Abstract
Wheat grains were collected from various locations in six governorates of Egypt and subjected to isolation trials. The isolated fungi were identified as Aspergillus spp. followed by Alternaria spp., Fusarium spp., Rhizopus spp. and other unidentified fungi, in that respective order. All isolated A. flavus strains (16 isolates) were screened for their ability to produce aflatoxins (AFs) on synthetic medium under long (365 nm wavelength) UV irradiation to determine their mycotoxin production capabilities. Data revealed that seven toxigenic isolates of A. flavus exhibited high fluorescent color. A preliminary test using TLC technique detected high production of aflatoxins by A. flavus isolates 2, 3, 4 and 6. The concentrations of AFs produced by positive A. flavus strains were determined using high-performance liquid chromatography (HPLC), to confirm high production of AFs by the 4 isolates. The A. flavus strain with the highest AFs production was selected for further studies. In laboratory conditions, the inhibitory effects of various organic acids, salts and essential oils were evaluated against the linear growth of A. flavus. Results indicated complete inhibition (100%) of fungal growth at 1.0% concentrations of malic acid, potassium sorbate, thyme and carnation oils. The production of aflatoxins in stored wheat grains treated with different concentrations of essential oils, organic acids and salts was studied over 45-day period. Untreated stored wheat grains showed high concentrations of AFG1, AFG2, AFB1 and AFB2 produced by A. flavus compared to grains treated with essential oils (thyme and carnation), malic acid and potassium sorbate. It was observed that all types of produced AFs gradually decreased with increasing concentrations of oil, acid or salt reaching their minimum levels at the highest concentration used (8%). The data demonstrated that the lowest aflatoxin production was recorded in grains treated with 8% potassium sorbate.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Among cereal crops, wheat (Triticum aestivum L.) is considered one of the most important worldwide and is the primary strategic food crop in Egypt. Throughout its growth stages and storage periods, wheat plants are susceptible to various diseases resulting in a decrease in both quantity and quality of the yield and stored grains. Post-harvest losses of cereal grains can be measured quantitatively through the loss of nutritional or processing quality. These losses also have economic implications such as a decrease in value or restricted access to certain markets. One of the significant risks associated with cereal consumption is the presence of mycotoxins, heavy metals, pesticide residues and alkaloids. In the USA, mycotoxin contamination alone has been estimated to cause losses of $932 million in stored grains (Richard and Payne 2003). Various fungi that colonize cereals and their products in the field or during post-harvest can contaminate grains with mycotoxins (Rasmussen et al. 2010; Cheli et al. 2013). Maize, wheat and peanuts are particularly susceptible to mycotoxin-associated plant diseases during storage. To control the risk of mycotoxins in wheat, it is essential to consider multiple stages, including pre-harvest, post-harvest and storage processes. Certain fungal species, like Aspergillus, Fusarium, Penicillium and Alternaria are well known to be plant-pathogenic and able to produce mycotoxins (Placinta et al. 1999). For example, Aspergillus flavus infects grain crops in the field and can contaminate grains during storage under suitable temperature and humidity conditions (Campbell, & White 1995). Aflatoxins produced by toxigenic Aspergillus fungi such as A. parasiticus and A. flavus pose a significant problem for healthy cereals and can affect various food and feed crops including wheat, maize, peanuts, rice, cotton seeds and spices (Madbouly et al. 2012; El-Nagerabi et al. 2012). There are natural and synthetic agents that can help mitigate the toxic effects of mycotoxins such as antioxidants like selenium, vitamins, phenolic compounds, chlorophyll, carbohydrates, medicinal herbs and their extracts, essential oils and organic and inorganic mineral and biological agents (Farombi 2006). Additionally, Nguefack et al. (2004) reported that the carcinogenic effects of mycotoxin exposure can be chemically delayed or blocked. Currently, there are several approaches to reduce mycotoxin contamination in seeds and grains. These methods involve inhibiting fungal growth and subsequently preventing toxin production to eliminate aflatoxins from contaminated grains. Essential oils which contain components that suppress plant growth and act as antimicrobial agents have shown promise in controlling toxin-producing phytopathogens (Soliman and Badeaa 2002; Tepe et al. 2005). Common preservatives such as carnation and lemongrass oils (Smith-Palmer et al. 2001), organic acids (El Mougy et al. 2008 Abdel-Kader et al. 2022) and organic salts (Merck 2015) are also used as antifungal agents. The main goal of the present study is to evaluate the effectiveness of certain chemicals including carnation, lemongrass, thyme, malic acid and potassium sorbate in inhibiting aflatoxin production in artificially infested wheat grains stored for up to 45 days.
Materials and Methods
Isolation and identification of various fungi associated with apparently healthy and infected wheat grains
Wheat grain samples of the c.v. Gemiza-11 were collected after harvest from local markets in Alexandria, Beheira, Qualubia Sharkia, Giza and Bani-Seuif governorates of Egypt. To isolate the associated fungi, the grains were surface disinfected by submersing them in a 5% sodium hypochlorite solution for 2 min, rinsing them three times with sterile distilled water, and then allowing them to air dry on sterilized filter paper at room temperature. Five grains were aseptically transferred to PDA plates (9 cm diameter) and incubated for 5–7 days at 25 ± 2 °C. Twenty replicated plates were used for each sample. Purification of the growing fungi was done using the single spore technique medium. Purified fungi were stored on PDA slants at 5 °C for upcoming tests. Identification of the isolated fungal strains was based on cultural and microscopic characteristics using the taxonomic key described by Klich (2002) and Samson, et al., (2004).
Toxicological analysis of A. flavus isolates
Sixteen fungal isolates of A. flavus were preliminarily examined for their capability to build up Aflatoxins (AFs). Coconut agar medium (CAM), which contains homogenized 100 g of sliced coconut for 5 min with the addition of 300 ml of hot water, at pH 7.0 (Davis, et al. 1987) was used for this test. Growth disks of the sixteen isolates of A. flavus were placed individually in the center of Petri dishes containing autoclaved CAM and then incubated for 7 days at 28 ± 2 °C. They were inspected under a UV lamp in a dark room to check the fluorescence color, which indicates the existence of aflatoxin. If fluorescence color appeared, it could be confirmed as positive aflatoxin production by the tested fungus and further confirmation as an aflatoxigenic fungus would be needed.
Thin-layer chromatography (TLC) assay
Furthermore, the thin-layer chromatography (TLC) method after Filtenborg et al., (1983) for detecting the presence of aflatoxins in fungal growth culture was followed. This method was used for testing the seven isolates of Aspergillus flavus which showed a fluorescent color under long-wave UV light. The Petri plates containing yeast extract sucrose agar medium (YES) and inoculated with Aspergillus isolates were incubated for 7 days at 28 °C and then examined for aflatoxins production following the method described by Samson et al. (2002) and Hans and Walter (1986). Fungal growth disks (5 mm diameter) were cut and immersed separately in 2 mL of chloroform for extraction of toxin. Ten microliters of the obtained extracts was spotted on TLC plates. Thereafter, 10 μL of a mycotoxin standard solution of aflatoxins B1 and G1 (Sigma-Aldrich, Dorset, UK) was used as reference standards and spotted along with the fungal sample extracts. The TLC plate was placed I vertically in a jar containing the solvent system of chloroform/toluene/acetone (75:15:10) and stand for 1 h and then examined under long-wave UV light (365 nm). Then, the running sample extracts were compared with the used reference standards spot.
Inspection and detecting of aflatoxins produced by different isolates of A. flavus
The correlation between fluorescence and aflatoxin production was defined for the four strains of A. flavus (Nos. 3, 4, 5 and 6). The fungal strains were grown on YES liquid medium (Abdollahi and Buchanan 1981). Preparation of spore suspensions at concentration of 106 spores /ml using a hemocytometer was separately carried out for tested isolates. Certain volumes (fifty mL) of sterile YES medium were inoculated individually with one ml of each spore suspension and incubated at 25 ± 20C for 14 days. The entire cultures were filtered using filter paper No.3, and the obtained filtrates were extracted by separating funnel by adding chloroform (50 ml × 3) after Fente et al., (2001). The determination of aflatoxins was done in the resulted extraction according to AOAC (2016) methods using HPLC.
Efficacy of plant resistance inducers against the growth of mycotoxin producer fungi A. flavus in vitro
Aspergillus flavus fungi isolate (No. 4) which produced the highest mycotoxins was used in this study. The inhibitory effect of resistance inducers, organic acids (humic, malic acids), organic salts (potassium sorbate, sodium benzoate) as well as thyme and carnation essential oils was evaluated against the linear growth and its reduction of fungal isolates in vitro. The tested organic acids, salts and essential oils were used at a concentration of 0.5%, 1.0% and 2.0%. All the used chemicals were purchased from Sigma-Aldrich. Certain weights or volumes were added individually to flasks containing 100 ml sterilized PDA medium to reach the suggested concentrations and then rotated gently to ensure the equal distribution of added materials. About 20 ml of prepared supplemented medium was poured in sterilized Petri plates (9 cm diameter). Plates were separately inoculated at the center with disks (5 mm) of tested fungal isolate. Separate PDA plates free of salts, acids or essential oils were used as control treatment. All plates were incubated at 25 ± 20C until the tested fungi reach full growth in check treatment. The mean of the 2 perpendicular fungal growth diameters was measured. Then, the percentage of fungal growth reduction was calculated as the relatively of fungal growth diameter in treatment to the fungal growth diameter in check control.
Determination of aflatoxins production in stored wheat grains
The production of aflatoxins in wheat grains stored for up to 45 days and treated with different concentrations of essential oils, organic acid and salt was evaluated using the HPLC method. Essential oils of carnation and thyme were bought from Cairo Company for Oils and Aromatic Extractions CID in Egypt. The samples of essential oils were maintained in black glass vials at 4 °C until use. High concentrations of the tested essential oils were emulsified by solving them in deionized water (dH2O) and adding a small drops of the emulsifier Tween 80. Other tested chemical substances from Sigma-Aldrich, such as malic acid and potassium sorbate, were also purchased. Serial dilutions of the testing solutions were prepared at concentrations of 4.0%, 6.0% and 8.0% for carnation, thyme oils, malic acid and potassium sorbate, before treating the wheat grains. One hundred grams of wheat grains were placed into conical flasks (500 ml) with 10 ml of dH2O and autoclaved. The proposed concentrations of the tested agents were added to each flask containing wheat grains individually and shaken manually for a few minutes. The next day, the treated wheat grains were inoculated with 2 mL containing 106 spores/ml of A. flavus strain and shaken well again. All inoculated treated and untreated wheat grains were stored at 28 ± 2ºC for 45 days. Then, the wheat grains were autoclaved and tested for aflatoxin production according to the CB methods described by AOAC, (2016).
Aflatoxins detecting by high-performance liquid chromatography (HPLC)
The final extracts were derivatized by adding 200 µl of hexane to the residue followed by a volume of 50 µl of trifluoroacetic acid (TFA) and mixing for 30 s using a vortex. The obtained mixture was then left to be constant for 5 min. Additionally, admixture of 1.95 ml of H2O/CH3CN (9:1 v/v) was added and mixed well for 30 s using a vortex and left to stand for 10 min for layer separation (AOAC 2016). Samples from each treatment were analyzed for AFs by HPLC using an Agilent 1260 series. The ZORBAX Eclipse Plus C18 column (4.6 × 250 mm, 5 μm) was used for the analysis. The solvent system consisted of 6:3:1 water/methanol/acetonitrile with a flowing rate of 1 mL/min. The injection volume was 20 μl. The FLD was adapted with an excitation/release wavelength of 360/450 nm. The temperature degree of the column was kept at 40 °C. The concentrations of aflatoxins were estimated from individual five-point standard curves (regression coefficient > 0.995 for all curves), using peak area for quantification. The limits of detection (LODs) were 0.01 and 0.05 mg/kg for AFB1 and AFG1, consequently, and the limits of quantification (LOQs) were 0.01 mg/kg for both AFB2 and AFG2.
Statistical analysis
The data were analyzed using SPSS software version 14.0. Analysis of variance was done, and the mean values were compared by Duncan’s multiple range test at P < 0.05.
Results
Isolation and identification of various fungi associated with apparently healthy and infected wheat grains
Isolation of associated fungi from collected wheat grain samples of c.v. Gemiza-11 was carried out under laboratory conditions. The data showed that the most frequently isolated fungi belonged to the genera Aspergillus followed by Alternaria, Fusarium, Rhizopus and other unidentified fungi, in respective order. The highest mean count was for Aspergillus flavus (16 isolates).
Toxicological analysis of A. flavus strains
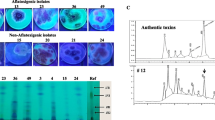
The ability of 16 isolates of A. flavus to produce aflatoxin(s) on synthetic medium (CAM) was primarily screened under long-wave UV irradiation (365 nm wavelength) to determine their mycotoxin-producing capability. Data presented in Table 1 reveal that seven isolates of A. flavus showed high fluorescent color and were considered toxigenic isolates. Not all tested isolates could produce fluorescent color under UV light. The remaining tested fungal isolates showed slight or non-fluorescent color and were neglected from further studies. Additionally, fungal diffusible metabolites may produce similar fluorescence under UV light, but fluorescence does not always indicate the presence of aflatoxins. Therefore, confirming the detection of aflatoxin(s) in culture filtrate broth should be done for the toxigenic isolates of A. flavus. Generally, producing strains of tested Aspergillus isolates showed variously colored fluorescent substances during their growth on standard CAM. The highest intensity of fluorescent color was visually recorded for fungi isolated from Alexandria, Beheira, Sharkia, Giza and Bani-Seuif governorates which corresponded to isolate numbers 1, 3, 5, 7, 9, 13 and 15 of A. flavus.
Thin-layer chromatography (TLC) assay
The fungal isolates of Aspergillus flavus (seven isolates) that produced the fluorescent color were used in this test. Data illustrated in Fig. 1 show the presence of aflatoxins referred in the standard used. Examination of aflatoxins (AFs) production of the tested 16 A. flavus isolates using ultraviolet light (UV) indicate that only four isolates (Nos. 3, 4, 5 and 6) which represent 25% of the total examined isolates were positive and the rest isolates were negative for producing aflatoxins. Detection by UV at 365 nm acknowledged aflatoxigenic fungal isolate by the presence of blue fluorescent colonies through the center of the Petri dish containing CAM medium.
Seven strains among sixteen isolates of A. flavus that showed fluorescence color were used to confirm the correlation between fluorescence color and aflatoxin production using HPLC. The data in Table 2 revealed that fungal isolated Nos. 2, 3, 4 and 6 produced a higher amount of AFs compared to the other tested isolates. Based on the capability to produce the highest amount and different types of aflatoxin(s) in cultural filtrate extract, the highly toxigenic A. flavus isolate No. 4 (Table 2) was chosen for further studies.
Efficacy of plant resistance inducers against the growth of mycotoxin producer fungi A. flavus in vitro
The inhibitor effect against the mycelial growth of tested A. flavus was noticed at all tested concentrations of the used chemicals (Table 3). The presented data in Table 3 showed that the fungal mycelial growth with humic acid treatment decreased significantly with the increase in concentrations to reach a minimum mycelial growth of 20.7 mm with the highest concentration used 2%. Malic acid revealed a higher effect on fungal growth with complete fungal growth inhibition recorded at a concentration of 1.0%. As for organic salts, potassium sorbate had superior effect, causing 100% growth inhibition at concentration of 1.0% compared to sodium benzoate which caused the same effect at a concentration of 2.0%. A high inhibitory effect with the two tested essential oils at a concentration of 1.0% and 100% growth suppression was observed.
Determination of aflatoxins production in stored wheat grains
The production of aflatoxins in stored wheat grains is detailed in Table 4. The data show that untreated wheat grains had higher levels of aflatoxins compared to treated grains. The impact of the various tested agents on AFG1, AFG2, AFB1 and AFB2 produced by A. flavus in stored wheat grains is presented in the table. It was noted that the reduction of AFB1 and AFB2 was more significant than that of AFG1 and AFG2 for all tested agents and concentrations. Additionally, all types of aflatoxins decreased with higher concentrations of the tested agents, reaching their lowest levels at 8% concentration. The applied agents (thyme and carnation oils, malic acid and potassium sorbate) had an effect on aflatoxin production of AFG1, AFG2, AFB1 and AFB2. Notably, a significant reduction was observed for AFB1 and AFB2 with all applied (Fig. 2). The levels of aflatoxin production for AFG1, AFG2, AFB1 and AFB2 in untreated grains were 23.54, 18.58, 12.22 and 10.72 ng/g in stored wheat grains for 45 days. Furthermore, in Table 1 data demonstrated that levels of AFG1, AFG2, AFB1 and AFB2 decreased within the ranges of 10.15 to 8.33, 13.93 to 0.00, 3.96 to 0.00 and 0.10 to 0.00 in treated wheat grains with 4%, 6% and 8% of thyme oil, carnation oil, malic acid and potassium sorbate after 45 days of storage, respectively. The results revealed a significant reduction in aflatoxin compared to untreated wheat grains (Fig. 2).
Discussion
In this study, the fungi isolated from wheat grain samples were identified as A. flavus with the help of taxonomical characteristic described by Klich (2002) and Samson et al. (2004). Similar findings have been reported by researchers worldwide. A recent study by Channa et al. (2017) found that collected wheat samples from different fields were naturally contaminated with Aspergillus spp. They noted that the wheat variety SKD-1 had a high contamination rate of 10% A. flavus; meanwhile, the Sonalika variety displayed high frequencies of mycotoxin-producing fungi with 25% A. flavus and 40% Aspergillus spp. All of these previous reports supported the results obtained in our study. Mycotoxins are a significant concern in grain storage due to different environmental factors such as moisture, temperature, storage duration, contamination levels as well as broken grains and impurities, insect infestations, oxygen levels, harvest and processing damages, and grain and seed transportation (Scudamore 2005). Aflatoxins, in particular, have significant attention due to their strong carcinogenic reactions in susceptible sensitive animals and toxicological effects in humans. Many researchers have reported that different Aspergillus spp. isolates can produce mycotoxins in feeds and food products (Koehler et al. 1985). Given the hazardous nature of these compounds, it was crucial to assess the ability of the Aspergillus isolates obtained in this study to produce aflatoxins.
In the current study, sixteen isolates were tested for aflatoxin using ultraviolet light (UV). The results showed that only four isolates were aflatoxigenic (positive), as indicated by the blue fluorescent color observed within the fungal colonies grown on CAM medium under UV light at 365 nm, confirming aflatoxin production. The A. flavus isolate, which produced the highest amount and various types of aflatoxins in cultural filtrate extract, was selected for further investigation. It is important to note that fungal metabolites may exhibit similar fluorescence under UV light, but this does not always indicate the presence of aflatoxins. Therefore, confirmation of aflatoxin detection in culture filtrates is necessary for toxigenic isolates of A. flavus. The total amount of aflatoxin produced in the A. flavus culture filtrates varied among isolates which can be attributed to environmental conditions affecting aflatoxin production, as reported by Jarvis (1971). Similarly, Austwick and Ayerst (1963) found that only a fraction of A. flavus isolates were capable of producing mycotoxins, while Diener and Davis (1966) observed varying degrees of aflatoxin production among isolates. Those findings align with these recorded in the current study. Our study also demonstrated significant inhibitory effect on the growth of tested fungal isolates when exposed to different concentrations of humic and malic organic acids under in vitro conditions. Previous research has shown the effectiveness of humic acid in controlling plant diseases. For example, Scheuerell and Mahaffee, (2006) found that a combination of compost tea, kelp extract and humic acid effectively suppressed gray mold in Geranium plants caused by Botrytis cinereain. Additionally, Charest et al. (2005) reported that compost bacteria impacted by humic matters could suppress the growth of Pythium ultimum in vitro, focusing the attention on the role of humic substances in enhancing the antagonistic microbes. It is also observed that the biocontrol effectivity against plant-pathogenic microbes as well as plant growth was improved through the biosolubilization of humic acid (Prakash et al. 2010).
Moreover, benzoic acid and its salt have been known to suppress the growth of various fungi. The preserving acid foods such as fruit juices and pharmaceutical preparations have been widely used attributed to their antifungal action. The efficacy of some organic acids (ascorbic, benzoic, citric and sorbic) and salts (potassium sorbate and sodium benzoate) was successfully evaluated against mold fungi Geotricum candidum, Penicillium digitatum and P. italicum on lemon fruits under in vitro and in vivo conditions (El-Mougy et al. 2008). Additionally, they found that these chemicals had an inhibitory effect on fungal growth and fruit rot incidence. Furthermore, in a study of Hassan et al. (2015) some organic acids such as propionic, acetic, formic, lactic, tartaric, citric, oxalic and malic acids were studied as antifungal agents on the growth of four fungi: Aspergillus flavus, Penicillium purpurogenum, Rhizopus nigricans and Fusarium oxysporum in vitro. They found that malic acid and oxalic acid at 5% had a weak inhibitory effect on the tested fungal growth, with rates of 5.31% and 6.45%, respectively.
Otherwise, essential oils are considered as promising alternative compounds that could suppress the growth of plant pathogens. They suggested their utilization for controlling plant disease as the principal antimicrobial compounds. There has been a substantial need for the finding of new natural antimicrobials (Sagdiç et al. 2003). Plant products with antimicrobial properties have particularly had attention as prospective applications to suppress microbial growth (Lanciotti et al. 2004). This leads to suggestion that essential oils could be used as alternative antibacterial and antifungal treatments (Jenny 2000). Additionally, Karapinar (1985) stated that some plant extracts and essential oils reveal antimicrobial properties. Also, studies on the efficacy of some essential oils to control the growth of producing mycotoxins as the main or as adjuvant antimicrobial compounds revealed that clove, cinnamon and oregano had the ability to prevent the growth of Aspergillus parasiticus and Fusarium moniliforme (Juglal et al. 2002). Similarly, in the present study, the commercial essential oils used contain constituents considered as active compounds against microorganisms, such as thymol, carvacrol, eugenol, flavonoids, phenolic compounds and others. Also, in the current investigation, the ability of the highest producer strain of A. flavus isolated from wheat grains to produce aflatoxins (AFs) AFG1, AFG2, AFB1 and AFB2 in stored wheat grains for 45 days was evaluated. Before storage, the wheat grains were treated with carnation oil, thyme oil, malic acid and potassium sorbate at different concentrations.
Environmental variations that cause a crop contamination often a result of atmospheric conditions and agricultural operations that stimulate crops to be attacked by aflatoxigenic fungi such as A. flavus (Nawar 2008). Additionally, air temperature and humidity in the field as well as unsuitable post-harvest processes, such as handling, marketing and storing, contribute to crop contamination. Over the past few years, many attempts have been made to develop modern antifungal agents to manage the increase of the fungus Aspergillus in grains used for human utilization (Soliman and Badeaa 2002; Sukatta et al. 2008; Abdel-Rahman et al. 2019). The most effective method for reducing mycotoxin level is to suppress the growth of mold and mycotoxin-forming using various methods. Carnation and thyme essential oils, malic acid and potassium sorbate have been found to reduce production of all types of AFs by the toxigenic fungus A. flavus. The lowest levels of AFs were achieved when their concentrations were increase up to 8% compared to the untreated control. In this concern, earlier reports have also shown that aromatic oils including lemongrass, citronella, clove, peppermint, thyme and oregano had antifungal activity against various pathogenic fungal species (Viuda-Martos et al. 2007; Leyva Salas et al. 2017). Additionally, natural products, such as cinnamon, clove and other essential oils, phenolic compounds and certain spices have been registered as active suppressants of fungal growth and aflatoxin formation (Hasan and Mahmoud 1993; Soliman and Badeaa 2002; Sukatta, et al. 2008, Pizzolitto et al. 2015, Youssef et al. 2016; Ibrahim et al. 2017).
Considering the vast diversity of chemical components included in aromatic oils, it is likely that their antifungal and antibacterial effectiveness is not attributed to one special mechanism, but rather to the presence of various pathways within the cell (Carson et al. 2002). The physical nature of aromatic essential oils, such as their low mass and strong lipophilic properties, allows them to penetrate the cell wall faster than other substances (Pawar and Thaker 2006). Conner and Beuchat, (1984a, b a, b); Chouhan et al. (2017) have stated that the antimicrobial effectiveness of essential oils or their derivatives such as thymol, carvacrol and vanillin may cause a damage to the cell system, including energy production and synthesis of structural components. Phenolics have also been found to alter the qualities of enzymes responsible for spore germination or be involved with the amino acids responsible for germination (Nychas 1995; Pillai and Ramaswamy 2012). Repairable damage to the cell membrane, cytomembrane and cellular organelles has been observed when Aspergillus parasiticus and A. flavus were exposed to various essential oils (Kupferwasser et al. 2003; Rasooli and Owlia 2005; Helal et al. 2007). Sharifi-Rad et al. (2017) have also noted that organic acids like salicylic acid have antifungal and antibacterial effects. They suggested that salicylic acid may prevent or delay the production of aflatoxin by reducing fungal growth for a few days. Hassan et al. (2015) examined the sensitivity of four pathogenic fungi: Aspergillus flavus, Penicillium purpurogenum, Rhizopus nigricans and Fusarium oxysporum to the production of toxins when exposed to various organic acids. These acids included propionic, acetic, formic, lactic, tartaric, citric, oxalic and malic acids. The researchers found that these acids have antifungal properties and inhibited AFs production by approximately 50%. Panahirad et al. (2014) also noted that organic salicylic acid could suppress the growth of A. flavus and the formation of mycotoxins, potentially serving as an alternative to chemical fungicidal agents. Additionally, potassium sorbate and calcium propionate were found to suppress both aflatoxin production and fungal growth (Bullerman, 1983, Shi-Jenq 1986; Mahjoub and Bullerman 1986; Al-Ashmawy and Ibrahim 2009). In a recent study by Abdel-Kader et al. (2021), it was discovered that storing maize grains with a concentration of 0.25% carnation oil reduced the production of AFB1 and AFB2 by 93% and 99%, respectively. Furthermore, lemongrass oil at concentrations ranging from 2 to 6% almost completely suppressed the production of the same aflatoxins by 99.12% to 99.98% and 99.98% to 99.99%, respectively. Additionally, storing maize grains treated with concentrations of 0.25% to 6% of potassium sorbate and salicylic acid proved to be remarkably effective in controlling aflatoxin during storage for up to 30 days compared to the control group.
Conclusion
The current findings suggest that essential oils, such as carnation oil and thyme oil along with malic acid and potassium sorbate, were effective in inhibiting aflatoxigenic fungi and the production of their toxins during storage. These substances, when used at concentrations between 4.0% and 8.0%, could be utilized to prevent the growth of aflatoxigenic fungi and, as a result, the production of their toxins during the storage of wheat grains.
Availability of data and materials
All created and/or analyzed data during the present study are attainable in the manuscript, and the corresponding author has no interception to the availability of data and materials.
References
Abdel-Kader MM, El-Mougy NS, Soliman KM, Abd Elfatah SI (2021) Suppression of Aflatoxin production in artificially infested maize grains with Aspergillus flavus during storage conditions. J Microbiol Biotechnol Food Sci 10(5):e2243
Abdel-Kader MM, Khail MSA, El-Mougy NS (2022) Efficacy of fungicide alternatives against late wilt disease of maize and their influence on plant morphogenesis and yield characters. Hellenic Plant Prot J 15:57–71. https://doi.org/10.2478/hppj-2022-0007
Abdel-Rahman GN, Sultan YY, Salem SH, Amer MM (2019) Identify the natural levels of mycotoxins in Egyptian roasted peanuts and the destructive effect of gamma radiation. J Microbiol Biotechnol Food Sci 8:1174–1177
Abdollahi A, Buchanan RL (1981) Regulationofaflatoxin biosynthesis: characterization of glucose as an apparent inducer of aflatoxin production. J Food Sci 46:143–146. https://doi.org/10.1111/j.1365-2621.1981.tb14549.x
Al-Ashmawy MA, Ibrahim JI (2009) Influence of potassium sorbate on the growth of yeasts and moulds in yogurt. Int J Dairy Technol 62(2):224–227. https://doi.org/10.1111/j.1471-0307.2009.00463.x
AOAC (2016). Official methods of analysis association of official analytical chemistry. 13th Ed., USA, 971 p. https://www.techstreet.com/standards/official-methods-of-analysis-of-aoac-international-20th-edition-2016?product_id=1937367
Austwick PK, Ayerst G (1963) Toxic products in groundnuts: groundnut microflora and toxicity. J Chem Ind 2:55–61
Bullerman LB (1983) Effects of potassium sorbate on growth and aflatoxin production by Aspergillus parasiticus and Aspergillus flavus. J Food Prot 46(11):940–942. https://doi.org/10.4315/0362-028X-46.11.940
Campbell KW, White DG (1995) Evaluation of corn genotypes for resistance to Aspergillus ear rot, kernel infection, and aflatoxin production. Plant Dis 79:1039–1045
Carson CF, Mee BJ, Riley TV (2002) Mechanism of action of Melaleuca alternifolia (tea tree) oil on Staphylococcus aureus determined by time-kill, lysis, leakage, and salt tolerance assays and electron microscopy. Antimicrob Agents Chemother 46(6):1914–1920. https://doi.org/10.1128/AAC.46.6.1914-1920.2002
Channa MJ, Ghanghro AB, Rahman AA Ur (2017) Screening and assessment of mycotoxin production among common wheat varieties of Sindh, Pakistan. World Appl Sci J 35(1):137–141
Charest MH, Beauchamp CJ, Antoun H (2005) Effects of the humic substances of deinking paper sludge on the antagonism between two compost bacteria and Pythium ultimum. FEMS Microbiol Ecol 52:219–227. https://doi.org/10.1016/j.femsec.2004.11.017
Cheli F, Pinotti L, Rossi L, Dell’ Orto V (2013) Effect of milling procedures on mycotoxin distribution in wheat fractions: A review. LWT Food Sci Technol 54:307–314. https://doi.org/10.1016/j.lwt.2013.05.040
Chouhan S, Sharma K, Guleria S (2017) Antimicrobial activity of some essential oils—present status and future perspectives. Medicines 4(3):58. https://doi.org/10.3390/medicines4030058
Conner DE, Beuchat LR (1984a) Effects of essential oils from plants on growth of food spoilage yeasts. J Food Sci 49(2):429–434. https://doi.org/10.1111/j.1365-2621.1984.tb12437.x
Conner DE, Beuchat LR (1984) Sensitivity of heat-stressed yeasts to essential oils of plants. Appl Environ Microbiol 47(2):229–233. https://doi.org/10.1128/aem.47.2.229-233.1984
Davis ND, Iyer SK, Diener UL (1987) Improved method of screening for aflatoxin with a coconut agar medium. Appl Environ Microbiol 53(7):1593–1595. https://doi.org/10.1128/aem.53.7.1593-1595.1987
Diener UL, Davis ND (1966) Aflatoxin production by isolates of Aspergillus flavus. Phytopathology 56:1391–1393
El-Mougy NS, El-Gamal NG, Abd-El-Kareem F (2008) Use of organic acids and salts to control postharvest diseases of lemon fruits in Egypt. Arch Phytopathology Plant Prot 41(7):467–476. https://doi.org/10.1080/03235400600813532
El-Nagerabi SA, Al-Bahry SN, Elshafie AE, Alhilali S (2012) Effect of Hibiscus sabdariffa extract and Nigella sativa oil on the growth and aflatoxin B1 production of Aspergillus flavus and Aspergillus parasiticus strains. Food Cont 25(1):59–63. https://doi.org/10.1016/j.foodcont.2011.09.033
Farombi O (2006) Aflatoxin contamination of foods in developing countries: implications for hepatocellular carcinoma and chemo-preventive strategies. Afr J Biotechnol 5(1):1–14
Fente CA, Ordaz JJ, Vazquez BI, Franco CM, Cepeda A (2001) New additive for culture media for rapid identification of aflatoxin-producing Aspergillus strains. Appl Environ Microbiol 67(10):4858–4862. https://doi.org/10.1128/AEM.67.10.4858-4862.2001
Filtenborg O, Frisvad JC, Svendsen JA (1983) Simple screening method for molds producing intracellular mycotoxins in pure cultures. Appl Environ Microbiol 45(2):581. https://doi.org/10.1128/aem.45.2.581-585.1983
Hans PV, Walter HP (1986) Determination of mycotoxins. Pure Appl Chem 58(2):315–326. https://doi.org/10.9790/2402-1007022026
Hasan HAH, Mahmoud AL (1993) Inhibitory effect of spice oils on lipase and mycotoxin production. Zentralbl Mikrobiol 148(8):543–548. https://doi.org/10.1016/S0232-4393(11)80218-0
Hassan R, El-Kadi S, Sand M (2015) Effect of some organic acids on some fungal growth and their toxins production. J Agric Chem Biotechnol 2(1):1–11
Helal GA, Sarhan MM, Abu Shahla ANK, Abou El-Khair EK (2007) Effects of Cymbopogon citratus L. essential oil on the growth, morphogenesis and aflatoxin production of Aspergillus flavus ML2-strain. J Basic Microbiol 47(1):5–15. https://doi.org/10.1002/jobm.200610137
Ibrahim F, Asghar MA, Iqbal J, Ahmed A, Khan AB (2017) Inhibitory effects of natural spices extracts on Aspergillus growth and aflatoxin synthesis. Aust J Crop Sci 11(12):1553
Jenny J (2000) Essential oils: a new idea for postharvest disease control. Good Fruit Veg Mag 11(3):50–54
Juglal S, Govinden R, Odhav B (2002) Spice oils for the control of co-occurring mycotoxin-producing fungi. J Food Prot 65(4):683–687. https://doi.org/10.4315/0362-028X-65.4.683
Karapinar M (1985) The effects of citrus oil and some Turkish spices on growth and aflatoxin production by Aspergillus parasiticus NRRL 2999. Int J Food Microbiol 12:239–245. https://doi.org/10.1016/0168-1605(85)90014-5
Klich MA (2002) Identification of common Aspergillus species. Centraalbureau voor schimmel cultures, Utrecht. https://www.scirp.org/%28S%28351jmbntvnsjt1aadkposzje%29%29/reference/referencespapers.aspx?referenceid=1444674
Koehler PE, Beuchat LR, Chinnan MS (1985) Influence of temperature and water activity on aflatoxin production by Aspergillus flavus in cowpea seeds and meals. J Food Prot 48:1040–1043. https://doi.org/10.4315/0362-028X-48.12.1040
Kupferwasser LI, Yeaman MR, Nast CC, Kupferwasser D, Xiong YQ, Palma M, Bayer AS (2003) Salicylic acid attenuates virulence in endovascular infections by targeting global regulatory pathways in Staphylococcus aureus. J Clinic Invest 112(2):222–233
Lanciotti R, Gianotti A, Patrignani N, Belleti N, Guerzoni ME, Gardini F (2004) Use of natural aroma compounds to improve shelf-life of minimally processed fruits. Trends Food Sci Technol 15:201–208. https://doi.org/10.1016/j.tifs.2003.10.004
Leyva Salas M, Mounier J, Valence F, Coton M, Thierry A, Coton E (2017) Antifungal microbial agents for food biopreservation—a review. Microorganisms 5(3):37. https://doi.org/10.3390/microorganisms5030037
Madbouly AK, Ibrahim MIM, Sehab AF, Abdel-Wahhab MA (2012) Co-occurrence of mycoflora, aflatoxins and fumonisins in maize and rice seeds from markets of different districts in Cairo, Egypt. Food Addit Contam Part B Surveill 5:112–120. https://doi.org/10.1080/19393210.2012.676078
Mahjoub A, Bullerman LB (1986) Effects of natamycin and potassium sorbate on growth and aflatoxin production in olives. Archives de l’Institut Pasteur de Tunis 63(4):513–525
Merck (2015) The Merk index online, Combridge, UK, Royal society of chemistry https://merckindex.rsc.org/
Nawar LS (2008) Prevention and control of fungi contaminated stored pistachio nuts imported to Saudi. Saudi J Bio Sci 15(1):105–112
Nguefack J, Leth V, Amvam Zollo PH, Mathur SB (2004) Evaluation of five essential oils from aromatic plants of Cameroon for controlling food spoilage and mycotoxin producing fungi. Int J Food Microbiol 94(3):329–334. https://doi.org/10.1016/j.ijfoodmicro.2004.02.017
Nychas GJE (1995) Natural antimicrobials from plants. In: New methods of food preservation (pp. 58–89). Springer, Boston, MA. https://doi.org/10.1007/978-1-4615-2105-1_4
Panahirad S, Zaare-Nahandi F, Mohammadi N, Alizadeh-Salteh S, Safaie N (2014) Effects of salicylic acid on Aspergillus flavus infection and aflatoxin B1 accumulation in pistachio (Pistacia vera L.) fruit. J Sci Food Agric 94(9):1758–1763. https://doi.org/10.1002/jsfa.6488
Pawar VC, Thaker VS (2006) In vitro efficacy of 75 essential oils against Aspergillus niger. Mycoses 49(4):316–323. https://doi.org/10.1111/j.1439-0507.2006.01241.x
Pillai P, Ramaswamy K (2012) Effect of naturally occurring antimicrobials and chemical preservatives on the growth of Aspergillus parasiticus. J Food Sci Technol 49(2):228–233. https://doi.org/10.1007/s13197-011-0275-6
Pizzolitto RP, Barberis CL, Dambolena JS, Herrera JM, Zunino MP, Magnoli CE, Dalcero AM (2015) Inhibitory effect of natural phenolic compounds on Aspergillus parasiticus growth. J Chem. https://doi.org/10.1155/2015/547925
Placinta CM, D’Mello JPF, Macdonald AMC (1999) A review of worldwide contamination of cereal grains and animal feed with Fusarium mycotoxins. Animal Feed Sci Technol 78:21–37. https://doi.org/10.1016/S0377-8401(98)00278-8
Prakash KRN, Niveditha N, Tejaswini KV (2010) Optimization of humic acid by Trichoderma viridi and it’s effect on sorghum plant. J Biopestic 3:155–157
Rasmussen RR, Binderup ML, Larsen TO, Rasmussen PH (2010) Mycotoxins in maize silage detection of toxins and toxicological aspects. Kgs. Lyngby, Denmark: Technical University of Denmark (DTU). https://core.ac.uk/download/pdf/13737013.pdf
Rasooli I, Owlia P (2005) Chemoprevention by thyme oils of Aspergillus parasiticus growth and aflatoxin production. Phytochemistry 66(24):2851–2856. https://doi.org/10.1016/j.phytochem.2005.09.029
Richard JL, Payne GA (2003) Mycotoxins: risks in plant, animal, and human systems. CAST Council of Agricultural Science and Technology, Ames, Iowa, USA, Task Force Report, ISBN 1‐887383‐22‐0, ISSN 0194‐4088, No. 139. 199 p. https://www.cast-science.org/publication/mycotoxins-risks-in-plant-animal-and-human-systems/
Sagdiç O, Karahan AG, Ozcan M, Ozcan G (2003) Effect of some spices extracts on bacterial inhibition. Food Sci Technol Int 9:353–359. https://doi.org/10.1177/1082013203038976
Samson R, Hoekstra E, Frisvad J, Filtenborg O (2002) Introduction to food borne fungi. 6th Edition, Cen-traalbureau voor Schimmelcultures, Utrecht, Netherlands. https://www.scirp.org/(S(i43dyn45teexjx455qlt3d2q))/reference/ReferencesPapers.aspx?ReferenceID=1444676
Samson RA, Hoekstra ES, Frisvad JC (2004) Introduction to food-and airborne fungi. 7 th Edition, Centraalbureau voor Schimmel cultures, Utrecht (CBS). https://www.scirp.org/(S(i43dyn45teexjx455qlt3d2q))/reference/ReferencesPapers.aspx?ReferenceID=1444676
Scheuerell SJ, Mahaffee WF (2006) Variability associated with suppression of gray mold (Botrytis cinerea) on geranium by foliar applications of nonaerated and aerated compost teas. Plant Dis 90:1201–1208. https://doi.org/10.1094/PD-90-1201
Scudamore KA (2005) Identifying mycotoxins is paramount in the fight against their spread. Word Grain 23:36–39
Sharifi-Rad J, Sureda A, Tenore GC, Daglia M, Sharifi-RAD M, Valussi M, Sharifi-Rad R (2017) Biological activities of essential oils: from plant chemoecology to traditional healing systems. Molecules 22(1):70. https://doi.org/10.3390/molecules22010070
Shi-Jenq L (1986) Effect of food preservatives on fungal interaction and aflatoxin production. Ph. D. University of Nebraska-Lincoln, ProQuest Dissertations Publishing, 8614459. https://digitalcommons.unl.edu/dissertations/AAI8614459/
Smith-Palmer A, Stewart J, Fyfe L (2001) The potential application of plant essential oils as natural food preservatives in soft cheese. Food Microbiol 18(4):463–470. https://doi.org/10.1006/fmic.2001.0415
Soliman KM, Badeaa RI (2002) Effect of oil extracted from some medicinal plants on different mycotoxigenic fungi. Food Chem Toxicol 40(11):1669–1675. https://doi.org/10.1016/S0278-6915(02)00120-5
Sukatta U, Haruthaithanasan V, Chantarapanont W, Dilokkunanant U, Suppakul P (2008) Antifungal activity of clove and cinnamon oil and their synergistic against postharvest decay fungi of grape in vitro. Kasetsart J (Nat Sci) 42:169–174
Tepe B, Daferera D, Sokmen A, Sokmen M, Polissiou M (2005) Antimicrobial and antioxidant activities of the essential oil and various extracts of Salvia tomentosa Miller (Lamiaceae). Food Chem 90(3):333–340. https://doi.org/10.1016/j.foodchem.2003.09.013
Viuda-Martos M, Ruiz-Navajas Y, Fernández-López J, Pérez-Álvarez JA (2007) Antifungal activities of thyme, clove and oregano essential oils. J Food Saf 27(1):91–101
Youssef MM, Pham Q, Achar PN, Sreenivasa MY (2016) Antifungal activity of essential oils on Aspergillus parasiticus isolated from peanuts. J Plant Prot Res 56(2):139–142. https://doi.org/10.1515/jppr-2016-0021
Acknowledgements
This investigation was supported by In-House Project No. 12050106 of National Research Centre, Egypt, entitled “Integrated Management of Diseases Affecting Wheat Crop during Vegetative Growth and production of Mycotoxins in Stored Grains.”
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). The authors declare that no funds, grants or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All the authors shared in the experimental design, hands-on laboratory work, wrote the obtained results, analyzed data, made discussions, wrote the manuscript, reviewed the manuscript and approved the final form of manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethics approval and consent to participate
The study was conducted on natural isolated fungi from wheat grains and certain essential oils, organic acid and salt that are available in the environment and the ethical approval is not demanded.
Consent for publication
Not applicable.
Additional information
Communicated by Maria Rosa Simon.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abdel-Kader, M.M., Ibrahim, M.I.M., Khalil, M.S.A. et al. Pre-storage treatments for suppressing of aflatoxins production in wheat grains. CEREAL RESEARCH COMMUNICATIONS (2024). https://doi.org/10.1007/s42976-024-00560-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42976-024-00560-0