Abstract
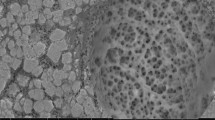
Activated carbon (AC) is a versatile and extensively employed adsorbent in environmental remediation. It possesses distinct properties that can be enhanced to selectively target specific pollutants through modifications, including chemical impregnation or incorporation into composite materials. In this study, porous calcium alginate beads (PCAB) were synthesized by incorporating AC and natural alginate through ion gelation in a Ca(II) ion-containing solution, with the addition of sodium lauryl sulfate as a surfactant. The prepared PCAB was tested for Cu(II) removal. PCAB exhibited a spherical shape with higher porosity and surface area (160.19 m2.g−1) compared to calcium alginate beads (CAB) (0.04 m2.g−1). The adsorption kinetics followed the pseudo-first-order model for PCAB and the pseudo-second-order model for CAB. The Langmuir isotherm model provided the best fit for adsorption on PCAB, while the Freundlich model was suitable for CAB. Notably, PCAB demonstrated a maximum adsorption capacity of 75.54 mg.g−1, significantly higher than CAB's capacity of 9.16 mg.g−1. Desorption studies demonstrated that 0.1 M CaCl2 exhibited the highest efficiency (90%) in desorbing Cu(II) ions from PCAB, followed by 0.1 M HCl and 0.1 M NaCl. PCAB showed efficient reusability for up to four consecutive adsorption–desorption cycles. The fixed-bed column experiment confirmed the match with the Thomas model to the breakthrough curves with qTH of 120.12 mg.g−1 and 68.03 mg.g−1 at a flow rate of 1 mL.min−1 and 2 mL.min−1, respectively. This study indicated that PCAB could be an effective adsorbent for Cu(II) removal, offering insights for further application and design considerations.












Similar content being viewed by others
Data availability
The authors confirm that the data supporting the findings of this study are available within the article.
References
Nemati F, Jafari D, Esmaeili H (2021) Highly efficient removal of toxic ions by the activated carbon derived from Citrus limon tree leaves. Carbon Lett 31:509–521. https://doi.org/10.1007/s42823-020-00181-7
Baladi E, Davar F, Hojjati-Najafabadi A (2022) Synthesis and characterization of g–C3N4–CoFe2O4–ZnO magnetic nanocomposites for enhancing photocatalytic activity with visible light for degradation of penicillin G antibiotic. Environ Res 215:114270. https://doi.org/10.1016/j.envres.2022.114270
Sulaiman S, Azis RS, Ismail I, Man HC, Yusof KFM, Abba MU, Katibi KK (2021) Adsorptive Adsorption removal of copper (II) ions from aqueous solution using a magnetite nano-adsorbent from mill scale waste: synthesis, characterization, adsorption and kinetic modelling studies. Nanoscale Res Lett. https://doi.org/10.1186/s11671-021-03622-y
Katiyar R, Patel AK, Nguyen TB, Singhania RR, Chen CW, Dong C (2021) Adsorption of copper (II) in aqueous solution using biochars derived from Ascophyllum nodosum seaweed. Bioresour Technol 328:124829. https://doi.org/10.1016/j.biortech.2021.124829
Wang B, Lan J, Bo C, Gong B, Ou J (2023) Adsorption of heavy metal onto biomass-derived activated carbon: review. RSC Adv 13:4275–4302. https://doi.org/10.1039/D2RA07911A
Xia C, Joo S-W, Hojjati-Najafabadi A, Xie H, Wu Y, Mashifana T, Vasseghian Y (2023) Latest advances in layered covalent organic frameworks for water and wastewater treatment. Chemosphere 329:138580. https://doi.org/10.1016/j.chemosphere.2023.138580
Yang W, Kong Y, Yin H, Cao M (2023) Study on the adsorption performance of ZIF-8 on heavy metal ions in water and the recycling of waste ZIF-8 in cement. J Solid State Chem 326:124217. https://doi.org/10.1016/j.jssc.2023.124217
Mashkoor F, Shoeb M, Mashkoor R, Anwer AH, Zhu S, Jeong H, Baek S-S, Jung J, Jeong C (2023) Synergistic effects of tungstate trioxide hemihydrate decorated reduced graphene oxide for the adsorption of heavy metals and dyes and postliminary application in supercapacitor device. J Clean Prod 418:138067. https://doi.org/10.1016/j.jclepro.2023.138067
Liu X, Yin H, Liu H, Cai Y, Qi X, Dang Z (2023) Multicomponent adsorption of heavy metals onto biogenic hydroxyapatite: Surface functional groups and inorganic mineral facilitating stable adsorption of Pb(II). J Hazard Mater 443:130167. https://doi.org/10.1016/j.jhazmat.2022.130167
Kovár F, Smutná K, Hruška A, Koutník I, Vráblová M (2023) Adsorption and permeability of heavy metals (Fe, Cu, Pb, Zn, Cr, and Cd) onto the adaxial cuticle of Ficus elastica leaf. Sci Hortic (Amsterdam) 321:112315. https://doi.org/10.1016/j.scienta.2023.112315
Raji Z, Karim A, Karam A, Khalloufi S (2023) A review on the heavy metal adsorption capacity of dietary fibers derived from agro-based wastes: opportunities and challenges for practical applications in the food industry. Trends Food Sci Technol 137:74–91. https://doi.org/10.1016/j.tifs.2023.05.004
Râpă M, Ţurcanu AA, Matei E, Predescu AM, Pantilimon MC, Coman G, Predescu C (2021) Adsorption of copper (II) from aqueous solutions with alginate/clay hybrid materials. Materials (Basel). https://doi.org/10.3390/ma14237187
Jiao W, Chen W, Mei Y, Yun Y, Wang B, Zhong Q, Chen H, Chen W (2019) Effects of molecular weight and guluronic acid/mannuronic acid ratio on the rheological behavior and stabilizing property of sodium alginate. Molecules 24:4374. https://doi.org/10.3390/molecules24234374
Tiwari R, Tiwari A, Kaushal M, Bajpal AK (2007) Removal of copper (II) ions by adsorption onto crosslinked calcium alginate beads. Biosci Biotechnol Res Asia 4:675–680
Kuczajowska-Zadrożna M, Filipkowska U, Jóźwiak T (2020) Adsorption of Cu (II) and Cd (II) from aqueous solutions by chitosan immobilized in alginate beads. J Environ Chem Eng 8:103878. https://doi.org/10.1016/j.jece.2020.103878
Cataldo S, Muratore N, Orecchio S, Pettignano A (2015) Enhancement of adsorption ability of calcium alginate gel beads towards Pd(II) ion. a kinetic and equilibrium study on hybrid Laponite and Montmorillonite-alginate gel beads. Appl Clay Sci 118:162–170. https://doi.org/10.1016/j.clay.2015.09.014
Zhan W, Xu C, Qian G, Huang G, Tang X, Lin B (2018) Adsorption of Cu(ii), Zn(ii), and Pb(ii) from aqueous single and binary metal solutions by regenerated cellulose and sodium alginate chemically modified with polyethyleneimine. RSC Adv 8:18723–18733. https://doi.org/10.1039/c8ra02055h
Aksu Demirezen D, Demirezen Yılmaz D, Yıldız YŞ (2023) Magnetic chitosan/calcium alginate double-network hydrogel beads: preparation, adsorption of anionic and cationic surfactants, and reuse in the removal of methylene blue. Int J Biol Macromol 239:124311. https://doi.org/10.1016/j.ijbiomac.2023.124311
Dao MU, Le HS, Hoang HY, Tran VA, Doan VD, Le TTN, Sirotkin A, Le VT (2021) Natural core-shell structure activated carbon beads derived from Litsea glutinosa seeds for removal of methylene blue: facile preparation, characterization, and adsorption properties. Environ Res 198:110481. https://doi.org/10.1016/j.envres.2020.110481
Ani JU, Akpomie KG, Okoro UC, Aneke LE, Onukwuli OD, Ujam OT (2020) Potentials of activated carbon produced from biomass materials for sequestration of dyes, heavy metals, and crude oil components from aqueous environment. Appl Water Sci 10:1–11. https://doi.org/10.1007/s13201-020-1149-8
Yang CY, Kao CL, Hung PY (2022) Preparation of activated carbon from waste cation exchange resin and its application in wastewater treatment. Carbon Lett 32:461–474. https://doi.org/10.1007/s42823-021-00275-w
Raninga M, Mudgal A, Patel VK, Patel J, Kumar Sinha M (2023) Modification of activated carbon-based adsorbent for removal of industrial dyes and heavy metals: a review. Mater Today Proc 77:286–294. https://doi.org/10.1016/j.matpr.2022.11.358
Park HG, Kim TW, Chae MY, Yoo I-K (2007) Activated carbon-containing alginate adsorbent for the simultaneous removal of heavy metals and toxic organics. Process Biochem 42:1371–1377. https://doi.org/10.1016/j.procbio.2007.06.016
Hassan AF, Abdel-Mohsen AM, Fouda MMG (2014) Comparative study of calcium alginate, activated carbon, and their composite beads on methylene blue adsorption. Carbohydr Polym 102:192–198. https://doi.org/10.1016/j.carbpol.2013.10.104
Kaygusuz H, Evingür GA, Pekcan Ö, von Klitzing R, Erim FB (2016) Surfactant and metal ion effects on the mechanical properties of alginate hydrogels. Int J Biol Macromol 92:220–224. https://doi.org/10.1016/j.ijbiomac.2016.07.004
Minh VX, Dung KTT, Lan PT, Hanh LTM, Dung NT (2020) Study on Ni(II) adsorption by calcium alginate beads. Vietnam J Chem 58:358–363. https://doi.org/10.1002/vjch.2019000195
Papageorgiou SK, Kouvelos EP, Favvas EP, Sapalidis AA, Romanos GE, Katsaros FK (2010) Metal–carboxylate interactions in metal–alginate complexes studied with FTIR spectroscopy. Carbohydr Res 345:469–473. https://doi.org/10.1016/j.carres.2009.12.010
Nguyen THA, Le VT, Doan VD, Tran AV, Nguyen VC, Nguyen AT, Vasseghian Y (2022) Green synthesis of Nb-doped ZnO nanocomposite for photocatalytic degradation of tetracycline antibiotic under visible light. Mater Lett 308:131129. https://doi.org/10.1016/j.matlet.2021.131129
Song Y, Yang LY, Wang YG, Yu D, Shen J, Ouyang XK (2019) Highly efficient adsorption of Pb(II) from aqueous solution using amino-functionalized SBA-15/calcium alginate microspheres as adsorbent. Int J Biol Macromol 125:808–819. https://doi.org/10.1016/j.ijbiomac.2018.12.112
Le VD, Dao MU, Le HS, Tran DL, Doan VD, Nguyen HT (2020) Comparative study on adsorption of cationic and anionic dyes by nanomagnetite supported on biochar derived from Eichhornia crassipes and Phragmites australis stems. Environ Technol 41:2817–2832. https://doi.org/10.1080/09593330.2019.1584250
Yuan B, Huang X, Yang S, Yang Y, Lin Z, Semiat R, Paul Chen J (2023) Development of a magnetic calcium-alginate hydrogel-sphere encapsulated with Fe–Mn–Zr ternary metal composite for heavy metal adsorption. Sep Purif Technol 306:122531. https://doi.org/10.1016/j.seppur.2022.122531
Yu F, Pan J, Li Y, Yang Y, Zhang Z, Nie J, Ma J (2022) Batch and continuous fixed-bed column adsorption of tetracycline by biochar/MOFs derivative covered with κ-carrageenan/calcium alginate hydrogels. J Environ Chem Eng 10:107996. https://doi.org/10.1016/j.jece.2022.107996
Doan VD, Tran TKN, Nguyen A-T, Tran VA, Nguyen TD, Le VT (2021) Comparative study of Ni(II) adsorption by magnetic activated carbon/chitosan beads prepared from spent coffee grounds, shrimp shells and green tea extract. Environ Nanotechnol Monit Manag 16:100569. https://doi.org/10.1016/j.enmm.2021.100569
Wang S, Kwak JH, Islam MS, Naeth MA, Gamal El-Din M, Chang SX (2020) Biochar surface complexation and Ni(II), Cu(II), and Cd(II) adsorption in aqueous solutions depend on feedstock type. Sci Total Environ 712:136538. https://doi.org/10.1016/j.scitotenv.2020.136538
Cai CX, Xu J, Deng NF, Dong XW, Tang H, Liang Y, Fan XW, Li YZ (2016) A novel approach of utilization of the fungal conidia biomass to remove heavy metals from the aqueous solution through immobilization. Sci Rep 6:36546. https://doi.org/10.1038/srep36546
Gotoh T, Matsushima K, Kikuchi K-I (2004) Preparation of alginate–chitosan hybrid gel beads and adsorption of divalent metal ions. Chemosphere 55:135–140. https://doi.org/10.1016/j.chemosphere.2003.11.016
Zhao F, Qin X, Feng S (2016) Preparation of microgel/sodium alginate composite granular hydrogels and their Cu 2+ adsorption properties. RSC Adv 6:100511–100518. https://doi.org/10.1039/C6RA21546G
Germanos G, Youssef S, Farah W, Lescop B, Rioual S, Abboud M (2020) The impact of magnetite nanoparticles on the physicochemical and adsorption properties of magnetic alginate beads. J Environ Chem Eng 8:104223. https://doi.org/10.1016/j.jece.2020.104223
Zhang P, Zhang X, Yuan X, Xie R, Han L (2021) Characteristics, adsorption behaviors, Cu(II) adsorption mechanisms by cow manure biochar derived at various pyrolysis temperatures. Bioresour Technol 331:125013. https://doi.org/10.1016/j.biortech.2021.125013
Melo DQ, Vidal CB, Da Silva AL, Teixeira RNP, Raulino GSC, Medeiros TC, Fechine PBA, Mazzeto SE, De Keukeleire D, Nascimento RF (2014) Removal of Cd2+, Cu2+, Ni2+, and Pb 2+ ions from aqueous solutions using tururi fibers as an adsorbent. J Appl Polym Sci 131:1–12. https://doi.org/10.1002/app.40883
Acknowledgements
This work was supported by the Vietnam Academy of Science and Technology under the grant numbers “VAST.03.05/21-22” and “QTBY01.04/21-22”.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
There are no conflicts of interests or competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vu, X.M., Le, T.M.H., Bui, V.C. et al. A promising composite adsorbent of activated carbon and natural alginate for Cu(II) ion removal from aqueous solutions. Carbon Lett. 34, 769–782 (2024). https://doi.org/10.1007/s42823-023-00598-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42823-023-00598-w