil abiotic parameters and the abundance values of the TOP20 OTUs (see OTU list in Table 3) showed a distinct separation of soil NSnM from the others (Fig. 3).
Canonical correlation analysis (CCA) between the 20 now most abundant bacterial OTUs of soil samples, environmental factors and sampling areas. NSnM, Nádudvar Solonetz Meadow; NSnA, Nádudvar Solonetz Arable; NChA, Nádudvar Chernozem Arable; AScP, Apaj Solonchak, Pasture; SGlP, Szappanszék Gleysol Pasture
Thus, the outlying nature of soil sample NSnM, which was taken from a meadow, was evident again. The sharp separation of this soil sample was caused by the high abundance of OTUs which could be identified as a Ktedonobacterales bacterium (Chloroflexota) (OTU 8), an Acidobacteraceae bacterium (Candidatus Solibacter sp., OTU 14), a genus Actinoallomurus-related bacterium (OTU 13), a “Spartobacteria”-related bacterium (OTU 14) and a Bradyrhizobium-related bacterium (OTU 11). Nevertheless, none of the investigated environmental parameters explained the outlying nature of soil sample NSnM. The other four soil samples were grouped closer to each other. Still, the arable soil NSnA had a slightly separate position on the CCA plot. This separation was caused mainly by the high abundance of Sphingomonas-related OTUs (OTU1 and OTU12) and a positive correlation with the soil moisture content was also observable. In case of pasture soil, SGlP OTU 2, OTU 5 and OTU 10 reached their maximum abundance and showed a significant positive correlation (p < 0.05) with the high sand content of this soil. In soil AScP, OTU 18, which was identified as an Iaimiaceae-related actinobacterium, showed a positive correlation with the high Na+ concentration.
Discussion
The main aim of the present study was to reveal the bacterial community composition of Hungarian salt-affected soils and to observe how land use affects the community structure. Nevertheless, these two characteristics considerably affect physicochemical parameters of soils, which eventually shape the structure of their bacterial communities.
Results of the bacterial community analysis have shown that besides land use and land cover, soil texture had a crucial effect on the presence or lack of some bacterial classes in the communities. The best example of this observation is the different occurrence of classes Blastocatellia and Acidobacteriia in the investigated soils. Both classes belong to the phylum Acidobacteriota, and it was observed that members of the class Acidobacteriia were exclusively abundant in soil NSnM and were marginal in the other samples. Contrarily, members of the class Blastocatellia were marginal in soil NSnM but were abundant in the other ones. Recently, the distinct habitat preferences of Acidobacteriia and Blastocatellia in tundra soil have been observed by Ivanova et al. (2020). It was reported that these two classes of the phylum Acidobacteriota have opposite habitat preferences, since Blastocatellia were primarily abundant in unfixed sands, while Acidobacteriia preferred more developed soils with continuous plant cover. However, our results show that higher sand content may be the main factor, which causes the high abundance of Blastocatellia in the Acidobacteriota community, rather than the extent of plant cover. Still, the Blastocatellia-related Brevitalea genus (OTU2 and OTU3) was most abundant in the case of soil SGIP, which was under fully developed plant cover. Members of this genus were isolated from a Namibian semi-arid savanna soil (Wüst et al. 2016). The sand content of soil SGIP was extremely high compared to other soils investigated in this study, and most probably this characteristic caused the high abundance of Blastocatellia bacteria here. The soil AScP had the second highest sand content and had slightly patchy vegetation cover, while soils NSnA and NChA had markedly lower sand content but were used as arable lands. Considering the fact that soils under intensive agricultural management (e.g. ploughing, tilling) could result in similar (weak) conditions for microbes like high sand content, it may be concluded that high sand content is the main factor, which determines whether Blastocatellia or Acidobacteriia will be dominant in the acidobacterial community. This observation is in agreement with the finding of Xia et al. (2020), according to which soil texture is one of the most important factors in shaping soil bacterial communities. Moreover, in a study by Mencel et al. (2022), a significant correlation was found between biochemical parameters (enzymatic activity and microbial abundance) and organic matter components. In another study, it was observed that bacterial community structure was affected by environmental factors such as soil organic matter, soil moisture and EC (He et al. 2021). Similar to Blastocatellia, members of Subgroup 6 were marginal in soil NSnM, but abundant in the other soil samples. However, it should be noted that the ratio of Blastocatellia was higher at the pastures, while the abundance of the Subgroup 6 members (e.g. OTU17-Vicinamibacteria) showed opposite preference, since in the soil of arable crops their abundance was 2–3 times higher. This result can be (at least partly) explained by the observations that these acidobacteria typically inhabit grassland soils and are positively correlated with nutrient availability (Naether et al. 2012; Navarrete et al. 2015). Thus, the abundance maximum of these bacteria in the arable soils can be explained by the high amount of nutrients originated from fertilizers used at these plots. As it was mentioned above, members of the class Acidobacteriia were exclusively abundant in soil NSnM, which was an almost undisturbed meadow soil with high silt and clay content.
The most abundant Pseudomonadota phylum-related OTUs belonged to the genus Sphingomonas and were typically observed with high relative abundance values in the arable soils NSnA and NChA. Although members of the genus are known to have significant role in the degradation of organic contaminants (e.g. aromatic hydrocarbons) in soils (Leys et al. 2004), a growing number of studies have shown their plant growth promoting ability as well, especially in the case of maize (Chen et al. 2021; Wang et al. 2022a, b). Moreover, it was also observed that nitrate applied as inorganic fertilizer can significantly decrease the bacterial diversity in the rhizosphere of maize, while recruiting bacteria such as members of the genus Sphingomonas and its close relatives (e.g. Sphingobium and Novosphingobium) (Zhang et al. 2021). In a recent study, Megyes et al. (2021) also found that the sole application of inorganic fertilizers (NPK) in case of a maize monoculture significantly affected soil bacterial and archaeal community structures. Other Pseudomonadota-related OTUs were much less abundant in the samples and were typically present with > 2% relative abundance only in one of the samples. A good example of this phenomenon is the genus Bradyrhizobium, which occurred in significant quantities only in soil NSnM. Here these bacteria most probably played key role in nitrogen fixation either as plant root symbionts or as free living bacteria (Ormeño-Orrillo and Martínez-Romero 2019). The relatively high abundance of these bacteria in soil NSnM can be explained by the facts that this was an undisturbed soil with continuous plant cover, which usually positively affect the abundance of genus Bradyrhizobium in soils (Zhalnina et al. 2013).
Members of the class Ktedonobacteria (phylum Chloroflexota) were exclusively abundant in soil NSnM. These bacteria are ubiquitous in terrestrial environments; still, our knowledge of their habitat preference and ecological role is limited (Yabe et al. 2017a; Zheng et al. 2019). Typically, they show low abundances in common terrestrial environments (e.g. soil, sand and bark) and can be abundant in extreme environments such as geothermal sediments (Yabe et al. 2017a). However, OTUs belonging to class Ktedonobacteria reached a relative abundance of over 9% in soil NSnM, which is comparable to that of was observed in geothermal sediments by Yabe et al. (2017a). Recently, it was suggested by Zheng et al. (2021) that members of the Ktedonobacteria lineage have a high cellulolytic potential. Based on a genome-wide analysis, it was found that many of these bacteria harbour carbohydrate-active enzymes (e.g. endo- and exocellulases), hinting at their role in cellulose degradation (Zheng et al. 2021). Consequently, the high abundance of these potentially cellulolytic bacteria in soil NSnM can be explained, since this site was used as a meadow with full and rich vegetation cover, and the decomposing plant material could fuel the ktedonobacterial community here. The dominant ktedonobacterial OTU (4.8%) of soil NSnM was most closely related to Dictyobacter aurantiacus, although at considerably low level of 16S rRNA gene similarity (86.4%). Members of the genus Dictyobacter have been described only recently, and most of them are able to degrade cellulose and xylan and do not grow at pH higher than 9 (Wang et al. 2019; Yabe et al. 2017b).
Interestingly, members of the class Anaerolineae were abundant in soil SGlP, while playing marginal role in the microbial communities of the other soil samples. Unfortunately, little is known about the role of these bacteria in soils. Zhao et al. (2020a, b) investigated soil bacterial communities along a salinity gradient in the Yellow River Delta and found that members of the class Anaerolineae preferred soils with low-salt content.
Members of the class Verrucomicrobiae were most abundant in soil NSnM (12%) and the least abundant in soil SGlP (1%). It is well known that Verrucomicrobiota are ubiquitous in soil, and the highest relative abundances can usually be observed in soils from humid grasslands and prairies (Bergmann et al. 2011). It was also observed by Bergmann et al. (2011) that significantly lower abundancies can be observed in soils of arid/semi-arid grasslands and agricultural lands. Our results further confirm this observation.
The phylum Gemmatimonadota was primarily found in arable soils NSnA and NChA (with relative abundances of 13.6 and 10.3%, respectively). This high abundance of Gemmatimonadota bacteria is unusual, since globally they comprise ca. 2% of soil bacterial communities (DeBruyn et al. 2011). Although these bacteria are ubiquitous members of soil microbial communities, still little is known about their ecological role. DeBruyn et al. (2011) suggested that they are adapted to low-moisture conditions but cannot tolerate moisture fluctuations. Nevertheless, members of the Gemmatimonadota are often reported among the most dominant bacteria in the rhizosphere of maize (Qaisrani et al. 2019; Wen et al. 2016). Moreover, Zhu et al. (2018) observed that sustainable agricultural management practices (e.g. returning all crop residues to the soil) further increased the relative abundance of the phylum Gemmatimonadota in the soil microbial community of a maize cropping system. Based on all these, it is well explained why Gemmatimonadota bacteria showed the highest relative abundances in soils NSnA and NChA.
Similar to Gemmatimonadota, members of the class Phycisphaerae (phylum Planctomycetota) showed the highest relative abundances in soils NSnA and NChA. The first representatives of Phycisphaerae were described more than a decade ago from marine alga (Fukunaga et al. 2009), and their role in soil environment is still largely unknown. The stable-isotope probing-based study of Wang et al. (2015) suggested that these bacteria primarily act as heteropolysaccharide degraders in soils. Thus, it can be speculated that these bacteria benefited from decaying crop residues in soils NSnA and NChA, causing their high relative abundance.
Members of the class Bacilli together with the whole phylum of Bacillota were exclusively abundant in soil SGlP. Members of the genus Bacillus are often the major isolates in studies aiming to cultivate halophilic phosphate-solubilizing bacteria from salt-affected soils (Jiang et al. 2018, 2020). The fact that halophilic Bacillus strains often show alkaliphilic characteristics as well explains why Bacilli were considerably abundant in soil SGlP, which showed the highest pH value among the investigated soils (Arora and Vanza 2018). In such an environment, these spore-forming bacteria have a competitive advantage over other bacteria.
Conclusion for future biology
Based on the observed bacterial communities, it can be concluded that land usage and soil texture were the key factors, which shaped bacterial community compositions of the investigated soils. At those sites where the salt-affected soil was not disturbed (pasture and meadow soils), soil texture and the ratio of vegetation cover were the determinative factors, which shaped bacterial community structures, mainly at the level of phylum Acidobacteriota. In salt-affected soils with either high sand content or with patchy vegetation cover, members of the classes Blastocatellia and Vicinamibacteria were the abundant acidobacteria, while in the slightly disturbed meadow soil having higher clay content, members of the class Acidobacteriia overwhelmingly dominated the acidobacterial community. Besides, at arable lands, the cultivated plant (maize at the Nádudvar site) and the usage of fertilizers together were most probably responsible for the observed low bacterial diversity and the high abundance of some characteristic maize rhizosphere-associated bacteria. Overall, results of the present study provided further microbiological arguments against the irrational agricultural use (e.g. as arable lands) of salt-affected soils. Future microbiological studies on salt-affected soils should focus on how we can increase the resistance and resilience of the associated microbial communities in order to preserve soil stability.
References
Abrol IP, Yadav JSP, Massoud FI (1988) Salt-affected soils and their management, soils bulletin. Food & Agriculture Organisation, Rome
Ali S, Charles TC, Glick BR (2014) Amelioration of high salinity stress damage by plant growth-promoting bacterial endophytes that contain ACC deaminase. Plant Physiol Biochem 80:160–167. https://doi.org/10.1016/j.plaphy.2014.04.003
Arora S, Vanza MJ (2018) Halophilic microbial ecology for agricultural production in salt affected lands. In: Lichtfouse E (ed) Sustainable agriculture reviews 33: climate impact on agriculture, sustainable agriculture reviews. Springer, Cham, pp 203–229. https://doi.org/10.1007/978-3-319-99076-7_7
Bergmann GT, Bates ST, Eilers KG, Lauber CL, Caporaso JG, Walters WA, Knight R, Fierer N (2011) The under-recognized dominance of Verrucomicrobia in soil bacterial communities. Soil Biol Biochem 43:1450–1455. https://doi.org/10.1016/j.soilbio.2011.03.012
Borsodi AK, Mucsi M, Krett G, Szabó A, Felföldi T, Szili-Kovács T (2021) Variation in sodic soil bacterial communities associated with different alkali vegetation types. Microorganisms 9(8):1673. https://doi.org/10.3390/microorganisms9081673
Buzás I (1988) Manual of soil and agrochemical analysis. In: Talaj És Agrokémiai Vizsgálati Módszerkönyv (Physico-chemical and chemical analytical methods for soils). Mezőgazdasági Kiadó, Budapest, Hungary
Buzás I (1993) Manual of soil and agrochemical analysis. In: 2. Physical, water management and mineralogical analysis of the soil. INDA 4231, Budapest. Hungary
Chen L, Hao Z, Li K, Sha Y, Wang E, Sui X, Mi G, Tian C, Chen W (2021) Effects of growth-promoting rhizobacteria on maize growth and rhizosphere microbial community under conservation tillage in Northeast China. Microb Biotechnol 14:535–550. https://doi.org/10.1111/1751-7915.13693
Ci D, Tang Z, Ding H, Cui L, Zhang G, Li S, Dai L, Qin F, Zhang Z, Yang J, Xu Y (2021) The synergy effect of arbuscular mycorrhizal fungi symbiosis and exogenous calcium on bacterial community composition and growth performance of peanut (Arachis hypogaea L.) in saline alkali soil. J Microbiol 59:51–63. https://doi.org/10.1007/s12275-021-0317-3
DeBruyn JM, Nixon LT, Fawaz MN, Johnson AM, Radosevich M (2011) Global biogeography and quantitative seasonal dynamics of Gemmatimonadetes in soil. Appl Environ Microbiol 77:6295–6300. https://doi.org/10.1128/AEM.05005-11
Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R (2011) UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27:2194–2200. https://doi.org/10.1093/bioinformatics/btr381
Egnér H, Riehm H, Domingo WR (1960) Untersuchungen uber die chemische Bodenanalyse als Grundlage fur die Beurteilung des Nährstoffzustandes der Böden. Chem Extraktionsmethoden Zur Phosphor- Kaliumbestimmung 26:199–215
FAO (2006) Guidelines for soil description, 4th edn. FAO, Rome
Fukunaga Y, Kurahashi M, Sakiyama Y, Ohuchi M, Yokota A, Harayama S (2009) Phycisphaera mikurensis gen. nov., sp. nov., isolated from a marine alga, and proposal of Phycisphaeraceae fam. nov., Phycisphaerales ord. nov. and Phycisphaerae classis nov. in the phylum Planctomycetes. J Gen Appl Microbiol 55:267–275. https://doi.org/10.2323/jgam.55.267
Gangwar RK, Makádi M, Bresilla B, Zain M, Weldmichael TG, Demeter I, Táncsics A, Cserháti M, Szegi TA (2022) Effects of land uses and soil types on microbial activity and community structure. Int Agrophys 36:323–336. https://doi.org/10.31545/intagr/155096
Guan Y, Jiang N, Wu Y, Yang Z, Bello A, Yang W (2021) Disentangling the role of salinity-sodicity in shaping soil microbiome along a natural saline-sodic gradient. Sci Total Environ 765:142738. https://doi.org/10.1016/j.scitotenv.2020.142738
Hammer Ø, Harper DAT, Ryan PD (2001) PAST: paleontological statistics software package for education and data analysis. Palaeontol Electron 4:1–9
He S, Hu W, Jin X, Han J (2021) Soil bacterial community composition and diversity respond to soil environment in the Ebinur Lake Wetland. Arch Microbiol 203:1175–1182. https://doi.org/10.1007/s00203-020-02112-6
Ivanova AA, Zhelezova AD, Chernov TI, Dedysh SN (2020) Linking ecology and systematics of acidobacteria: Distinct habitat preferences of the Acidobacteriia and Blastocatellia in tundra soils. PLoS ONE 15:e0230157. https://doi.org/10.1371/journal.pone.0230157
Jiang H, Qi P, Wang T, Wang M, Chen M, Chen N, Pan L, Chi X (2018) Isolation and characterization of halotolerant phosphate-solubilizing microorganisms from saline soils. 3 Biotech 8:1–8. https://doi.org/10.1007/s13205-018-1485-7
Jiang H, Wang T, Chi X, Wang M, Chen N, Chen M, Pan L, Qi P (2020) Isolation and characterization of halotolerant phosphate solubilizing bacteria naturally colonizing the peanut rhizosphere in salt-affected soil. Geomicrobiol J 37:110–118. https://doi.org/10.1080/01490451.2019.1666195
Khan N, Ali S, Shahid MA, Mustafa A, Sayyed RZ, Curá JA (2021) Insights into the interactions among roots, rhizosphere, and rhizobacteria for improving plant growth and tolerance to abiotic stresses: a review. Cells 10:1551. https://doi.org/10.3390/cells10061551
Klindworth A, Pruesse E, Schweer T, Peplies J, Quast C, Horn M, Glöckner FO (2013) Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res 41:e1. https://doi.org/10.1093/nar/gks808
Kozich JJ, Westcott SL, Baxter NT, Highlander SK, Schloss PD (2013) Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl Environ Microbiol 79:5112–5120. https://doi.org/10.1128/AEM.01043-13
Kunin V, Engelbrektson A, Ochman H, Hugenholtz P (2010) Wrinkles in the rare biosphere: Pyrosequencing errors can lead to artificial inflation of diversity estimates. Environ Microbiol 12:118–123. https://doi.org/10.1111/j.1462-2920.2009.02051.x
Leys NM, Ryngaert A, Bastiaens L, Verstraete W, Top EM, Springael D (2004) Occurrence and phylogenetic diversity of Sphingomonas strains in soils contaminated with polycyclic aromatic hydrocarbons. Appl Environ Microbiol 70:1944–1955. https://doi.org/10.1128/Aem.70.4.1944-1955.2004
Lozupone CA, Knight R (2007) Global patterns in bacterial diversity. Proc Natl Acad Sci 104:11436–11440. https://doi.org/10.1073/pnas.0611525104
Megyes M, Borsodi AK, Árendás T, Márialigeti K (2021) Variations in the diversity of soil bacterial and archaeal communities in response to different long-term fertilization regimes in maize fields. Appl Soil Ecol 168:104120. https://doi.org/10.1016/j.apsoil.2021.104120
Mencel J, Futa B, Mocek-Płóciniak A, Mendyk Ł, Piernik A, Kaczmarek T, Glina B (2022) Interplay between selected chemical and biochemical soil properties in the humus horizons of grassland soils with low water table depth. Sustainability 14:16890. https://doi.org/10.3390/su142416890
Naether A, Foesel BU, Naegele V, Wüst PK, Weinert J, Bonkowski M, Alt F, Oelmann Y, Polle A, Lohaus G (2012) Environmental factors affect acidobacterial communities below the subgroup level in grassland and forest soils. Appl Environ Microbiol 78:7398–7406. https://doi.org/10.1128/AEM.01325-12
Navarrete AA, Venturini AM, Meyer KM, Klein AM, Tiedje JM, Bohannan BJ, Nüsslein K, Tsai SM, Rodrigues JL (2015) Differential response of Acidobacteria subgroups to forest-to-pasture conversion and their biogeographic patterns in the western Brazilian Amazon. Front Microbiol 6:1443. https://doi.org/10.3389/fmicb.2015.01443
Negrão S, Schmöckel SM, Tester M (2017) Evaluating physiological responses of plants to salinity stress. Ann Bot 119:1–11. https://doi.org/10.1093/aob/mcw191
Ormeño-Orrillo E, Martínez-Romero E (2019) A genomotaxonomy view of the Bradyrhizobium genus. Front Microbiol 10:1334. https://doi.org/10.3389/fmicb.2019.01334
Page AL, Miller RH, Keeney DR (1982) Methods of soil analysis. Part 2, chemical and microbiological properties, 2nd ed., agronomy monograph. American Society of Agronomy and Soil Science Society of America, Madison
Qaisrani MM, Zaheer A, Mirza MS, Naqqash T, Qaisrani TB, Hanif MK, Rasool G, Malik KA, Ullah S, Jamal MS (2019) A comparative study of bacterial diversity based on culturable and culture-independent techniques in the rhizosphere of maize (Zea mays L.). Saudi J Biol Sci 26:1344–1351. https://doi.org/10.1016/j.sjbs.2019.03.010
Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, Glöckner FO (2013) The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res 41:D590-596. https://doi.org/10.1093/nar/gks1219
Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, Sahl JW, Stres B, Thallinger GG, Van Horn DJ, Weber CF (2009) Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75:7537–7541. https://doi.org/10.1128/AEM.01541-09
Singh BK, Quince C, Macdonald CA, Khachane A, Thomas N, Al-Soud WA, Sørensen SJ, He Z, White D, Sinclair A (2014) Loss of microbial diversity in soils is coincident with reductions in some specialized functions. Environ Microbiol 16:2408–2420. https://doi.org/10.1111/1462-2920.12353
Tindall BJ, Rosselló-Móra R, Busse H-J, Ludwig W, Kämpfer P (2010) Notes on the characterization of prokaryote strains for taxonomic purposes. Int J Syst Evol Microbiol 60:249–266. https://doi.org/10.1099/ijs.0.016949-0
Tuteja N (2007) Mechanisms of high salinity tolerance in plants. Methods Enzymol 428:419–438. https://doi.org/10.1016/S0076-6879(07)28024-3
Wagg C, Schlaeppi K, Banerjee S, Kuramae EE, van der Heijden MG (2019) Fungal-bacterial diversity and microbiome complexity predict ecosystem functioning. Nat Commun 10:4841. https://doi.org/10.1038/s41467-019-12798-y
Walkley A, Black IA (1934) An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci 37:29
Wang X, Sharp CE, Jones GM, Grasby SE, Brady AL, Dunfield PF (2015) Stable-isotope probing identifies uncultured Planctomycetes as primary degraders of a complex heteropolysaccharide in soil. Appl Environ Microbiol 81:4607–4615. https://doi.org/10.1128/AEM.00055-15
Wang C, Zheng Y, Sakai Y, Toyoda A, Minakuchi Y, Abe K, Yokota A, Yabe S (2019) Tengunoibacter tsumagoiensis gen. nov., sp. nov., Dictyobacter kobayashii sp. nov., Dictyobacter alpinus sp. nov., and description of Dictyobacteraceae fam. nov. within the order Ktedonobacterales isolated from Tengu-no-mugimeshi, a soil-like granular mass of micro-organisms, and emended descriptions of the genera Ktedonobacter and Dictyobacter. Int J Syst Evol Microbiol 69:1910–1918. https://doi.org/10.1099/ijsem.0.003396
Wang S, Sun L, Ling N, Zhu C, Chi F, Li W, Hao X, Zhang W, Bian J, Chen L, Wei D (2020) Exploring soil factors determining composition and structure of the bacterial communities in saline-alkali soils of Songnen Plain. Front Microbiol 10:2902. https://doi.org/10.3389/fmicb.2019.02902
Wang Z, Song S, Song T, Yuan L, Zhang C (2022a) Responses of edaphic factors and microbial community to terrestrial succession and experimental warming in coastal salt marshes. Pedobiologia 93–94:150821. https://doi.org/10.1016/j.pedobi.2022.150821
Wang F, Wei Y, Yan T, Wang C, Chao Y, Jia M, An L, Sheng H (2022b) Sphingomonas sp. Hbc-6 alters physiological metabolism and recruits beneficial rhizosphere bacteria to improve plant growth and drought tolerance. Front Plant Sci 13:1002772. https://doi.org/10.3389/fpls.2022.1002772
Wen X, Dubinsky E, Yao WU, Rong Y, Fu C (2016) Wheat, maize and sunflower cropping systems selectively influence bacteria community structure and diversity in their and succeeding crop’s rhizosphere. J Integr Agric 15:1892–1902. https://doi.org/10.1016/S2095-3119(15)61147-9
WRB, IU of SS (IUSS) WG (2015) World reference base for soil resources 2014, update 2015: international soil classification system for naming soils and creating legends for soil maps. World Soil Resource Report No 106
Wüst PK, Foesel BU, Geppert A, Huber KJ, Luckner M, Wanner G, Overmann J (2016) Brevitalea aridisoli, B. deliciosa and Arenimicrobium luteum, three novel species of Acidobacteria subdivision 4 (class Blastocatellia) isolated from savanna soil and description of the novel family Pyrinomonadaceae. Int J Syst Evol Microbiol 66:3355–3366. https://doi.org/10.1099/ijsem.0.001199
Xia Q, Rufty T, Shi W (2020) Soil microbial diversity and composition: links to soil texture and associated properties. Soil Biol Biochem 149:107953. https://doi.org/10.1016/j.soilbio.2020.107953
Yabe S, Sakai Y, Abe K, Yokota A (2017a) Diversity of Ktedonobacteria with actinomycetes-like morphology in terrestrial environments. Microbes Environ 32:61–70. https://doi.org/10.1264/jsme2.ME16144
Yabe S, Sakai Y, Abe K, Yokota A, Také A, Matsumoto A, Sugiharto A, Susilowati D, Hamada M, Nara K (2017b) Dictyobacter aurantiacus gen. nov., sp. nov., a member of the family Ktedonobacteraceae, isolated from soil, and emended description of the genus Thermosporothrix. Int J Syst Evol Microbiol 67:2615–2621. https://doi.org/10.1099/ijsem.0.001985
Yoon SH, Ha SM, Kwon S, Lim J, Kim Y, Seo H, Chun J (2017) Introducing EzBioCloud: a taxonomically united database of 16S rRNA and whole genome assemblies. Int J Syst Evol Microbiol 67:1613–1617. https://doi.org/10.1099/ijsem.0.001755
Zhalnina K, de Quadros PD, Gano KA, Davis-Richardson A, Fagen JR, Brown CT, Giongo A, Drew JC, Sayavedra-Soto LA, Arp DJ, Camargo FAO, Daroub SH, Clark IM, McGrath SP, Hirsch PR, Triplett EW (2013) Ca. Nitrososphaera and Bradyrhizobium are inversely correlated and related to agricultural practices in long-term field experiments. Front Microbiol 4:104. https://doi.org/10.3389/fmicb.2013.00104
Zhang Y, Cao C, Guo L, Wu Q, Cui Z (2015) Soil properties, bacterial community composition, and metabolic diversity responses to soil salinization of a semiarid grassland in northeast China. J Soil Water Conserv 70:110–120. https://doi.org/10.2489/jswc.70.2.110
Zhang HQ, Zhao XQ, Shi Y, Liang Y, Shen RF (2021) Changes in soil bacterial communities with increasing distance from from maize roots affected by ammonium and nitrate additions. Geoderma 398:115102. https://doi.org/10.1016/j.geoderma.2021.115102
Zhao S, Liu JJ, Banerjee S, Zhou N, Zhao ZY, Zhang K, Hu MF, Tian CY (2020a) Biogeographical distribution of bacterial communities in saline agricultural soil. Geoderma 361:114095. https://doi.org/10.1016/j.geoderma.2019.114095
Zhao Q, Bai J, Gao Y, Zhao H, Zhang G, Cui B (2020b) Shifts in the soil bacterial community along a salinity gradient in the Yellow River Delta. Land Degrad Dev 31:2255–2267. https://doi.org/10.1002/ldr.3594
Zheng Y, Saitou A, Wang CM, Toyoda A, Minakuchi Y, Sekiguchi Y, Ueda K, Takano H, Sakai Y, Abe K (2019) Genome features and secondary metabolites biosynthetic potential of the class Ktedonobacteria. Front Microbiol 10:893. https://doi.org/10.3389/fmicb.2019.00893
Zheng Y, Maruoka M, Nanatani K, Hidaka M, Abe N, Kaneko J, Sakai Y, Abe K, Yokota A, Yabe S (2021) High cellulolytic potential of the Ktedonobacteria lineage revealed by genome-wide analysis of CAZymes. J Biosci Bioeng 131:622–630. https://doi.org/10.1016/j.jbiosc.2021.01.008
Zhu JK (2002) Salt and drought stress signal transduction in plants. Annu Rev Plant Biol 53:247–273. https://doi.org/10.1146/annurev.arplant.53.091401.143329
Zhu XC, Sun LY, Song FB, Liu SQ, Liu FL, Li XN (2018) Soil microbial community and activity are affected by integrated agricultural practices in China. Eur J Soil Sci 69:924–935. https://doi.org/10.1111/ejss.12679
Acknowledgements
The authors would like to thank to Gábor Mészáros (KITE Pvt. Ltd. Hungary), for permission to use the study sites and the cultivation data for the sites and AgriDron Ltd. for the continuous support.
Funding
Open access funding provided by Hungarian University of Agriculture and Life Sciences. This work was supported by the Tempus Public Foundation (Government of Hungary) Stipendium Hungaricum Scholarship Program, No. 2015-SH-500096, and the Ministry of Innovation and Technology within the framework of the Thematic Excellence Programme 2021, National Defence, National Security Subprogramme (TKP2021-NVA-22).
Author information
Authors and Affiliations
Contributions
RKG contributed to sampling, investigation, formal analysis, data curation and writing—original draft; AT contributed to investigation, formal analysis, visualization and writing—original draft; MM performed investigation, formal analysis and writing—original draft; MF performed visualization; MC and MF performed investigation; EM performed writing—review and editing; TS contributed to conceptualization, investigation, visualization, writing—original draft, and writing—review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gangwar, R.K., Táncsics, A., Makádi, M. et al. Bacterial community composition of Hungarian salt-affected soils under different land uses. BIOLOGIA FUTURA (2024). https://doi.org/10.1007/s42977-024-00235-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42977-024-00235-1