Abstract
Background
Hemodynamic changes during laparoscopy have not been well defined in very young children. In this study, intraoperative monitoring of hemodynamic parameters and oxygenation was evaluated during laparoscopy in infants weighing < 10 kg.
Patients and methods
Thirty infants (5.07 ± 2.51 kg), undergoing laparoscopy (LAP Group, n = 15) or open surgery (Open Group, n = 15), were enrolled. Cerebral regional (crScO2), renal regional oxygenation (rrScO2), peripheral oxygen saturation (SpO2), heart rate (HR), blood pressure, transcutaneous CO2 (TcCO2), end-tidal CO2 (EtCO2), and core temperature (TC) were analyzed at five intervals: T0 = basal; T1 = anesthesia induction; T2 = CO2 PP insufflation in the LAP group; T3 = intraoperative; T4 = cessation of CO2 PP in the LAP group; and T5 = before extubation.
Results
The LAP group showed differences in crScO2 at T1, T3, and T4 compared with T0 (p < 0.05) and in rrScO2 from T1 to T4 versus T0 (p < 0.05), without hypoxemia. In addition, in the LAP group, an increase in TcCO2 from T2 to T5 versus T0 (p < 0.01) was related to anesthesia time (p = 0.01) and sex (p = 0.03). The SpO2 values were not different during laparoscopy; on the contrary, the OPEN group exhibited a decrease in SpO2 at T4 (p < 0.001) and an increase at T5 versus T0 (p = 0.001) compared with T0. HR and TC changes were detected in both groups (p < 0.01). No significant variations were recorded in blood pressure or end-tidal CO2.
Conclusion
Limited effects on oxygenation, cardiac output, and thermoregulation were detected in infants during laparoscopy. Many factors, including the child’s age, may play different roles in the regulation of these parameters. Close monitoring is essential to guarantee the infant’s safety.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Laparoscopy in the pediatric population is now common [1,2,3,4,5,6]. The great majority of surgical procedures in infants and young children, that in the past would have required an open operation, can now be safely and effectively conducted using the laparoscope [6]. These include a wide spectrum of gastrointestinal tract interventions, uro-gynecological procedures, herniotomy, laparoscopic reconstructive procedures, e.g., oesophageal anastomosis for oesophageal atresia and surgery for choledochal cysts and biliary atresia [6, 7]. Evidence to date suggests that minimal access surgery results in a more rapid recovery with a shorter hospital stay, decreased postoperative pain, reduced wound complications, minimal scarring, and an earlier return to normal activities including feeding, bowel movements, and work/school when compared with traditional open surgery [6,7,8].
Some critical factors associated with mini-invasive surgery (MIS), such as carbon dioxide (CO2) insufflation, CO2 absorption, and intra-abdominal pressure, are also the same factors that limit this approach in neonates and infants [9, 10]. CO2 is usually accepted as the optimal gas for insufflation; however, increases in PaCO2 and resultant alterations in cerebral blood flow may occur with its use [9, 10]. In addition, an increase in intra-abdominal pressure (IAP) due to peritoneal insufflation may result in increased systemic vascular resistance and decreased cardiac output [9, 10]. Changes from fetal to neonatal life lead to a lower functional residual capacity of the lung, lower blood pressures, and higher heart rates [11, 12]. Consequently, neonates and infants have fewer physiologic heamodynamic and respiratory reserves than older children. For these reasons, MIS may be challenging in these patients; however, the effects on systemic and cerebral haemodynamics during laparoscopy are not yet well defined in very small children [7, 13,14,15,16,17].
The aim of this study was to evaluate intraoperative changes in hemodynamic parameters, peripheral and cerebral oxygenation during pediatric laparoscopy in infants. Results from this study will provide important knowledge and help define the optimal monitoring parameters to guarantee infant safety during minimally invasive surgical procedures.
Patients and methods
Patients
Thirty infants weighing less than 10 kg, scheduled for abdominal surgical procedures, were consecutively enrolled in the study between February 01, 2016 and October 01, 2018, at the Pediatric Surgery Unit of the Children’s Hospital “G. Di Cristina”, Palermo (Italy). The treatment group (LAP Group, n = 15) underwent laparoscopic surgery, while the control group underwent traditional open surgery (OPEN Group, n = 15). Surgery was performed, by an experienced surgeon, under general anesthesia.
The study was performed according to the Declaration of Helsinki and with the approval of the Institutional Review Board of the Children’s Hospital, ARNAS Civico-Di Cristina-Benfratelli, Palermo, Italy (CE n.registro 353 Civico 2016). The parents and/or legal guardian, after receiving information about the study, gave their written consent.
Methods
Surgical intervention
All surgeries were performed in the same operating theater with a stable temperature of 22 ± 1 °C. Patients were placed on a warm air mattress with a controlled temperature (38 ± 1 °C). The laparoscopic approach was accomplished, following a standard protocol, via the transabdominal approach using one 3 mm telescope and two, 3 mm or 2 mm operative instruments, placed into the lower abdomen. The pneumoperitoneum (PP) was created through the 3 mm infraumbilical camera trocar placed via an open approach. A PP CO2 pressure of 8 mmHg was used.
Intraoperative transcranial near-infrared spectroscopy (NIRS; Oximeter Medtronic INVOS 5100C, Minneapolis USA) was used to assess cerebral regional (crScO2) and renal regional oxygenation (rrScO2); while pulse oximetry was used to measure peripheral oxygen saturation with an SatO2 Monitor Infinity Delta (Dräger, Lübeck, Germany), heart rate (HR) was measured with an HR Monitor Infinity Delta (Dräger, Lübeck, Germany), and diastolic and systolic pressures were, respectively, measured with DP/SP Monitor Infinity Delta (Dräger, Lübeck, Germany). Transcutaneous CO2 was monitored with a TcCO2. Monitor TCMA4 Combi m, (Radiometer Medical ApS, Brønshøj, Denmark), end-tidal CO2 and core body temperature (TC) were measured continuously with an oropharyngeal probe during the entire procedure.
The following parameters were analyzed, every minute in 5 min intervals (mean values were used for the statistical analysis), at five intervals: basal (T0) in the LAP and Open groups; anesthesia induction (T1) in the LAP and OPEN groups; CO2 PP insufflation (T2) in the LAP group; intraoperative (T3) in the LAP and OPEN groups; cessation of CO2 PP (T4) in the LAP group; before extubation (T5) in the LAP and OPEN groups. The operative times and anesthesia duration were also recorded.
Anesthesia protocol
All of the children received the same anesthesia protocol. Inhalation induction was performed with 6% sevorane, and 1 mcg/kg fentanyl de-escalation to reach a deep anesthetic state; for muscle relaxation, 0.6 mg/kg rocuronium was administered. After tracheal tube positioning, patients underwent pressure control ventilation–volume-guaranteed (PCV–VG) mechanical ventilation with a tidal volume of 6–7 ml/kg, PEEP 3-5 cmH2O. The respiratory rate was adjusted to achieve an end-tidal CO2 of 35–40 cmH2O, and I:E ratio of 1:2–1:1.5 (avoiding dynamic hyperinflation) with a low-flow breathing system and an inspired mixture of air and oxygen (fresh gas flow of 4 l min−1 with 0.4 FiO2 during anesthesia). Anesthesia was maintained via administration of sevofluorane gas (MAC 1.5), a bolus of 1 mcg/kg fentanyl and 0.3 mg/kg rocuronium. The respiratory rate (RR) was adjusted to achieve an EtCO2 of 35–45 cmH2O. The anesthesiologists were not blinded to the data readings to prevent intraoperative alterations in the infants.
The anesthesiology protocol to prevent hypotension included the administration of a 10 ml/kg NaCl 0.9% bolus both before induction of general anesthesia and before peritoneum insufflation. The maintenance of blood volume was managed with NaCl 0.9% 4 ml/kg fluid therapy. Thirty minutes before the end of the intervention, all patients received 7.5 mg/kg paracetamol, as an analgesic. The analgesia was consolidated with surgical wound infiltration prevention using 0.2% ropivacaina and 2% lidocaine for both open and laparoscopic surgical patients.
Statistical analysis
Quantitative variables were described as the mean (SD) and compared between the different time intervals with population averaged, mixed multilevel models to take into account the clustered nature of the data. Probability values of less than 0.05 were considered statistically significant. All statistical analyses were performed using the SPSS statistical package (SPSS, Chicago IL, USA) and Stata 15.1.
Results
Thirty infants (7 females and 23 males) with an average weight of 5.0 ± 2.51 kg were evaluated. In Table 1, the clinical features of the patients, interventions, surgical procedure, and anesthesia times, according to the treatment group, are reported. No major complications were recorded.
Cerebral and renal region oxygenation
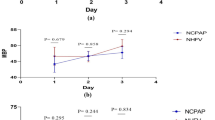
In the LAP group, a significant increase in crScO2 was noted at T1 (p = 0.002), T3 (p = 0.005), and T4 (p = 0.03) compared with T0. Significant changes were also recorded in rrScO2 from T1 to T4 versus T0 (p = 0.03; p = 0.006; p = 0.003 and p < 0.001, respectively), Table 2, Fig. 1. The changes in crScO2 and rrScO2 did not correlate with age, sex, anesthesia, or operative times. No significant changes in crScO2 or rrScO2 (p > 0.05) were observed in the OPEN group during the procedure (Table 2, Fig. 1).
Peripheral oxygen saturation
The SpO2 values were not statistically different during the laparoscopic surgical intervals (p > 0.05), Table 2, Fig. 1. The OPEN group had a significant decrease in SpO2 at T4 compared with T0 (p < 0.001) and a significant increase at T5 versus T0 (p = 0.001). These changes were related to operative times (p = 0.05) and age (p = 0.01) (Table 2, Fig. 1).
Heart rate
In the LAP group, a significant decrease in HR was recorded at T3 compared with T5 (p = 0.01), and a significant increase at T5 compared with T0 (p = 0.05) was noted, Table 2, Fig. 1. In the OPEN group, a significant increase in HR was observed at T3 (p = 0.01) and T5 (p = 0.02) compared with T0, Table 2, Fig. 1. The changes in HR in both groups were related to age (p < 0.001). No other correlations were detected.
Diastolic and systolic blood pressure
No significant changes were recorded in diastolic and systolic blood pressures in either the LAP or OPEN groups (p > 0.05, Table 2).
Transcutaneous CO2
A significant increase in TcCO2 from T2 to T5 versus T0 (p < 0.01, Table 1, figure) in the LAP group was observed. These changes were related to anesthesia time (p = 0.01) and sex (p = 0.03). Table 2, Fig. 1. No relevant changes in TcCO2 during surgery (p > 0.05, Table 2) were observed in the OPEN groups.
End-tidal CO2
No significant changes were recorded in End-tidal CO2 in either the LAP or OPEN groups (p > 0.05, Table 2).
Body temperature
In the LAP group, a significant increase in TC was noted at T3 (p = 0.005), T4 (p ≤ 0.001), and T5 (p ≤ 0.001) as compared with T0, Table 2, Fig. 1. A significant increase in TC was also detected in the OPEN group at T3 (p < 0.001) and T5 (p < 0.001) in comparison with T0, Table 2, Fig. 1. The changes in TC were not correlated with age, sex, anesthesia, or operative times (p > 0.05), Table 2.
Discussion
The successful application of laparoscopic surgery in the pediatric age has also led to its increased use in neonates and infants, as it offers the advantages of fewer postoperative complications, early discharge, and minimal scarring [1,2,3,4,5,6,7,8, 11,12,13,14,15,16,17]. However, laparoscopy may be associated with hemodynamic alterations, which are generated by the high intra-abdominal pressure brought on by PP creation and by the existence of insufflation gas that is absorbed by the blood. These systemic hemodynamic alterations may result in changes in end-organ blood flow and oxygen delivery. In addition, the impact of anesthesia on hemodynamic changes has also been described [9, 10, 18].
There are limited studies on the effects of pneumoperitoneum during laparoscopy in the pediatric age, particularly in small children [13,14,15,16,17,18,19,20,21,22,23,24,25,26]. For this reason, the aim of this study was to monitor adaptive changes in cerebral and systemic oxygenation and hemodynamic parameters in infants weighing less than 10 kg. We found that a CO2 pneumoperitoneum with 8 mm Hg IAP has minimal effects on cerebral oxygenation, without hypoxemia. The data on the relationship between laparoscopic surgery and regional effects on cerebral oxygenation in infants are non-homogenous. Tygat et al. [14] reported that regional brain oxygenation remained unaltered during surgical treatment of hypertrophic pyloric stenosis in young infants; Bishay et al. [15] showed a significant decrease in cerebral oxygenation in infants who underwent thorascopic surgery for treatment of esophageal atresia and congenital diaphragmatic hernia. As reported by Oztan [18], cerebral oxygen saturation is not only strongly related to the concentration of CO2 delivered during the pneumoperitoneum, but also to the hydration status. We propose that the stability of this parameter depends on the anesthesiology protocol used to prevent hypotension before the induction of anesthesia and peritoneum insufflation. Therefore, we recommend that patients be well hydrated throughout the procedure. In addition, the use of NIRS-based cerebral oximetry in pediatric anesthesia, as in this study, may be a helpful monitoring tool for detecting and correcting changes in oxygenation. Indeed, we previously reported on changes in cerebral oxygenation during laparoscopic procedures in pediatric patients when stringent monitoring was not adopted [21].
Having observed no decrease in renal rSO2 during laparoscopy suggests that laparoscopy and the increased pressure caused by the CO2 pneumoperitoneum does not decrease renal oxygenation to unacceptable intra-abdominal pressures with regard to renal perfusion.
Increased HR during laparoscopy has been well described in several studies. This has been attributed to the neurohumoral effect of CO2, which stimulates the sympathoadrenal system causing a significant release of catecholamines and cortisol and thus increases HR and arterial pressure [17]. We noted a variation in HR in both groups and these changes were significantly related to patient age. These data suggest that because of the age-related homeostatic vulnerability, hemodynamic status should be closely monitored in infants.
Pathophysiological hemodynamic values may also be affected by both the procedure and the anesthesia times. In particular, we observed that the LAP group exhibited a significant increase in TcCO2 differences related to anesthesia times. The monitoring of TcCO2 allowed the evaluation of the difference in CO2 partial pressures between the blood and alveoli and, therefore, the ventilation/perfusion ratio which can be an indication of pulmonary flow and the possible response to the elimination of CO2 on the volumic expansion with fluid. However, TcCo2 might not correlate with PaCO2. In most patients, controlled ventilation, end-tidal CO2 closely reflects arterial CO2 tension [19]; however, we did not monitor arterial CO2 and arterial pH levels. Arterial blood gas analysis monitoring should be performed during long laparoscopic procedures to exclude hypercapnia [19, 21, 22].
The accurate measurement of TC is an essential aspect of intraoperative management in children. However, little attention has been paid to the effect that surgical factors have on thermogenesis, such as surgical stress and anesthetic agents [27]. In neonates and infants, thermoregulation includes basic heat production resulting from increased cellular metabolic activity and extra-heat production such as nonshivering termogenesis, which is heat produced by brown adipose tissue, and the more limited shivering termogenesis, muscle produced heat [28]. In our infants, a significant rise in TC was noted in both groups. As reported by McHoney [29], laparoscopy in children is associated with an intraoperative hypermetabolic response characterized by increased oxygen consumption and core temperature; these changes are more markedly recorded in younger children. In very small children, the hypermetabolic response seems to be activated during open surgery. The influence of extra-heat production on intraoperative TC changes should not be underestimated.
We recognize that there are some limitations to this study starting from the limited sample size; therefore, a larger cohort of patients is mandatory to confirm the results. The ‘broad’ range in type of intervention influences anesthesia choice and operative time, and consequently, the exact impact of these parameters on hemodynamics could not be defined. Further studies focused on the duration of surgery are mandatory to detect the appearance of early signs related to hemodynamic adaptation. Finally, arterial blood gas analysis monitoring would be useful during laparoscopic procedures to estimate hypercapnia. Despite these limitations, studies like ours, focused on specific populations of very small children, are very important to support the safety of laparoscopy when close monitoring is adopted.
In conclusion, the results of the present study showed that infants tolerated CO2 insufflation without significant effects on cerebral oxygenation, with minimal effects on cardiac output and thermoregulation. Many factors, including the child’s age, play different roles in the regulation of hemodynamics and oxygenation and during laparoscopy; each of these requires further research. Knowledge of pathophysiological changes, standardization of intraoperative surgical evaluation, and anesthesia management are all necessary to prevent adverse hemodynamic outcomes.
References
Zitsman JL (2006) Pediatric minimal-access surgery: update 2006. Pediatrics 118:304–308
Zitsman JL (2003) Current concepts in minimal access surgery for children. Pediatrics 111:1239–1252
Georgeson KE, Owings E (2000) Advances in minimally invasive surgery in children. Am J Surg 180:362–364
Mattei P (2007) Minimally invasive surgery in the diagnosis and treatment of abdominal pain in children. Curr Opin Pediatr 19:338–348
Mansuria SM, Sanfilippo JS (2004) Laparoscopy in the pediatric and adolescent population. Obstet Gynecol Clin N Am 31:469–483
Gupta R, Singh S (2009) Challenges in paediatric laparoscopic surgeries. Indian J Anaesth 53:560–566
Blinman T, Ponsky T (2012) Pediatric minimally invasive surgery: laparoscopy and thoracoscopy in infants and children. Pediatrics 130:539–549
Chao TE, Mandigo M, Opoku-Anane J, Maine R (2016) Systematic review of laparoscopic surgery in low- and middle-income countries: benefits, challenges, and strategies. Surg Endosc 30:1–10
Truchon R (2004) Anaesthetic considerations for laparoscopic surgery in neonates and infants: a practical review. Best Pract Res Clin Anaesthesiol 18:343–355
Srivastava A, Niranjan A (2010) Secrets of safe laparoscopic surgery: anaesthetic and surgical considerations. J Minim Access Surg 6:91–94
Gill AW (2018) Postnatal cardiovascular adaptation. Arch Dis Child Fetal Neonatal Ed. https://doi.org/10.1136/archdischild-2017-314453
Morton SU, Brodsky D (2016) Fetal physiology and the transition to extrauterine life. Clin Perinatol 43:395–407
Tytgat SH, van Herwaarden MY, Stolwijk LJ, Keunen K, Benders MJ, de Graaff JC, Milstein DM, van der Zee DC, Lemmers PM (2016) Neonatal brain oxygenation during thoracoscopic correction of esophageal atresia. Surg Endosc 30:2811–2817
Tytgat SH, Stolwijk LJ, Keunen K, Milstein DM, Lemmers PM, van der Zee DC (2015) Brain oxygenation during laparoscopic correction of hypertrophic pyloric stenosis. J Laparoendosc Adv Surg Tech A 25:352–357
Bishay M, Giacomello L, Retrosi G, Thyoka M, Nah SA, McHoney M, De Coppi P, Brierley J, Scuplak S, Kiely EM, Curry JI, Drake DP, Cross KM, Eaton S, Pierro A (2011) Decreased cerebral oxygen saturation during thoracoscopic repair of congenital diaphragmatic hernia and esophageal atresia in infants. J Pediatr Surg 46:47–51
Simpao AF, Ahumada LM, Gálvez JA, Bonafide CP, Wartman EC, Randall England W, Lingappan AM, Kilbaugh TJ, Jawad AF, Rehman MA (2017) The timing and prevalence of intraoperative hypotension in infants undergoing laparoscopic pyloromyotomy at a tertiary pediatric hospital. Paediatr Anaesth 27:66–76
De Waal EE, Kalkman CJ (2003) Haemodynamic changes during low-pressure carbon dioxide pneumoperitoneum in young children. Paediatr Anaesth 13:18–25
Oztan MO, Aydin G, Cigsar EB, Sutas Bozkurt P, Koyluoglu G (2018) Effects of carbon dioxide insufflation and Trendelenburg position on brain oxygenation during laparoscopy in children. Surg Laparosc Endosc Percutan Tech. https://doi.org/10.1097/sle.0000000000000593
Tuna AT, Akkoyun I, Darcin S, Palabiyik O (2016) Effects of dioxide insufflation on regional cerebral oxygenation during laparoscopic surgery in children: a propsective study. Braz J Anesthesiol 66:249–253
Westgarth-Taylor C, de Lijster L, van Bogerijen G, Millar AJ, Karpelowsky J (2013) A prospective assessment of renal oxygenation in children undergoing laparoscopy using near-infrared spectroscopy. Surg Endosc 27:3696–3704
Pelizzo G, Carlini V, Iacob G, Pasqua N, Maggio G, Brunero M, Mencherini S, De Silvestri A, Calcaterra V (2017) Pediatric laparoscopy and adaptive oxygenation and hemodynamic changes. Pediatr Rep 9:7214
Pelizzo G, Bernardi L, Carlini V, Pasqua N, Mencherini S, Maggio G, De Silvestri A, Bianchi L, Calcaterra V (2017) Laparoscopy in children and its impact on brain oxygenation during routine inguinal hernia repair. J Minim Access Surg 13:51–56
Miyano G, Nakamura H, Seo S, Sueyoshi R, Okawada M, Doi T, Koga H, Lane GJ, Yamataka A (2016) Pneumoperitoneum and hemodynamic stability during pediatric laparoscopic appendectomy. J Pediatr Surg 51:1949–1951
Maesani M, Pares F, Michelet D, Abdat R, Hilly J, Diallo T, Greff B, Malbezin S, Bonnard A, Dahmani S (2016) Haemodynamic and cerebral oxygenation during paediatric laparoscopy in fluid optimized patients. Br J Anaesth 116:564–566
Lorenzo AJ, Karsli C, Halachmi S, Dolci M, Luginbuehl I, Bissonnette B, Farhat WA (2006) Hemodynamic and respiratory effects of pediatric urological retroperitoneal laparoscopic surgery: a prospective study. J Urol 175:1461–1465
Halachmi S, El-Ghoneimi A, Bissonnette B, Zaarour C, Bagli DJ, McLorie GA, Khoury AE, Farhat W (2003) Hemodynamic and respiratory effect of pediatric urological laparoscopic surgery: a retrospective study. J Urol 170:1651–1654
Nour BM, Boudreaux JP, Rowe MI (1984) An experimental model to study thermogenesis in the neonatal surgical patient. J Pediatr Surg 19:764–770
Asakura H (2004) Fetal and neonatal thermoregulation. J Nippon Med Sch 71(6):360–370
McHoney MC, Corizia L, Eaton S, Wade A, Spitz L, Drake DP, Kiely EM, Tan HL, Pierro A (2006) Laparoscopic surgery in children is associated with an intraoperative hypermetabolic response. Surg Endosc 20:452–457
Acknowledgements
The authors thank Dr. L. Kelly for English revision of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Pelizzo, G., Puglisi, A., Di Mitri, M. et al. Laparoscopy in infants: close intraoperative hemodynamic monitoring for patient safety. J Ped Endosc Surg 1, 15–22 (2019). https://doi.org/10.1007/s42804-019-00004-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42804-019-00004-1