Abstract
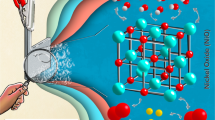
Compared with the traditional Haber Bosch process, green and pollution-free electrocatalytic nitrogen reduction (NRR) has received considerable attention in the electrocatalysis field in the last decade. To address the issue of its low reactivity as well as the existence of competitive reactions, efficient electrocatalysts are particularly important. In this paper, NiO nanomaterials were synthesized by a simple water bath reaction. The effect of different calcination temperatures on the structure of NiO catalyst and its catalytic activity was studied. Uniform NiO-600 nanoparticles (56 ± 9.3 nm) obtained at 600 ℃ showed the best electrocatalytic NRR activity with an NH3 yield of 12 μg h−1 mg−1 and a Faraday efficiency of 5.5% at -0.5V (vs.RHE). The small particle size of the nanoparticles provided more active sites and the oxygen-rich vacancies facilitated the adsorption and activation of N2, which improved the catalytic activity of NiO-600. This study highlights the need for calcination temperature regulation and the huge impact on catalyst structure.






Similar content being viewed by others
Data availability
Data are available upon reasonable request.
References
Wen J, Zuo LQ, Sun HD, Wu XW, Huang T, Liu ZC, Wang J, Liu LL, Wu YP, Liu X et al (2021) Nanomaterials for the electrochemical nitrogen reduction reaction under ambient conditions. Nanoscale Advances 3(19):5525–5541
Yang MQ, Wang MJ, Zhang MN, Sun X, Xuan XX (2023) Nanostructured carbon electrocatalysts for clean energy conversion and storage: a mini review on the structural impact. Frontiers in Materials 9:1131651
Foster SL, Bakovic SIP, Duda RD, Maheshwari S, Milton RD, Minteer SD, Janik MJ, Renner JN, Greenlee LF (2018) Catalysts for nitrogen reduction to ammonia. Nat Catal 1(7):490–500
Carreon ML (2019) Plasma catalytic ammonia synthesis: state of the art and future directions. J Phys D-Appl Phys 52(48):483001
Martin AJ, Shinagawa T, Perez-Ramirez J (2019) Electrocatalytic reduction of nitrogen: from haber-bosch to ammonia artificial leaf. Chem 5(2):263–283
Wang QR, Guo JP, Chen P (2019) Recent progress towards mild-condition ammonia synthesis. J Energy Chem 36:25–36
Wan Y, Xu J, Lv R (2019) Heterogeneous electrocatalysts design for nitrogen reduction reaction under ambient conditions. Mater Today 27:69–90
Shen P, Li X, Luo Y, Guo Y, Zhao X, Chu K (2022) High-efficiency N2 electroreduction enabled by Se-vacancy-rich WSe2–x in water-in-salt electrolytes. ACS Nano 16(5):7915–7925
Jiang JZ, Wang YJ, Wu J, Wang H, Zou YL, Zou J, Wang HT (2024) Charge transfer interfaces across black phosphorus/Co, N Co-doped carbon heterojunction for enhanced electrocatalytic water splitting. J Mater Sci Technol 178:171–178
Lee DK, Wee SJ, Jang KJ, Han MK, Surendran S, Cho SY, Kim JY, Lee SK, Sim U (2021) 3D-printed cobalt-rich tungsten carbide hierarchical electrode for efficient electrochemical ammonia production. J Korean Ceram Soc 58:679–687
Kim D, Surendran S, Janani G, Lim Y, Choi H, Han MK, Yuvaraj S, Kim TH, Kim JK, Sim U (2022) Nitrogen-impregnated carbon-coated TiO2 nanoparticles for N2 reduction to ammonia under ambient conditions. Mater Lett 314:131808
Yang ML, Jin ZX, Cao XX, Wang XM, Ma HY, Pang HJ, Yang GX (2023) Polyoxometalates-derived ternary metal oxides electrocatalyst for N2 reduction under ambient conditions. Tungsten. https://doi.org/10.1007/s42864-023-00206-4
Bin Shahid U, Chen YF, Gu S, Li WZ, Shao MH (2022) Electrochemical nitrogen reduction: an intriguing but challenging quest. Trends in Chemistry 4(2):142–156
Cao N, Zheng G (2018) Aqueous electrocatalytic N2 reduction under ambient conditions. Nano Res 11(6):2992–3008
Marinho AL, Comminges C, Habrioux A, Célérier S, Bion N, Morais C (2023) Reactivity of nitrogen atoms from ZiF-8 structure deposited over Ti3C2 MXene in the electrochemical nitrogen reduction reaction. Chem Commun 59(67):10133–10136
Jiang JZ, Xiong ZG, Wang HT, Liao GD, Bai SS, Zou J, Wu PX, Zhang P, Li X (2022) Sulfur-doped g-C3N4/g-C3N4 isotype step-scheme heterojunction for photocatalytic H2 evolution. J Mater Sci Technol 118:15–24
Zou J, Wu SL, Liu Y, Sun YJ, Cao Y, Hsu JP, Wee ATS, Jiang JZ (2018) An ultra-sensitive electrochemical sensor based on 2D g-C3N4/CuO nanocomposites for dopamine detection. Carbon 130:652–663
Yang XJ, Xu BJ, Chen JG, Yang X (2023) Recent progress in electrochemical nitrogen reduction on transition metal nitrides. Chemsuschem 16(5):e202201715
Yang M, Chen YY, Wang HT, Zou YL, Wu PX, Zou J, Jiang JZ (2022) Solvothermal preparation of CeO2 nanoparticles-graphene nanocomposites as an electrochemical sensor for sensitive detecting pentachlorophenol. Carbon Lett 32(5):1277–1285
Gou GC, Wei WJ, Yang JX, Liu JH, Liu Y, Li J, Shi K (2022) Effect of anion species on preparation and properties of pitch-based activated carbon fibers by in-situ catalytic activation of metal nanoparticles. Carbon Letters 32(6):1507–1518
Wang LJ, Yang TY, Feng B, Xu XY, Shen YY, Li ZH, Jiang JZ (2023) Constructing dual electron transfer channels to accelerate CO2 photoreduction guided by machine learning and first-principles calculation. Chin J Catal 54:265–277
Ma BY, Zhao HT, Li TS, Liu Q, Luo YS, Li CB, Lu SY, Asiri AM, Ma DW, Sun XP (2021) Iron-group electrocatalysts for ambient nitrogen reduction reaction in aqueous media. Nano Res 14(3):555–569
Zhang G, Li X, Chen K, Guo Y, Ma D, Chu K (2023) Tandem electrocatalytic nitrate reduction to ammonia on MBenes. Angew Chem Int Ed 62(13):e202300054
Tian Y, Chang B, Wang GH, Li LL, Gong LG, Wang B, Yuan RS, Zhou WJ (2022) Magnetron sputtering tuned “pi back-donation” sites over metal oxides for enhanced electrocatalytic nitrogen reduction. J Mater Chem A 10(6):2800–2806
Du CF, Yang L, Tang KW, Fang W, Zhao XY, Liang QH, Liu XH, Yu H, Qi WH, Yan QY (2021) Ni nanoparticles/V4C3Tx MXene heterostructures for electrocatalytic nitrogen fixation. Mater Chem Front 5(5):2338–2346
Bai F, Qu X, Li C, Liu S, Sun J, Chen X, Yang WS (2022) Nickel nanoflowers with controllable cation vacancy for enhanced electrochemical nitrogen reduction. ACS Appl Mater Interfaces 14(24):28033–28043
Wan YC, Zhou HJ, Zheng MY, Huang ZH, Kang FY, Li J, Lv RT (2021) Oxidation state modulation of bismuth for efficient electrocatalytic nitrogen reduction to ammonia. Adv Func Mater 31(30):2100300
AbdelHamid AA, Yu Y, Yang J, Ying JY (2017) Generalized synthesis of metal oxide nanosheets and their application as Li-ion battery anodes. Adv Mater 29(32):1701427
Kitano M, Kanbara S, Inoue Y, Kuganathan N, Sushko PV, Yokoyama T, Hara M, Hosono H (2015) Electride support boosts nitrogen dissociation over ruthenium catalyst and shifts the bottleneck in ammonia synthesis. Nat Commun 6:6731
Chen JG, Crooks RM, Seefeldt LC, Bren KL, Bullock RM, Darensbourg MY, Holland PL, Hoffman B, Janik MJ, Jones AK et al (2018) Beyond fossil fuel-driven nitrogen transformations. Science 360(6391):eaar6611
Nangan S, Ding Y, Alhakemy AZ, Liu Y, Wen Z (2021) Hybrid alkali-acid urea-nitrate fuel cell for degrading nitrogen-rich wastewater. Appl Catal B 286:119892
Gao JQ, Xue JB, Jia SF, Shen QQ, Zhang XC, Jia HS, Liu XG, Li Q, Wu YC (2021) Self-doping surface oxygen vacancy-induced lattice strains for enhancing visible light-driven photocatalytic H2 evolution over black TiO2. ACS Appl Mater Interfaces 13(16):18758–18771
Guo WH, Zhang KX, Liang ZB, Zou RQ, Xu Q (2019) Electrochemical nitrogen fixation and utilization: theories, advanced catalyst materials and system design. Chem Soc Rev 48(24):5658–5716
Yang X, Tian Y, Mukherjee S, Li K, Chen X, Lv J, Liang S, Yan LK, Wu G, Zang HY (2023) Constructing oxygen vacancies via engineering heterostructured Fe3C/Fe3O4 catalysts for electrochemical ammonia synthesis. Angew Chem Int Ed 62(34):e202304797
Yan ZF, Zuo ZJ, Lv XY, Li Z, Li Z, Huang W (2012) Adsorption of NO on MoO3 (010) surface with different location of terminal oxygen vacancy defects: A density functional theory study. Appl Surf Sci 258(7):3163–3167
Zhu KY, Shi F, Zhu XF, Yang WS (2020) The roles of oxygen vacancies in electrocatalytic oxygen evolution reaction. Nano Energy 73:104761
Chang B, Deng L, Wang S, Shi D, Ai Z, Jiang H, Shao Y, Zhang L, Shen J, Wu Y (2020) A vanadium-nickel oxynitride layer for enhanced electrocatalytic nitrogen fixation in neutral media. J Mater Chem A 8(1):91–96
Guo W, Liang Z, Zhao J, Zhu B, Cai K, Zou R, Xu Q (2018) Hierarchical cobalt phosphide hollow nanocages toward electrocatalytic ammonia synthesis under ambient pressure and room temperature. Small Methods 2(12):1800204
Li T, Xia J, Xian H, Chen Q, Xu K, Gu Y, Luo Y, Liu Q, Guo H, Traversa E (2022) Fe (III) grafted MoO3 nanorods for effective electrocatalytic fixation of atmospheric N2 to NH3. Int J Hydrogen Energy 47(6):3550–3555
Wu X, Xia L, Wang Y, Lu W, Liu Q, Shi X, Sun X (2018) Mn3O4 nanocube: an efficient electrocatalyst toward artificial N2 fixation to NH3. Small 14(48):1803111
Chen JY, Cheng H, Ding LX, Wang HH (2021) Competing hydrogen evolution reaction: a challenge in electrocatalytic nitrogen fixation. Mater Chem Front 5(16):5954–5969
Acknowledgements
This work was supported by the Open Project of Key Laboratory of Green Chemical Engineering Process of Ministry of Education (No. GCX2023006) and the Innovation Project of Key Laboratory of Novel Biomass-based Environmental and Energy Materials in Petroleum and Chemical Industry (No. 2022BEEA03).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, X., Xing, X., Xiong, W. et al. Facile synthesis of Ni-based oxides nanocatalyst: effect of calcination temperature on NRR properties of NiO. Carbon Lett. (2024). https://doi.org/10.1007/s42823-023-00681-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42823-023-00681-2