Abstract
Young shoots of cereals are widely regarded as superfoods with health benefits attributed to their potential antioxidant activity and antioxidant-related effects (e.g. anticancer). The current study aimed to examine the chemical characteristics of Hordeum vulgare methanolic and aqueous extracts and assess their antioxidant activity using the DDPH and ORAC. Furthermore, the inhibitory effect of xanthine oxidase was screened. TLC bioautography was employed to determine the polarity of the compounds present in the extracts that exhibited the most potent free radical scavenging activity. Total flavonoid content of the methanolic and aqueous extracts was 0.14 mg QE/g and 0.012 mg QE/g, respectively. The antioxidant activity of the methanolic extract was found to be more potent, with a value of 0.97 ± 0.13 mmol TE/g than the aqueous extract which had no activity. This study presents novel findings on the xanthine inhibitory activity of H. vulgare. The methanolic extract demonstrated moderate inhibition of xanthine oxidase with a value of 23.24%. The results of our study were compared with the phytochemical and pharmacological analysis of Triticum aestivum, and further comparison was made with the data reported in the literature. Inconsistencies were observed in the chemical and pharmacological properties of H. vulgare, which could be a result of using herbal material harvested in different vegetative phases and various methods used for extraction. The findings of our study indicate that the timing of the harvest and extraction method may play crucial role in attaining the optimal phytochemical composition of H. vulgare, hence enhancing its pharmacological activity.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Barley (Hordeum vulgare L.) is a plant that has been grown since ancient times. The cultivation and utilisation of this particular crop as human food source and animal feed may be traced back to the period 8000–7000 BC. Barley is used primarily in the production of animal feed and various industrial products, with a notable emphasis on its application in the brewing of beer. Additionally, it has excellent potential as a healthy food source due to its abundance of dietary fibre, protein, calcium, phosphorus, and modest amounts of B vitamins (Fatemi et al. 2022).
During the past three decades, numerous animal studies have reported the beneficial effects associated with the consumption of barley leaves. These effects include the prevention of ulcers, antioxidant properties, hypolipidemic, antidepressant, and antidiabetic effects (Ohtake et al. 1985; Yu et al. 2002; Yamaura et al. 2012). Many research groups have investigated the antioxidant effect of the plant. Their results have conclusively demonstrated that products derived from barley leaves serve as valuable dietary sources of antioxidants (Kim et al. 2007; Macháň et al. 2014; Panthi et al. 2020).
Plant-derived food and dietary supplements are widely recognised for their potential to neutralise the free oxygen radicals. Reactive species tend to accumulate in the human organism as a result of regular metabolism, leading to the occurrence of progressive reactions and adverse changes (Lobo et al. 2010). The scavenging of reactive oxygen species (ROS) by antioxidants plays a vital role in reducing the risk of carcinogenesis caused by oxidative stress. Antioxidants possess the ability to inhibit the cell proliferation secondary to protein phosphorylation (Glatthaar et al. 1986). Strong antioxidant activities have been found in sour cherries, citruses, prunes, and herbs (Szent-Györgyi 1988; Nemes et al. 2015). Green tea-based products have been extensively studied in the recent past for antioxidant properties since they contain up to 30% of phenolic compounds of dry weight (Lin et al. 1998).
The objective of our study was to evaluate the biological activity of young barley leaf, specifically focussing on its antioxidant capacity and the presence of bioactive chemicals. A comparative analysis was conducted to assess the biological activity and chemical composition of barley in relation to wheatgrass extract. Based on our results, we analysed several factors that might affect the chemical composition and biological activity of the plant extracts.
Materials and methods
Plant material
The young shoots of Hordeum vulgare L. and Triticum aestivum L. were harvested after a growth period of eight months in Hódmezővásárhely (Hungary) on 4 May 2022. The plant shoots reached a height of 30–40 cm. The plant materials were identified by Ferenc Lantos. The voucher specimens were deposited in the herbarium of the Institute of Pharmacognosy, University of Szeged (barley: 2022/VI-12, wheatgrass 2022/VI-13).
Extraction
Fresh leaves were cut in 2–4 cm in length then homogenised with either water or methanol, respectively, using a mechanical blender. The homogenised plants were subjected to ultrasonication-assisted extraction for ten minutes. The filtrate was obtained by using a press machine and subsequent filtration process employing filter paper (Whatman, grade 0905). Additional purification was achieved by centrifugation (5,000g) for 15 min. The supernatants were collected. The methanolic extracts were evaporated in vacuo, while the aqueous extracts were freeze-dried using Christ Alpha 1–4 freeze dryer. The extractable matter was expressed as the drug extract ratio (DER). All available plant materials were subjected to a one-step extraction.
Comparative analysis of extract content was performed using normal phase thin-layer chromatography (SiO2 60 F254, 10 cm in height). Two different mobile phases were applied, respectively (toluene-ethyl acetate-formic acid 5:4:1 and ethyl acetate–dichloromethane–formic acid–acetic acid–water 100:25:10:10:10). For visualisation UV254 nm was applied. Further visualisation was performed after spraying TLC (thin-layer chromatography) chromatograms with vanillin sulfuric acid and heated on 105°C for 5 min.
Total flavonoid determination
The total flavonoid content of the extracts was determined by using spectrophotometry. The dry methanolic extracts were redissolved in methanol, and the lyophilizates were redissolved in water. The concentration was set at 1 mg/mL, respectively.
External calibration was performed using quercetin solution with the concentrations of 5, 10, 25, and 50 μg/mL. The reagent was 2% aluminium chloride solution prepared with either methanol or water, respectively. Two mL of extract was combined with an equal volume of reagent. The absorbance was measured after 60 min at 420 nm by using Heliosβ ThermoSpectronic spectrophotometer. Either methanol or water was used as a blank. The measurements were carried out in triplicate.
The total flavonoid content of the extracts was calculated and expressed as mg of quercetin equivalent in one gram of extract (QE mg/g extract).
Total polyphenol determination
Total polyphenol determination was carried out according to Pharmacopoeia Europea 11.0. The freeze-dried aqueous extracts were used for analysis, 0.700 mg in weight, respectively. Pyrogallol was used as a reference compound (EDQM 2022).
Thin-layer chromatography (TLC) bioautography assay
Bioautography assay for antioxidant evaluation was performed using the modified method of Wang et al. (Wang et al. 2012). The lyophilizates were dissolved in water, and the dry residues obtained with methanolic extraction were dissolved in methanol. The concentrations were set at ten mg/mL concentration. For screening, normal phase silica gel plates (SiO2 60 F254, 20 × 10 cm) were used. Ten µL of the extracts were loaded, respectively, using a microliter syringe (Hamilton, 25 µL) in eight mm long bands ten mm from the side and ten mm from the bottom of the plate.
The TLCs were developed in glass chamber using mobile phases ethyl acetate–dichloromethane–formic acid–acetic acid–water 100:25:10:10:10 and toluene–formic acid–water 5:4:1, respectively. After development, the plates were dried on a cold airflow. The visualisation was performed by spraying the TLCs using 20% (m/v) DPPH (2,2-diphenyl-1-picrylhydrazyl) methanolic solution.
DPPH assay
DPPH assay was done on 96-well microtiter plates. For measurements, barley extracts were dissolved in methanol and the concentration was set up to one mg/mL. Microdilution series were prepared starting with 150 μL. To each well 50 μL of DPPH (100 μM) was added. Methanolic solution of ascorbic acid at 0.01 mg/mL concentration was used as a positive control.
The microplate was stored at room temperature under dark conditions. The absorbance was measured after 30 min at 550 nm using a microplate reader (FluoSTAR OPTIMA, BMG Labtech, Germany). Antiradical activity of the samples was expressed as EC50. The EC50 values were calculated using GraphPad 8.02.
ORAC assay
The ORAC (oxygen radical absorbance capacity) assay was carried out on a 96-well microplate. First, the extracts were dissolved in DMSO at a concentration of one mg/mL. The extracts were diluted with buffer to 0.1 mg/mL. To each well 20 µL of extracts (final concentration 0.01 mg/mL) with 60 µL of AAPH ((2,2′-azobis(2-methyl-propionamidine)dihydrochloride) (12 mM final concentration) and 120 µL of fluorescein solution (70 nM final concentrations), then the fluorescence was measured for 3 h with 1.5 min cycle intervals with FluoSTAR OPTIMA (BMG Labtech, Germany) plate reader. Trolox was used as standard. AAPH free radical and trolox standard (( ±)-6-hydroxy-2,5,7,8-tetramethyl-chromane-2-carboxylic acid) were purchased from Sigma-Aldrich Hungary. Fluorescein was purchased from Fluka Analytical (Tokyo, Japan). The antioxidant capacity was expressed as mmol trolox equivalent per g of dry extract (mmol TE/g), with help of GraphPad Prism 8.02 (GraphPad Software, La Jolla, CA, USA).
Xanthine oxidase assay
The inhibitor activity of the extracts on xanthine oxidase enzyme was measured by applying the adapted method provided by Sigma-Aldrich. The method is based on continuous spectrophotometric rate determination. The absorbance of xanthine oxidase enzyme-induced uric acid production from xanthine was measured at 290 nm for 3 min in 96-well plate, using the plate reader FluoSTAR OPTIMA (BMG Labtech, Germany).
The xanthine oxidase activity was evaluated using the following mixture: 150 μL potassium phosphate buffer (50 mM), 100 μL xanthine solution (0.15 mM), and 50 μL of xanthine oxidase (XO) enzyme (0–1–0.2 U/mL) were added. For extracts XO-inhibition 140 μL potassium phosphate buffer, 100 μL xanthine (0.15 mM), 50 μL of xanthine oxidase enzyme (0.1–0.2 U/mL), and 10 μL extract (12 mg/mL in DMSO) were added.
Data analysis
The normal distribution of the data was checked by using Shapiro–Wilk test. Either independent t-test or ANOVA was used for evaluation of the dataset. In case of ANOVA, Tukey’s HSD post hoc test was applied to show which groups are significantly different from each other. The differences were considered significant when p < 0.05 (*), p < 0.01 (**), and p < 0.001 (***). Statistical analyses were carried out using R (version 3.6.1, The R Foundation for Statistical Computing, Vienna, Austria, http://www.r-project.org).
Results
Barley and wheatgrass extracts
The extractable matter was expressed as drug extract ratio (DER). Extraction with methanol resulted in a lower DER in general, while the aqueous extraction yielded a higher extractable matter. The DER values are summarised in Table 1. The methanolic extracts of both species were deep green in colour and syrupy in consistency, while the lyophilizates of the aqueous extracts were dark green powders with a sweet taste.
The composition of the extracts was screened using normal phase thin-layer chromatography (Fig. 1a–d). The TLC plates were developed in two mobile phase systems in order to separate compounds according to their polarity on a wider scale. For TLC visualisation, UV254 was applied (Fig. 1a, b). The most polar spots appear to be photosynthetic carotenoids (e.g. chlorophylls). Spraying the TLC with vanillin sulfuric acid and heating on 105 °C for five minutes afforded the detection of organic compounds (Fig. 1c, d).
Thin-layer chromatograms of extracts. TLC plates a, c, and e were developed in ethyl acetate–dichloromethane–formic acid–acetic acid–water 100:25:10:10:10, while plates b, d, and f were developed in toluene–formic acid–water 5:4:1 mobile phase. Chromatograms a and b were evaluated under UV254. Vanillin sulfuric acid (plates b and c) and DPPH (plates e and f) were used for visualisation. W-M, wheatgrass methanolic extract; B-M, barley methanolic extract; W-A, wheatgrass aqueous; B-A, barley aqueous extract
The polyphenol content of the extracts
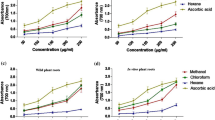
The determination of the total flavonoid content of the extracts was conducted by the utilisation of spectrophotometry. External calibration was performed using a methanolic and aqueous solution of 2% aluminium chloride as reagent. The linearities of the calibration curves were in the range of 5–50 µg/mL quercetin. The correlation coefficient was higher than 0.995 for quercetin in the concentration range (Fig. 2a).
Calibration curve for determination of QE in µg/mL of extracts (a) and the total flavonoid content in quercetin equivalent in one mg extract (b). The values are expressed as mean ± SD of values. W-M, wheatgrass methanolic extract; B-M, barley methanolic extract; W-A, wheatgrass aqueous extract; B-A, barley aqueous extract; ***, means statistically significant (p < 0.001) using Tukey’s HSD Test
After redissolving the methanolic extract of both species at a concentration of one mg/mL, the extracts were deep green colour considered unsuitable for spectrophotometric analysis. To reduce the interference of the background colour originating from the matrix, the extracts were diluted to a concentration of 0.1 mg/mL. Aqueous extracts did not have a dark green colour; therefore, the aqueous extracts were set at a concentration of 1 mg/mL.
Based on our results, methanol was proved to be more potent solvent for extraction to reach higher flavonoid content. In case of methanolic extract of wheatgrass, the total flavonoid content was 0.26 ± 0.01 mg QE in one gram of extract, while for barley it was almost half of it with 0.14 mg QE. Based on a one-way ANOVA, the observed difference in flavonoid content proved to be significant (F(3, 8) = [174.5], p < 0.001). The flavonoid content of the aqueous extracts was lower, 12.42 ± 1.29 µg QE in wheatgrass and 11.88 ± 1.59 µg QE in barley aqueous extracts, respectively (Fig. 2b). Tukey’s HSD test for multiple comparisons found that the mean value of total flavonoid content was not significantly different between barley and wheatgrass aqueous extracts.
For aqueous extracts, the total polyphenol content was evaluated and expressed as pyrogallol equivalent. The barley was found to contain 3.03 ± 0.29% of polyphenols. The total polyphenol content of the wheatgrass aqueous extract proved to be lower with a value of 2.25 ± 0.04%. Due to the low water solubility, the total polyphenol content of methanolic extracts could not be determined.
Thin-layer chromatography (TLC) bioautography assay
Chemical compounds with antioxidant activity react with DPPH. The bioautography assay allowed us to estimate the polarity of compounds in the extracts responsible for antioxidant activity. Spraying plates with DPPH reagent resulted in a purple background in TLC, while those compounds whit antioxidant activity by reacting with DPPH caused the purple colour to fade or disappear. The utilisation of two mobile phase systems allowed the evaluation of the compounds on a broader spectrum of polarity.
In the chromatograms, the faded spots could be observed either near the start points or with lower retention factor values. The chromatogram in Fig. 1e showed faded spots in range of Rf = 0.7–0.8 (B-M), which also appears on chromatogram Fig. 1f near the front. The more polar solvent system (Fig. 1f) allowed us to identify a notable spot in B-M at Rf = 0.24, while a less pronounced spot was observed at Rf = 0.34. The overall observation from the TLC bioautography assay indicated that none of the examined extracts exhibited a presence of compounds with strong antioxidant capacity, both in terms of quantity and diversity.
Free radical scavenging activity (ORAC and DPPH assays)
The free radical scavenging effect of barley extracts based on the DPPH assay was weak compared to ascorbic acid (the EC50 of ascorbic acid was 0.71 ± 0.07 mg/mL); however, the EC50 value of aqueous extract was lower (EC50 = 54.33 ± 3.49 mg/mL) compared to methanolic extract (EC50 = 394.50 ± 5.50 mg/mL) (Fig. 3a). Independent t-test afforded to conclude that differences in means of aqueous extract and methanolic extract were significant (t(2) = 90.5, p < 0.001); thus, aqueous extract was more potent antioxidant compared to methanolic extract.
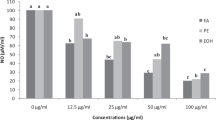
Antioxidant activity screening using DPPH (a) and ORAC (b) assays, and the xanthine oxidase inhibitory activity of the extract (c). Presented values are expressed as mean ± SD. B-M, methanolic extract of barley; B-A, aqueous extract of barley; W-M, methanolic extract of wheatgrass; W-A, aqueous extract of wheatgrass; RU, rutin; QE, quercetin; ALLOP, allopurinol; *p < 0.05, ***p < 0.001
The ORAC assay allowed us to measure and express the antioxidant activity of the extracts as mmol of tocopherol equivalent in one gram of extracts. Two flavonoids, specifically rutin and quercetin, were applied as positive standards. Three extracts were evaluated for their antioxidant capacity: methanolic extract (0.97 ± 0.13 mmol TE/1 g extract), aqueous extract of barley (0.35 ± 0.04 mmol TE/g extract), and in addition, wheatgrass methanolic extract (0.56 ± 0.16 mmol TE/g extract). A one-way ANOVA was performed in order to compare the antioxidant activity of extracts. The differences in antioxidant activities found to be statistically significant (F(5, 12) = [187.9], p < 0.001). Tukey’s HSD test was applied for multiple comparison of antioxidant activities. All extracts exerted significantly lower antioxidant activity compared to rutin (6.10 ± 0.70 mmol TE) and quercetin (7.71 ± 0.76 mmol TE), respectively. Significant difference could only be observed between antioxidant activity of methanolic extract of barley and water extract of wheatgrass (p = 0.014, 95% C.I. [–2.66, –0.25]). The results demonstrated that the aqueous extract exhibited pro-oxidant properties, as indicated by a value of –0.49 ± 0.24 mmol TE/g extract (Fig. 3b).
Xanthine oxidase assay
The xanthine oxidase inhibitory activity of the extracts was assessed and compared to the standard compound allopurinol. Allopurinol exerted inhibition activity 97.56 ± 0.98%. One-way ANOVA was applied to compare the inhibition activities. Statistically significant difference could be observed between at least two compounds (F(4, 10) = [520.1], p < 0.001). The inhibitory activity of the extracts was significantly low compared to the positive control (Fig. 3c). The wheatgrass methanolic extract demonstrated the highest level of inhibition (35.50 ± 0.98%), while the aqueous extract of wheatgrass exhibited lower level of inhibition (28.85 ± 1.00%). The aqueous extract of barley had a moderate level of inhibition (23.24 ± 0.92%), while the methanolic extract of barley extracted the lowest inhibition (16.04 ± 3.11%). Tukey’s HSD test for multiple comparisons found that the extract prepared with methanol exerted significantly lower activity compared to aqueous extract in case of barley (p = 0.011, 95% C.I. [–15.47, –1.95]), while the difference in case of wheat methanolic and aqueous extracts was not significant. The methanolic extract of barley was significantly weaker compared to methanolic extract of wheat (p < 0.001, 95% C.I. [14.21, 27.73]) and even to aqueous extract of wheat (p < 0.001, 95% C.I. 7.56, 21.08]).
Discussion
There is a popular belief that barley grass could be a good source of food with beneficial effects and even it might be considered as a functional food (Ajmera 2020). Several preclinical studies have been carried out to explore the potential pharmacological effects of this plant, and these investigations have reported promising results. However, it is important to note that no clinical trials have been conducted to validate these hypotheses in humans. Furthermore, the results of phytochemical and preclinical investigations are not consistent.
One of the main focusses of barley pharmacological studies is the anticancer activity. There is an accepted fact that free oxygen radicals have damaging effect on DNA, potentially leading to the development of cancer (Valko et al. 2004). Compounds with the ability to interact free radicals may potentially mitigate the risk of cancer development. These compounds are generally considered as antioxidants. The investigation of the antioxidant activity of barley grass has been conducted by several research groups. Shrivastava et al. have reported that the aqueous extract of barley grass has antioxidant activity properties that are comparable to those of vitamin C (Shrivastava et al. 2022). The study conducted by Yan et al. examined the scavenging effect of water-soluble polysaccharides on the DPPH radical, as well as their impact on the TEAC (trolox equivalent antioxidant capacity) assay and FRAP (ferric reducing antioxidant power) assay. Polysaccharide isolation has been conducted at different stages of plant development. The antioxidant capacity of barley polysaccharides has been found to be significantly weaker antioxidants compared to vitamin C. According to Yan et al., the fraction BGP-Z12 has had the highest antioxidant activity, as determined by TEAC, with a value of 30 µmol trolox equivalent per gramme (Yan et al. 2022). The study reported by Thatiparthi et al. presents a comparison between the freeze-dried aqueous extract of barley with flavonoid rutin. According to Thatiparthi et al., the extract’s antioxidant activity, as determined by the DPPH assay and expressed as the IC50 value (358.0 ± 46.8 µg/mL), has been shown to be 40% less active compared to rutin (208.3 ± 10.52 µg/mL) (Thatiparthi et al. 2019).
The evaluation of antioxidant activity in our experiment was carried out using ORAC and DPPH assays. Furthermore, the polarity of the most potent antioxidants was estimated by means of bioautography. The findings of our study indicate a limited level of antioxidant activity in barley and wheatgrass, respectively. The ORAC assay results indicated that the barley extracts exhibited a relatively modest level of antioxidant activity, measuring less than 100 µmol TE/g, which is 16% of rutin and 12.6% of quercetin (Fig. 3b). Our results align with the conclusions documented by Thatiparthi et al. (2019) about the barley polysaccharides. In addition, the bioautography demonstrated that polar molecules were compounds with greater ability to react with DPPH (Fig. 1e, f). Shrivastava et al. (2022) have observed same range of activity for barley extracts just as ascorbic acid; however, in our experiment ascorbic acid was significantly superior to methanolic and aqueous extracts, respectively. Although the aqueous extract was significantly higher than the methanolic, it was still 70-fold weaker than the ascorbic acid. Numerous studies reporting good antioxidant activity for barley grass; interestingly, Woo et al. (2017) have reported findings that are inconsistent with the prevailing evidence: barley grass extract has caused elevation of intracellular reactive oxygen species.
The antiproliferative activity of barley has been investigated in several in vitro experiments. According to Xu et al. (2022), the water-soluble polysaccharide of the hulless barley grass powder has inhibited proliferation of colon cancer (i.e. HT29, Caco-2, CT26.WT) and breast cancer (4T1) cell lines in vitro in dose dependent manner. The study conducted by Kawka et al. examined the potential colon antitumor activity of commercially available ground barley grass meal (YGB INT) and powdered juice from young barley leaves (YGB GW). The efficacy of aqueous extracts of YGB INT and YGB GW food products has been tested on human colon adenocarcinoma cell lines (LS180 and HT29) as well as on human colon epithelial cell line (CCD841 CoN). The assessment of cytotoxicity has been evaluated using LDH, MTT, and BrdU assays. Both extracts have decreased the proliferation of the cancer cells. The calculated IC50 values obtained from the MTT assay ranged from 62 to 2,100 μg/mL. The findings obtained from the BrdU assay have suggested inhibition of DNA synthesis as a possible mechanism of antiproliferation. In contrast, both YGB INT and YGB GW have not demonstrated any impact on the morphology and growth pattern of a healthy human colon epithelial cell line. This observation may imply that barley grass extracts possess selectivity and safety properties (Kawka et al. 2019).
In addition to its antioxidant and anticancer activities, barley grass has been studied in further pharmacological assays. In a study conducted by Shrivastava et al., it has been shown that the administration of aqueous extracts of barley and wheat at a dose of 400 mg/kg resulted in the reduction of stress level in mice. In the forced swim test, both extracts have been similar to the positive control (i.e. 100 mg/kg imipramine). However, in the tail suspension test, the immobility time has been even higher in groups treated with extracts compared to imipramine, but still significantly lower compared to negative control (Shrivastava et al. 2022). Thatiparthi et al. have examined the antiobesity effect and antihyperlipidaemic effects of freeze-dried barley grass juice. The 60-day treatment resulted in significant decrease in body weight and BMI, as well as improvements in lipid profile and liver function markers (AST, ALT, ALP) in male Wistar rats compared to group on high fat diet (Thatiparthi et al. 2019).
The findings of our study on the inhibition of xanthine oxidase have expanded the potential pharmacological benefits associated with barley. Xanthine oxidase inhibitors play a crucial role in the prevention of gout development in those who are prone to accumulate urate in joints. The inhibitory effects of wheatgrass and barley grass extracts were found to be mild compared to allopurinol. It is suggested that the aqueous extract of barley might have a beneficial contribution in the prevention of gout.
To assess the pharmacological advantages of an extract, it is crucial to have knowledge on the phytochemical characteristics of those extracts. Most of the reporting pharmacological activities also deal with phytochemistry of barley. In the experiment conducted by Shrivastava et al., the plants have been harvested nine days after germination. Aqueous extraction has resulted in 12.79% yield for barley grass and 14.57% yield for wheatgrass. The extraction in our experiment yielded slightly diminished quantities for both plants. The DER was 11:1 for barley resulting a yield of 9.09% during water extraction. In contrast, wheatgrass demonstrates a DER of 12:1, yielding 8.33% during the same extraction process method. The assessment of chemical characterisation has involved the utilisation of total phenolic content and total flavonoid content. Our results were presenting significantly lower flavonoid content expressed in quercetin equivalent (133.14 mg QE/g vs. 12.42 mg QE/g for wheat aqueous extract, and 153.42 mg QE/g vs. 11.88 mg QE mg/g for barley extract). Particularly, the total phenolic contents of our extracts were approximately 10% of those values reported by Shrivastava et al. (2022). It is important to highlight that Shrivastava et al. employed Soxhlet extraction at temperatures ranging from 80 to 90 °C in order to obtain the aqueous extract. In contrast, in our experiment the extraction was performed at room temperature. The difference in the extraction procedure might contribute to the deviation in flavonoid content and antioxidant capacity, as well. The barley grass was harvested by Thatiparthi et al. on day 15 after germination. The extraction method used and the resulted in yield of 7.92% were comparable to our own findings. Significantly higher content has been reported for total phenolic compounds (225.33 ± 1.67 mg GA equivalent /g of extract), and flavonoids (203 ± 1.03 mg QE/g of extract) for extracts have been reported compared to our measurements (Thatiparthi et al. 2019). The study conducted by Wangcharoen et al. involved the evaluation of chlorophyll and total phenolic contents, antioxidant properties, and consumer acceptance of processed grass drinks. The analysis incorporated wheat, barley, and rice in its examination. Wheat and barley were harvested on seventh day, while rice on 20th day after germination. The total chlorophyll content and total phenolic content of wheat and barley juices, tested in 200 mL, have been reported as follows: wheat—chlorophyll content of 90.2 90 ± 2 µg and phenolic content 5.60 ± 0.44 mg GA equivalent; barley—chlorophyll content of 958 ± 4 µg and total phenolic content of 26.14 ± 0.52 mg GA equivalent. No clear method for obtaining juice has been reported (Wangcharoen and Phimphilai 2016). Yan et al. have pointed on very important fact that growth stage plays crucial role in chemical composition and the exerted activity of the extract. The extraction of polysaccharides from barley samples obtained at three different development stages (i.e. seedling stage, tillering stage, steam elongation stage). Barley grass extract from seedling stage has demonstrated the most potent antioxidant capacity measured by FRAP and DPPH assays (Yan et al. 2022). Not only the harvest timing, but the food processing method affects the chemical and antioxidant characteristics. Zhou et al. (2021) have established correlation between the particle size of barley grass powder and both the total flavonoid content and the organoleptic qualities of the product.
The findings reported by literature and our own research indicate that the timing of harvest and the method of extraction may play a critical role in determining the pharmacological efficacy. Early harvesting might be in favour of higher secondary metabolite content, resulting in notable increase in antioxidant activity. Delaying the harvesting time results in more mature barley plants with higher cellulose content, thus lower DER value and less rich in secondary metabolites. The selection of an appropriate solvent might cause deviation in the content and effect of extracts. Barley grass products must be prepared using solvents that can be applied in food industry. There is limited information about effectivity of ethanol, dichloromethane, and water barley grass extraction. Conditions (e.g. temperature, time of extraction) have not been discussed as factors to achieve more beneficial pharmacological effects of barley grass extract.
Conclusions for future biology
The potential for utilising barley grass juice as means to preserve and improve health appears to be a promising prospect. Multiple preclinical studies have indicated that barley juice and its derivatives have antioxidant properties, potentially leading to a decreased susceptibility to cancer. Additional pharmacological effects have been examined through in vitro and in vivo studies. However, the reported data about antioxidant activity and the effect on free oxygen radicals are diverse and there is a lack of clinical trials to support the health-promoting effects of barley.
The findings of our study indicate low antioxidant activity which implies that the primary contributors to the scavenging of free radicals in barley and wheatgrass extracts are the polar compounds. This study presents the initial documentation of a moderate inhibitory effect on xanthine oxidase by extracts of barley and wheatgrass. However, our results might suggest future studies about the timing of harvest and extraction method that are crucial factors in chemical composition and the pharmacological assay outcome. Future studies that thoroughly describe the chemistry of the extract might provide comparable and pharmacological results ready for systematic review.
References
Ajmera R (2020) What is barley grass? Everything you need to know. healthline. Available from: https://www.healthline.com/nutrition/barley-grass (June 29, 2023)
EDQM (2022) Tannins in herbal drugs 01/2008:20814. In: European Pharmacopoeia 11.0. EDQM Council of Europe, Strasbourg, 326
Fatemi F, Kianersi F, Pour-Aboughadareh A, Poczai P, Jadidi O (2022) Overview of identified genomic regions associated with various agronomic and physiological traits in barley under abiotic stresses. Appl Sci 12:5189. https://doi.org/10.3390/app12105189
Glatthaar BE, Hornig DH, Moser U (1986) The role of ascorbic acid in carcinogenesis. In: Poirier LA, Newberne PM, Pariza MW (eds) Essential nutrients in carcinogenesis. Springer, pp 357–377. https://doi.org/10.1007/978-1-4613-1835-4_27
Kawka K, Lemieszek M, Rzeski W (2019) Chemopreventive properties of young green barley extracts in in vitro model of colon cancer. Ann Agric Environ Med 26:174–181. https://doi.org/10.26444/aaem/102624
Kim M-J, Hyun J-N, Kim J-A, Park J-C, Kim M-Y, Kim J-G, Lee S-J, Chun S-C, Chung I-M (2007) Relationship between phenolic compounds, anthocyanins content and antioxidant activity in colored barley germplasm. J Agric Food Chem 55:4802–4809. https://doi.org/10.1021/jf0701943
Lin J-K, Lin C-L, Liang Y-C, Lin-Shiau S-Y, Juan I-M (1998) Survey of catechins, gallic acid, and methylxanthines in green, oolong, pu-erh, and black teas. J Agric Food Chem 46:3635–3642. https://doi.org/10.1021/jf980223x
Lobo V, Patil A, Phatak A, Chandra N (2010) Free radicals, antioxidants and functional foods: impact on human health. Pharmacogn Rev 4:118. https://doi.org/10.4103/0973-7847.70902
Macháň P, Ehrenbergerová J, Cerkal R (2014) Barley grain non-starch polysaccharides with malting and nutritional significance. Kvas Prum 60:258–265. https://doi.org/10.18832/kp2014025
Nemes A, Stefanovitsné Bányi É, Remenyik J (2015) Development of a new measurement method to determine plant antioxidant status. Acta Agrar Debr 17:105–112. https://doi.org/10.34101/actaagrar/63/1844
Ohtake H, Nonaka S, Sawada Y, Hagiwara Y, Hagiwara H, Kubota K (1985) Studies on the constituents of green juice from young barley leaves. Effect on dietarily induced hypercholesterolemia in rats. Yakugaku Zasshi 105:1052–1057. https://doi.org/10.1248/yakushi1947.105.11_1052
Panthi M, Subba RK, Raut B, Khanal DP, Koirala N (2020) Bioactivity evaluations of leaf extract fractions from young barley grass and correlation with their phytochemical profiles. BMC Complement Med Ther 20:64. https://doi.org/10.1186/s12906-020-2862-4
Shrivastava AK, Magar PT, Shrestha L (2022) Effect of aqueous extract of barley and wheat grass in stress induced depression in Swiss mice. J Ayurveda Integr Med 13:100630. https://doi.org/10.1016/j.jaim.2022.100630
Szent-Györgyi A (1988) Válogatott tanulmányok. Gondolat, Budapest
Thatiparthi J, Dodoala S, Koganti B, Kvsrg P (2019) Barley grass juice (Hordeum vulgare L.) inhibits obesity and improves lipid profile in high fat diet-induced rat model. J Ethnopharmacol 238:111843. https://doi.org/10.1016/j.jep.2019.111843
Valko M, Izakovic M, Mazur M, Rhodes CJ, Telser J (2004) Role of oxygen radicals in DNA damage and cancer incidence. Mol Cell Biochem 266:37–56. https://doi.org/10.1023/B:MCBI.0000049134.69131.89
Wang J, Yue Y-D, Tang F, Sun J (2012) TLC screening for antioxidant activity of extracts from fifteen bamboo species and identification of antioxidant flavone glycosides from leaves of Bambusa textilis McClure. Molecules 17:12297–12311. https://doi.org/10.3390/molecules171012297
Wangcharoen W, Phimphilai S (2016) Chlorophyll and total phenolic contents, antioxidant activities and consumer acceptance test of processed grass drinks. J Food Sci Technol 53:4135–4140. https://doi.org/10.1007/s13197-016-2380-z
Woo SM, Kwon S-C, Ko SG, Cho S-G (2017) Barley grass extract causes apoptosis of cancer cells by increasing intracellular reactive oxygen species production. Biomed Rep 6:681–685. https://doi.org/10.3892/br.2017.897
Xu Y, Zhang C, Qi M, Huang W, Sui Z, Corke H (2022) Chemical characterization and in vitro anti-cancer activities of a hot water soluble polysaccharide from hulless barley grass. Foods 11:677. https://doi.org/10.3390/foods11050677
Yamaura K, Shimada M, Fukata H, Nakayama N, Bi Y, Ueno K (2012) Antidepressant-like effects of young green barley leaf (Hordeum vulgare L.) in the mouse forced swimming test. Pharmacogn Res 4:22. https://doi.org/10.4103/0974-8490.91030
Yan J-K, Chen T-T, Wang Z-W, Wang C, Liu C, Li L (2022) Comparison of physicochemical characteristics and biological activities of polysaccharides from barley (Hordeum vulgare L.) grass at different growth stages. Food Chemistry 389:133083. https://doi.org/10.1016/j.foodchem.2022.133083
Yu Y-M, Chang W-C, Chang C-T, Hsieh C-L, Tsai CE (2002) Effects of young barley leaf extract and antioxidative vitamins on LDL oxidation and free radical scavenging activities in type 2 diabetes. Diabetes Metab 28:107–114
Zhou W, Cao X, Islam MdN, Zheng H, Li J, Liu F, Cao Y, Dai Y (2021) Comparison of hydrability, antioxidants, microstructure, and sensory quality of barley grass powder using ultra-micro-crushing combined with hot air and freeze drying. Food Sci Nutr 9:1870–1880. https://doi.org/10.1002/fsn3.2138
Funding
Open access funding provided by University of Szeged. This research was supported by the Ministry of Innovation and Technology of Hungary (grant number TKP2021-EGA-32).
Author information
Authors and Affiliations
Contributions
Conceptualization was prepared by FL and TK. Methodology was performed by VV, IG, PP, FL, ÁB, and TK. Formal analysis was done by VV, IG, PP, and ÁB. Investigation was done by VV, TK, IG, and FL. Writing—original draft was performed by VV, TK, and FL. Writing—review and editing was done by FL, PP, IG, and ÁB. Visualisation was performed by PP, IG, and ÁB. Supervision was performed by LF and TK. Project administration was done by VV, TK, and LF.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lantos, F., Váczi, V., Gyalai, I. et al. Investigation of in vitro biological activity of young Hordeum vulgare leaf in correlation with its bioactive compounds. BIOLOGIA FUTURA (2024). https://doi.org/10.1007/s42977-024-00227-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42977-024-00227-1