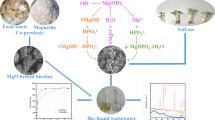
Abstract
Emergent plants have been remarkably effective in reducing phosphorus (P) discharge from ecological ditches; however, the treatment and recycling of these residues is a great challenge. In this study, magnetic biochars (MBs, i.e., MB-A, MB-C, and MB-T) were fabricated from three emergent plant residues (Acorus calamus L., Canna indica L., and Thalia dealbata Fraser, respectively) and modified with Fe(II)/Fe(III). Scanning electron microscopy-energy dispersive spectroscopy and X-ray diffraction spectra confirmed the successful loading of Fe3O4 and FeO(OH) onto the surfaces of the MBs. Batch adsorption experiments showed that MBs exhibited a higher P adsorption capacity than that of the raw biochars. Within the range of 0.8–43.0 mg L−1 in solution, the adsorption capacities of P by MB-A, MB-C, and MB-T were 304.6–5658.8, 314.9–6845.6, and 292.8–5590.0 mg kg−1, with adsorption efficiencies of 95.2–32.9%, 98.4–39.8%, and 91.5–32.5%, respectively. The primary mechanisms that caused P to adsorb onto the MBs were inner-sphere complexation and electrostatic attraction. Low pH conditions were more beneficial for the P adsorption of the MBs, while co-existing anions had a negative impact with the following order: HCO3− > SO42− > Cl−≈NO3−. The P-31 nuclear magnetic resonance results further demonstrated that the main adsorbed P species on the MBs was orthophosphate, followed by orthophosphate monoesters and DNA. Overall, MBs offer a resource utilization strategy for emergent plant residues and P-laden MBs are promising alternative P fertilizers.
Graphical Abstract

Highlights
-
Emergent plant biochar modified with Fe(II)/Fe(III) enhanced P adsorption capacity.
-
Canna indica residue-derived MB exhibited the best P adsorption efficiency.
-
MBs promoted P adsorption mainly via inner-sphere complexation and electrostatic attraction.
-
P species adsorbed by MBs were mainly orthophosphate followed by orthophosphate monoesters and DNA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Phosphorus (P) is a nonrenewable resource, and it is predicted that global P reserves will be depleted within 100 years if no solution is taken (Smit et al. 2009). Since P is essential for plant growth, large amounts of P continuously enter the environment with the development of agriculture, causing the wastage of P resources and water eutrophication (Conley et al. 2009). Recently, several countries have implemented strict thresholds for P concentrations in the environment. Total phosphorus (TP) emissions should not exceed 1 mg L−1 in class 1B and 0.5 mg L−1 in class 1A (MEP 2002). The US EPA advised that TP levels in streams entering lakes should be below 50 μg of P L−1 (Loganathan et al. 2014). In response to the upgrading of emission standards, the development of more effective measures to mitigate P pollution and promote P recycling is urgently required.
Ecological ditches are one of the most effective methods for mitigating agricultural P pollution by virtue of their ability to remove P (Kröger and Moore 2011; Kumwimba et al. 2017). Emergent plants, in particular, are frequently utilized in environmental remediation because of their high P uptake, low cost, and visual attractiveness (Singh et al. 2023). Emergent plants can adsorb P directly and store it in their biomass. The P uptake rates of emergent plants have been reported to be 1–4 kg of dry matter m−2 yr−1 or more (Iamchaturapatr et al. 2007; Christopher 2013; Wu et al. 2023). However, many emergent plants wilt and rot during winter, which poses a risk of secondary water contamination; thus, they must be harvested regularly (Yu et al. 2019). Current methods for treating emergent plants mainly include composting and incineration; however, these methods suffer from inefficiencies and long processing times (Gusain et al. 2018). Therefore, the next crucial step is to create efficient and eco-friendly alternative utilization strategies for emerging plant residues.
Biochar is expected to be a reliable P-adsorption material because of its cost-effectiveness and unique properties that can be easily obtained by biomass pyrolysis in an anaerobic or oxygen-limited environment (Klinar 2016). With in-depth research into engineered biochar, the feedstock options for raw biochar are continuously increasing (Cheng et al. 2023), however, these were found to have a limited P adsorption ability because of the negative charge on their surface (Shepherd et al. 2017; Liu et al. 2022). To overcome this problem, metal-modified biochars have received increasing attention owing to their strong affinity for P (Palansooriya et al. 2021; Padilla et al. 2023). Currently, metals including iron (Fe), calcium (Ca), and magnesium (Mg) are frequently utilized to modify biochar because of their low cost and lack of secondary pollution (Jiang et al. 2018; Mitrogiannis et al. 2018; Chen et al. 2022). In addition, Fe-based biochars have the advantage of magnetic recovery, offering the ability to separate these from solution (Huang et al. 2019; Palansooriya et al. 2021). According to previous studies, Fe-modified biochar can increase the surface positive charge, thereby improving the anion exchange capacity and redox properties of the biochar (Ou et al. 2023). Wang et al. (2020a, b)’s study validated that Fe-modified biochar had a greater adsorption capacity (1.84 mg g−1) for P than raw biochar (no effect) and Palansooriya et al. (2021) also found comparable outcomes in their study of Fe(III)-loaded chitosan biochar. Mixed metal oxides/hydroxides containing multiple metal components possess the benefits of parent metals and produce synergies that improve their adsorption properties (Wu et al. 2020). Although reports are available on the effect of Fe-modified materials since over two years ago, little attention has been paid to Fe(II) and Fe(III) modification of biochars for P adsorption.
Additionally, after P adsorption, biochar has the potential to be functionalized as a P fertilizer. Ai et al. (2023) used corn stalk biochar after P adsorption as a fertilizer and observed that it significantly promoted plant growth. Nevertheless, the variation in the bioavailability of the P species adsorbed by biochar in actual water is not clear because most studies tested the performance of biochar as a P adsorbent in phosphate solutions. In this study, to reduce P discharge into external water bodies and to reuse plant residues, we prepared Fe(II)/Fe(III) co-modified biochars using three types of emergent plant residues for application in P adsorption from wastewater. The aims of this study were: (i) to investigate the P adsorption capacity of biochars derived from emergent plant residues, (ii) to explore the P adsorption processes and mechanisms of magnetic biochars (MBs), and (iii) to identify the P species in P-laden biochars and provide data for the effective support of using these as P fertilizers. The results of this study offer an efficient adsorbent for P adsorption and a greater understanding of the P species adsorbed by MBs, which will be helpful for the further utilization of MBs in P adsorption, fertilizer, and resource cycling engineering.
2 Materials and methods
2.1 Preparation of RBs
Three emergent plants (Acorus calamus L., Canna indica L., and Thalia dealbata Fraser) were collected from ecological ditches in Pinghu County, Zhejiang Province, China. Emergent plant residues were cleaned with deionized (DI) water before use, dried in an oven for 2 h at 105℃, and further dried at 60℃. The dried residue was cut into fragments smaller than 5 cm, crushed, and filtered through a 0.15 mm pore size filter. The treated residue was pyrolyzed in a programmable electric tube furnace (OTF-1200X, Hefei kejing materials technology Co., Ltd, China). The system temperature was raised to 650 °C at a pace of 10 °C min−1 and the high temperature was maintained for 0.5 h under N2 conditions (Zhao et al. 2022). The yields of the three biochars were approximately 29%. These raw biochars (RBs) were denoted as RB-A, RB-C, and RB-T and were produced from the residues of Acorus calamus L., Canna indica L., and Thalia dealbata Fraser, respectively.
2.2 Preparation of MBs
The MBs were prepared as follows: 2.5 g of each RB was placed into 50 mL of mixed iron (Fe) solution (0.01 mol FeSO4·7H2O and 0.01 mol FeCl3), heated at 60℃ for 30 min under slow stirring, cooled to 40℃, and then 5 M NaOH was added to maintain the pH at 10–11 (Dai et al 2020). The mixture was stirred for 10 min and aged for 24 h. The black precipitate was collected and cleaned with DI water and ethanol. The MBs were finally obtained after filtration and drying at 60℃ for 24 h. Modified RB-A, RB-C, and RB-T were named MB-A, MB-C, and MB-T, respectively. By dispersing the MBs evenly in pure water, aggregation of the suspended powder was observed under the attraction of magnets. Thus, the MBs were confirmed to be highly magnetic and could be successfully separated from water via magnetic separation (Additional file 1: Fig S1).
2.3 Adsorption experiments
The P adsorption ability of the MBs was assessed using batch adsorption tests. All the solutions used for the adsorption experiments were obtained from a livestock breeding farm in Kaihua, Zhejiang Province, China. The experiments were conducted at 120 rpm and 25 °C for 24 h. To evaluate the adsorption capacity of the RBs, 100 mg of RBs were added to 40 mL of the diluted solution. Similarly, 100 mg of RBs were added to 40 mL of solution with the initial P concentration in the range from 0.8 mg L−1 to 43 mg L−1 to examine the adsorption abilities of the RBs. The adsorption kinetic tests were completed at 25 °C with the TP concentration of the solution at 20 mg L−1. Samples were periodically collected at a mixture time after 2, 4, 6, 8, 10, 20, 30, 60, 90, 180, 360, 720, and 1440 min. The P content of the solution was analyzed using the molybdenum blue colorimetric method (Lei et al. 2021). The experimental data were fitted with pseudo-first-order, pseudo-second-order, and intra-particle diffusion models to understand the adsorption kinetics.
The influence of the solution pH on adsorption was investigated by conducting experiments at pH levels between 3 and 11. The pH of the solution was adjusted using 0.2 M HCl or NaOH solutions. The method of pH drift was used to quantify the point of zero charge (pHPZC) of the adsorbent (Villanueva et al. 2014). In addition, possible interferences between co-existing anions (sulfate (SO42−), chlorine (Cl−), nitrate (NO3−), and bicarbonate (HCO3−)) and P adsorption by MBs were investigated. Different co-existing anions of the same concentration (1 M) were added to the solution under the same conditions, including pH. The P adsorption experiment followed the same procedure, with 100 mg of MBs added to 40 mL of solution and oscillated for 24 h at 120 rpm and 25 °C.
In the above experiments, the amount of P in the solution was determined using a UV–vis spectrometer (UV-9000, Shanghai Metash Instruments Co., Ltd., China) following the molybdate blue spectrophotometric method. The pH of each solution was measured using an HQ 11D pH meter (HACH, USA). The average values for each experiment were obtained from three replicates.
2.4 Characterization of biochars
Microscopic features and morphology of the biochars were characterized using scanning electron microscopy-energy dispersive spectroscopy (SEM–EDS; Gemini SEM–EDS 300; ZEISS, Germany). The crystal structures of the MBs were analyzed by X-ray diffraction (XRD; Bruker D8 Advance, Germany). The surface functional groups of the RMs and MBs were examined by Fourier transform infrared spectroscopy (FTIR, Nicolet is50 FTIR, Thermo Scientific, USA) with a wavenumber range of 400–4000 cm−1. X-ray photoelectron spectroscopy (XPS, Thermo Scientific Escalab 250xi, USA) was used to analyze the elemental states on RMs before and after adsorption.
2.5 Phosphorus species of the MBs before and after adsorption
The P species were determined using a slightly altered version of the Cade-Menun and Preston (1996) approaches. Specifically, P on the MBs before and after adsorption was extracted and examined using solution P-31 nuclear magnetic resonance (31P-NMR) spectroscopy. First, 2.0 g of samples and 40 mL of NaOH-Na2EDTA mixture solution (0.25 M NaOH and 0.05 M Na2EDTA) were put in centrifuge tubes, shaken gently at 20 °C for 16 h, then centrifuged at 5000 rpm for 20 min. One milliliter of the supernatant was used for inductively coupled plasma optical emission spectroscopy (ICP-OES) analysis. Freeze-dried samples were dissolved in 0.65 mL of deuterium oxide (including 0.84 mg mL−1 methylenediphosphonic acid (MDPA)), 0.65 mL of distilled water, 0.4 mL of 10 M NaOH, and 0.65 mL of NaOH-EDTA solution. The dissolved samples were vortexed for 10 min and centrifuged at 5000 rpm for 20 min, then 0.7 mL was transferred into 5-mm NMR tubes. The NMR parameters included 90° pulse, 0.68-s acquisition time, 4.0-s pulse delay, 10 Hz spinning, 20 °C, and 2400–2800 scans for different samples depending on the P concentration. The quantities of various P species were analyzed by calculating the relative integral of the peak area versus MDPA using MestReNova (version 7.0, Mestrelab Research, Spain). The position of orthophosphate was adjusted at 6.0 ppm, the peaks between 5.0 and 4.3 ppm were sequentially determined as orthophosphate monoesters, the peak around -0.7 ppm was determined as DNA, and the peaks from −4.0 to −4.2 ppm were determined as pyrophosphate (Liu et al. 2018).
2.6 Phosphorus release tests
The P release capability of the MBs after P adsorption (P-laden MBs) was measured using DI water as the release medium. The specific steps were as follows: 100 mg of P-laden MBs was added to 40 mL of DI water, and the mixture was shaken at 25 °C and 120 rpm for sampling at 1, 2, 3, 6, 10, 24, 48, 72, 120, 144, 168, and 192 h. The results were fitted to second-order release kinetics. The P-laden MBs were reintroduced into distilled water for cyclic P release at 72 h. The P content was determined using molybdate blue spectrophotometry. The P release experiments were conducted three times, and the average and standard deviation were calculated.
2.7 Statistical analysis
Data were analyzed using SPSS (version 26.0, IBM, USA) and the results were visualized using Origin 2021 (Origin Lab, USA). Significant differences in the P adsorption content were tested using a one-way analysis of variance (ANOVA) with the least significant difference (LSD) test at the 5% level (p < 0.05).
3 Results and discussion
3.1 Characteristics of biochars
SEM was used to observe the microstructures of RBs and MBs (Fig. 1). As shown in Fig. 1a–c, the surface morphology of the RBs appeared smooth and irregular, whereas the MBs exhibited disordered particles on the surface (Fig. 1d–f). The EDS results (Fig. 1j–l) confirmed that the Fe content in the MBs exceeded 20%, indicating that Fe-related complexes were successfully loaded onto the MBs surface. Simultaneously, the disordered particles on the MBs were iron oxides, which provide binding sites for P adsorption. Notably, the Fe content in MB-C reached 27.3%, indicating its potential for a higher adsorption capacity than that of the other MBs (Huang et al. 2021).
The XRD patterns of the RBs and MBs revealed six peaks corresponding to crystalline substances (synthetic minerals) (Fig. 2a). The RBs were mainly composed of potassium chloride (KCl) and calcite (CaCO3), whereas the MBs contained magnetite (Fe3O4) and goethite (FeO(OH)). These results show that Fe modification changed the predominant mineral composition of biochars, with the major substance changing from KCl or CaCO3 to Fe3O4 or FeO(OH). The transformation of Fe(III) into FeO(OH) and Fe3O4 was consistent with the previous report of: FeCl3 → Fe(OH)3 → FeO(OH) → Fe3O4 (Li et al. 2016). Fe(III) loaded onto biochar reacted directly with P to form precipitates, whereas FeO(OH) interacts with P to produce complexes (Yang et al. 2018; Min et al. 2020). Furthermore, Fe(II) also contributed to the doping of Fe3O4 on biochars, enhancing the magnetic separation and adsorption capabilities of the biochar (Reguyal et al. 2017; Zhou et al. 2017; Dong et al. 2018). The Fe oxide layer on the MBs surface was the primary reason for the enhanced P adsorption.
3.2 Phosphorus adsorption capacities and mechanism of biochars
The capability of RBs for P adsorption was investigated using a 2.5 mg L−1 P solution, however, it demonstrated a desorption phenomenon of P from raw materials (Fig. 3a), possibly owning to the negative surface charge on RBs, which was not conducive to P adsorption (Ahmad et al. 2014). Therefore, RBs were excluded from the subsequent P adsorption studies. The P-adsorption performance of the MBs is shown in Fig. 3b. At 0.8 mg L−1 (low concentration of P), the adsorption efficiency of all MBs was above 90%. As the solution's P content increased from 0.8 to 43.0 mg L−1, the P adsorption efficiency decreased owing to the limited availability of adsorption sites. However, at the initial concentration of 22.0 mg L−1, the adsorption efficiency of MB-A, MB-C, and MB-T was still 61.7%, 76.0%, and 57.2%, respectively. The results showed that the MBs had a stronger P-adsorption capacity than the RBs, and that MB-C was the most efficient among the three MBs.
To enhance our comprehension about the P adsorption process onto MBs, adsorption studies were conducted with varying contact times. Kinetic models play a significant role in elucidating the pathways and mechanisms associated with adsorption processes (Gong et al. 2019). As depicted in Fig. 3c, the P adsorption of all MBs was rapid during the first two hours before slowing down and reaching equilibrium at approximately 6 h. The initial rapid adsorption can be attributed to the large number of binding sites and the electrostatic attraction between the phosphates and positively charged Fe or Fe oxides (Shan et al. 2021). Notably, this behavior was consistent across different types MBs. The adsorption parameters for the pseudo-first-order and pseudo-second-order models are listed in Table 1. Among all MBs, the pseudo-second-order model exhibited a better fit (R2 ≥ 0.87) than the pseudo-first-order model (R2 ≥ 0.63), indicating that chemical adsorption was the rate-determining step (Yi et al. 2019).
The experimental data were fitted using an intra-particle diffusion model to enhance our understanding of the rate-limiting steps. This model was used to explain the diffusion of P onto the adsorbent particle pores (Tran et al. 2017). The P adsorption process was divided into three sections (Fig. 3d), and the related parameters are listed in Table 1. The results revealed that the slope of the first stage was the highest of the three stages, implying a gradual decrease in the reaction rate with increasing contact time (Malash and El-Khaiary 2010). The rapid adsorption stage was caused by an external mass transfer phase, which may have been influenced by the high density of P-binding sites on the surface of the MBs (Fang et al 2022). Subsequently, the rate of P adsorption decreased, and P began to diffuse within the solid biochar particles, which is known as an intra-particle diffusion process (Yu et al. 2021). The third stage of adsorption reached equilibrium when no accessible adsorption sites were present on the MBs (Yao et al. 2013). Additionally, the first lines did not pass through the origin, indicating that intra-particle diffusion was not the sole rate-limiting process (Li et al. 2019).
The functional groups of RBs, MBs and P-laden MBs were examined by FTIR (Fig. 4b–d). Wide absorption bands approximately 3390 cm−1 were observed in MBs samples and were linked to the stretching vibrations of −OH, which were vibrationally enhanced with the addition of Fe (Yin et al. 2018). The 1630 cm−1 peak corresponded to the stretching vibration of −OH, arising from the distortion and movement of adsorbed water molecules (Ajmal et al. 2018). The peak at approximately 1085 cm−1 was associated with the deformation vibration of P−O in iron phosphates, providing evidence for the presence of phosphate in the MBs (Zhang et al. 2019). After adsorption, these peaks became more pronounced, indicating successful adsorption of P onto the MBs surface. The characteristic vibration of C−O bonds was attributed to the peaks observed at 980 cm−1 and 797 cm−1 (Zhao et al. 2022). These two peaks weakened after P adsorption, suggesting that phosphate entered the exchange layer and was exchanged (Jiang et al. 2020). The peak at 580 cm−1 was attributed to the Fe–O stretch vibration of magnetite and it disappeared after P adsorption, which might be attributed to the strong adsorption of phosphate on the metal oxide surface (Wang et al. 2023). A novel peak at 1390 cm−1 was related to the stretching vibration of Fe–O–P, which belonged to inner-sphere surface complex (Wang et al. 2021a, b). P adsorption onto MBs was controlled by the inner-sphere surface complex formation process while maintaining structural and chemical stability.
Figure 5 shows that the P 2p peak intensity of MBs was stronger after P adsorption, demonstrating that P was adsorbed on the MBs surface. The high-resolution P 2p spectrum (Fig. 5b) showed a clear separation, revealing two individual peaks (2p1/2 and 2p3/2). Novel bonds were created by P adsorption. The primary component at binding energy (BE) = 133.0–133.4 eV was pentavalent tetra coordinated P (PO43−) and the peak around 134.0 eV was attributed to HPO42− (Xie et al. 2014). Moreover, the BE of the P 2p peaks of the three MBs differed, with MB-CP (P-laden MB-C) having the highest BE, indicating that it exhibited the strongest P binding (Gao et al. 2019). Additionally, Fe peaks were clearly visible in the XPS survey of the MBs, indicating that Fe was successfully loaded onto the MBs (Fig. 5c, d). The two peaks visible in the Fe 2p spectra were Fe 2p3/2 and Fe 2p1/2, with the former being narrower and stronger than the latter (Yamashita and Hayes 2008). The 711.4 eV and 713.8 eV of the Fe 2p3/2 spectra were associated with Fe(II) and Fe(III), respectively (Tang et al. 2023). The satellite peaks observed at approximately 719.0 eV and 732.0 eV were attributed to Fe 2p3/2 and Fe 2p1/2 respectively, confirming the Fe loaded on the MBs existed in the form of γ-Fe2O3 (Hao et al. 2018; Tu et al. 2020). After P adsorption, the peak position of Fe 2p remained similar, indicating that Fe maintained its oxidation state during the adsorption process (Yang et al. 2022). The binding energies of the main peaks were consistent with the slightly shifted satellite peaks. These phenomena revealed that the MBs adsorbed P by relying on active Fe sites.
pH is a key influencing factor that affects the charge on the adsorbent’s surface and the dissociation equilibrium of phosphate; therefore, the effect of starting pH levels on P adsorption by MBs and the equilibrium pH of the solution after P adsorption were explored (Fig. 6a–c). Moreover, we found that the pHPZC values of MB-A, MB-C and MB-T were 3.85, 3.33, and 3.28, respectively (Fig. 6d). As the initial pH rose from 3 to 11, the P adsorption capacity of MB-A, MB-C, and MB-T decreased from 7.8 to 5.5 mg g−1, 7.9 to 5.2 mg kg−1, and 6.4 to 5.4 mg g−1, respectively. When the solution pH fell below the pHPZC, a strong electrostatic attraction developed between the binding sites on the MBs and H2PO4− ions, increasing P adsorption (Hartley et al. 1997). As the pH increased, the charge on the surface of the adsorbent shifted in the negative direction and formed a repulsive charge with the phosphates, causing the P adsorption capacity to decline (Liu et al. 2021). Additionally, the solution pH increased after P adsorption, which could be attributed to the complexation processes between P and the hydroxyl groups on the MBs, which consisted of the formation of inner-sphere surface complexes (Xu et al. 2019). In the preliminary stage, electrostatic attraction occurred between the negatively charged phosphates and the electropositive MBs surfaces loaded with amorphous hematite with a high zeta potential. During the subsequent adsorption process, inner-sphere complexation through ligand exchange played a significant role between the surface-bound Fe oxides/hydroxyl groups and phosphates (representative equilibrium; Eq. (1) is given below, taking H2PO4− as an example).
As shown in Table 2, the P adsorption capacity was negatively affected by the ions SO42−, Cl−, NO3−, and HCO3−. Of these, HCO3− imposed the most significant effect on the P adsorption capacity, decreasing it by 30.3%, 11.5%, and 27.0% for MB-A, MB-C, and MB-T, respectively. Because HCO3− had a higher distribution coefficient, causing significant interference to phosphate adsorption (Pitakteeratham et al. 2013). Another non-negligible negative effect was from SO42− on P adsorption with obvious reduction rates of 19.1%, 9.7%, and 19.7% for MB-A, MB-C, and MB-T, respectively. This may be owing to SO42− competing for complexation reaction sites with phosphate (Drenkova-Tuhtan et al. 2013). Conversely, Cl− and NO3− were related to outer sphere compounds forming (Wang et al. 2020a, b). They showed a slight adverse impact on P adsorption, indicating that the affinity for Cl− and NO3− was relatively low and inner-sphere adsorption plays a primary role in P adsorption (Fang et al. 2022). MB-C adsorbed more P than MB-A and MB-T for the different co-existing anions, validating that MB-C had the strongest P adsorption ability.
3.3 Phosphorus species of MBs before and after adsorption
The NaOH-EDTA extracted 59.5–66.0% of the TP from the MBs before and after P adsorption, with similar P species extracted. The results of the 31P-NMR spectroscopy analysis showed that MBs contained only orthophosphate and pyrophosphate before adsorption, while novel peaks of orthophosphate monoesters (5.0−4.3 ppm) and DNA (~ 0.7 ppm) were observed in the MBs after adsorption (Table 3, Fig. 7) (Liu et al. 2018), indicating that solutions from livestock and poultry farms contain orthophosphate monoesters and DNA. Pyrophosphate has been reported to be directly involved in microbial activities and have a significant impact on P cycling (Xie et al. 2019). Furthermore, orthophosphate was the dominant P species in MBs both before and after adsorption, ranging from 0.30–1.37 mg g−1 in MBs and 7.99–9.44 mg g−1 in P-laden MBs. Orthophosphate is the major P species directly available for plant uptake(Nash et al. 2014), which suggests that MBs possess great potential as soil fertilizer to facilitate P resource recycling.
3.4 Phosphorus release from P-laden MBs
The desorption kinetics of the P-laden MBs revealed a slow release of P and the release rate gradually decreased within 192 h (Additional file 1: Fig S2). This might be attributed to the high concentration of P in the solution, where P release and adsorption approached a dynamic equilibrium. The data showed a good fit with the second-order kinetic model, with R2 values of 0.98, 0.96, and 0.97 for MB-AP, MB-CP, and MB-TP, respectively. The P release capacities of MB-AP, MB-CP, and MB-TP were 8.24, 9.61, and 7.27 mg L−1, respectively. In three consecutive cyclic release experiments, the amount of P released in each cycle was approximately consistent, indicating the potential slow-release capability of the P-laden MBs (An et al. 2021). Given that the P released into the soil by P-laden MBs is promptly absorbed by plants, possibly intensifying the further release of P at soil–water interfaces (Yao et al. 2013), the slow release of P will contribute to reduce P loss and enhance P bioavailability for plants (Wang et al. 2021a, 2021b). The release patterns of the three treatments were consistent, with MB-CP exhibiting the best P release capability.
3.5 Environmental implication
In this study, MBs with stronger P adsorption capacities and magnetism were prepared from three types of emergent plant residues, enhancing P adsorb and enabling efficient recovery using magnets. The 31P-NMR results showed that orthophosphate accounted for the largest proportion of the P constituents in the P-laden MBs and is considered an ideal P fertilizer. Developing sustainable fertilizer as an alternative to conventional P fertilizer will promote environmentally friendly P supply and plant uptake efficiency. We anticipate that MBs-based absorbent will facilitate the reuse of P in additional scenarios, which are also under investigation by our research group.
4 Conclusions
In this study, we provide a novel approach for utilizing emergent plant residues from ecological ditches as P-adsorption materials. By successfully loading Fe compounds onto the surface of the three biochars, as validated by adsorption kinetics, MB-C was observed to have the best P adsorption capacity. Combined with the SEM-EDS, XRD, FTIR, and XPS characterization data analysis, inner-sphere complexation and electrostatic attraction were confirmed to play major roles in controlling P adsorption by the MBs. The P species adsorbed by the MBs was mainly orthophosphate, proving the potential of the MBs as carbon-based P fertilizer. Overall, this study provides an innovative idea for utilizing emergent plant residues as sustainable P resources, as well as proposes a feasible and eco-friendly scheme to reduce P pollution and promote P recycling in agricultural.
Data availability
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
References
Ahmad M, Rajapaksha AU, Lim JE, Zhang M, Bolan N, Mohan D, Vithanage M, Lee SS, Ok YS (2014) Biochar as a sorbent for contaminant management in soil and water: a review. Chemosphere 99:19–33. https://doi.org/10.1016/j.jclepro.2018.11.087
Ai D, Ma H, Meng Y, Wei T, Wang B (2023) Phosphorus recovery and reuse in water bodies with simple ball-milled Ca-loaded biochar. Sci Total Environ 860:160502. https://doi.org/10.1016/j.scitotenv.2022.160502
Ajmal Z, Muhmood A, Usman M, Kizito S, Lu JX, Dong RJ, Wu SB (2018) Phosphate removal from aqueous solution using iron oxides: adsorption, desorption and regeneration characteristics. J Colloid Interface Sci 528:145–155. https://doi.org/10.1016/j.jcis.2018.05.084
An X, Wu Z, Yu J, Cravotto G, Liu X, Li Q, Yu B (2020) Copyrolysis of biomass, bentonite, and nutrients as a new strategy for the synthesis of improved biochar-based slow-release fertilizers. ACS Sustain Chem Eng 8(8):3181–3190. https://doi.org/10.1021/acssuschemeng.9b06483
Cade-Menun BJ, Preston CM (1996) A comparison of soil extraction procedures for 31 P NMR spectroscopy. Soil Sci 161:770–785. https://doi.org/10.1080/00103620600710116
Chen H, Gao Y, Li J, Fang Z, Bolan N, Bhatnagar A, Gao B, Hou D, Wang S, Song H, Yang X, Shaheen SM, Meng J, Chen W, Rinklebe J, Wang H (2022) Engineered biochar for environmental decontamination in aquatic and soil systems: a review. Carbon Res. https://doi.org/10.1007/s44246-022-00005-5
Cheng H, Yang S, Bolan N (2023) Biochar for future and futuristic biochar. Pedosphere 33:680–682. https://doi.org/10.1016/j.pedsph.2023.07.023
Christopher C (2013) Emergent macrophyte biomass production. Methods Biogeochem Wetlands 10:137–153. https://doi.org/10.2136/sssabookser10.c9
Conley DJ, Paerl HW, Howarth RW, Boesch DF, Seitzinger SP, Havens KE, Lancelot C, Likens GE (2009) Controlling eutrophication: nitrogen and phosphorus. Science 323:1014–1015. https://doi.org/10.1126/science.1167755
Dai J, Meng X, Zhang Y, Huang Y (2020) Effects of modification and magnetization of rice straw derived biochar on adsorption of tetracycline from water. Bioresource Technol 311:123455. https://doi.org/10.1016/j.biortech.2020.123455
Dong X, He L, Hu H, Liu N, Gao S, Piao Y (2018) Removal of 17β-estradiol by using highly adsorptive magnetic biochar nanoparticles from aqueous solution. Chem Eng J 352:371–379. https://doi.org/10.1016/j.cej.2018.07.025
Drenkova-Tuhtan A, Mandel K, Paulus A, Meyer C, Hutter F, Gellermann C, Sextl G, Franzreb M, Steinmetz H (2013) Phosphate recovery from wastewater using engineered superparamagnetic particles modified with layered double hydroxide ion exchangers. Water Res 47:5670–5677. https://doi.org/10.1016/j.watres.2013.06.039
Fang Y, Ali A, Gao Y, Zhao P, Li R, Li X, Liu J, Luo Y, Peng Y, Wang H, Liu H, Zhang Z, Pan J (2022) Preparation and characterization of MgO hybrid biochar and its mechanism for high efficient recovery of phosphorus from aqueous media. Biochar 4:40. https://doi.org/10.1007/S42773-022-00171-0
Gao R, Fu Q, Hu H, Wang Q, Liu Y, Zhu J (2019) Highly-effective removal of pb by co-pyrolysis biochar derived from rape straw and orthophosphate. J Hazard Mater 371:191–197. https://doi.org/10.1016/j.jhazmat.2019.02.079
Gong H, Tan Z, Zhang L, Huang Q (2019) Preparation of biochar with high absorbability and its nutrient adsorption–desorption behaviour. Sci Total Environ 694:133728. https://doi.org/10.1016/j.scitotenv.2019.133728
Gusain R, Pandey B, Suthar S (2018) Composting as a sustainable option for managing biomass of aquatic weed Pistia: a biological hazard to aquatic system. J Clean Prod 177:803–812. https://doi.org/10.1016/j.jclepro.2017.12.261
Hao S, Li Q, Qu J, An F, Zhang Y, Yu Z (2018) Neuron-inspired Fe3O4/conductive carbon filament network for high-speed and stable lithium storage. Acs Appl Mater Inter 10:17923–17932. https://doi.org/10.1021/acsami.8b03174
Hartley AM, House WA, Callow ME, Leadbeater BSC (1997) Coprecipitation of phosphate with calcite in the presence of photosynthesizing green algae. Water Res. https://doi.org/10.1016/S0043-1354(97)00103-6
Huang Q, Song S, Chen Z, Hu B, Chen J, Wang X (2019) Biochar-based materials and their applications in removal of organic contaminants from wastewater: state-of-the-art review. Biochar 1:45–73. https://doi.org/10.1007/s42773-019-00006-5
Huang H, Guo T, Wang K, Li Y, Zhang G (2021) Efficient activation of persulfate by a magnetic recyclable rape straw biochar catalyst for the degradation of tetracycline hydrochloride in water. Sci Total Environ 758:143957. https://doi.org/10.1016/j.scitotenv.2020.143957
Iamchaturapatr J, Yi SW, Rhee JS (2007) Nutrient removals by 21 aquatic plants for vertical free surface-flow (VFS) constructed wetland. Ecol Eng 29:287–293. https://doi.org/10.1016/j.ecoleng.2006.09.010
Jiang D, Chu B, Amano Y, Machida M (2018) Removal and recovery of phosphate from water by Mg-laden biochar: batch and column studies. Colloids Surf, A 558:429–437. https://doi.org/10.1016/j.colsurfa.2018.09.016
Jiang Z, Yan L, Wu J, Liu X, Zhang J, Zheng Y, Pei Y (2020) Low-temperature synthesis of carbonate-intercalated NiXFe-layered double hydroxides for enhanced adsorption properties. Appl Surf Sci 531:147281. https://doi.org/10.1016/j.apsusc.2020.147281
Klinar D (2016) Universal model of slow pyrolysis technology producing biochar and heat from standard biomass needed for the techno-economic assessment. Bioresource Technol 206:112–120. https://doi.org/10.1016/j.biortech.2016.01.053
Kröger R, Moore MT (2011) Phosphorus dynamics within agricultural drainage ditches in the lower Mississippi Alluvial Valley. Ecol Eng 37:1905–1909. https://doi.org/10.1016/j.ecoleng.2011.06.042
Kumwimba MN, Zhu B, Muyembe DK (2017) Assessing the influence of different plant species in drainage ditches on mitigation of non-point source pollutants (N, P, and sediments) in the Purple Sichuan Basin. Environ Monit Assess 189:1–14. https://doi.org/10.1007/s10661-017-5965-8
Lei J, Lin J, Zhan Y, Zhang Z, Ma J (2021) Effectiveness and mechanism of aluminum/iron co-modified calcite capping and amendment for controlling phosphorus release from sediments. J Environ Manage 298:113471. https://doi.org/10.1016/j.jenvman.2021.113471
Li R, Wang JJ, Zhou B, Awasthi MK, Ali A, Zhang Z, Lahori AH, Mahar A (2016) Recovery of phosphate from aqueous solution by magnesium oxide decorated magnetic biochar and its potential as phosphate-based fertilizer substitute. Bioresource Technol 215:209–214. https://doi.org/10.1016/j.biortech.2016.02.125
Li J, Li B, Huang H, Lv X, Zhao N, Guo G, Zhang D (2019) Removal of phosphate from aqueous solution by dolomite-modified biochar derived from urban dewatered sewage sludge. Sci Total Environ 687:460–469. https://doi.org/10.1016/j.scitotenv.2019.05.400
Liu J, Cade-Menun BJ, Yang J, Hu Y, Liu CW, Tremblay J, LaForge K, Schellenberg M, Hamel C, Bainard LD (2018) Long-term land use affects phosphorus speciation and the composition of phosphorus cycling genes in agricultural soils. Front Microbiol 9:1643. https://doi.org/10.3389/fmicb.2018.01643
Liu X, Fu J, Tang Y, Smith RL Jr, Qi X (2021) Mg-coordinated self-assembly of MgO-doped ordered mesoporous carbons for selective recovery of phosphorus from aqueous solutions. Chem Eng J 406:126748. https://doi.org/10.1016/j.cej.2020.126748
Liu M, Li R, Wang J, Liu X, Li S, Shen W (2022) Recovery of phosphate from aqueous solution by dewatered dry sludge biochar and its feasibility in fertilizer use. Sci Total Environ 814:152752. https://doi.org/10.1016/j.scitotenv.2021.152752
Loganathan P, Vigneswaran S, Kandasamy J, Bolan NS (2014) Removal and recovery of phosphate from water using sorption. Crit Rev Env Sci Tec 44:847–907. https://doi.org/10.1080/10643389.2012.741311
Malash GF, El-Khaiary MI (2010) Piecewise linear regression: a statistical method for the analysis of experimental adsorption data by the intraparticle-diffusion models. Chem Eng J 163:256–263. https://doi.org/10.1016/j.cej.2010.07.059
MEP (2002) Discharge standard of pollutants for municipal wastewater treatment plant (GB18918-2002). China Environment Press, Beijing
Min L, Zhang ZS, Zhe L, Haitao W (2020) Removal of nitrogen and phosphorus pollutants from water by FeCl3- impregnated biochar. Ecol Eng 149:105792. https://doi.org/10.1016/j.ecoleng.2020.105792
Mitrogiannis D, Psychoyou M, Koukouzas N, Tsoukalas N, Palles D, Kamitsos E, Pantazidis A, Oikonomou G, Baziotis I (2018) Phosphate recovery from real fresh urine by Ca(OH)2 treated natural zeolite. Chem Eng J 347:618–630. https://doi.org/10.1016/j.cej.2018.04.102
Nash DM, Haygarth PM, Turner BL, Condron LM, McDowell RW, Richardson AE, Watkins M, Heaven MW (2014) Using organic phosphorus to sustain pasture productivity: a perspective. Geoderma 221:11–19. https://doi.org/10.1016/j.geoderma.2013.12.004
Ou W, Lan X, Guo J, Cai A, Liu P, Liu N, Liu Y, Lei Y (2023) Preparation of iron/calcium-modified biochar for phosphate removal from industrial wastewater. J Clean Prod 383:135468. https://doi.org/10.1016/j.jclepro.2022.135468
Padilla J, Watts D, Novak J, Cerven V, Ippolito J, Szogi A, Johnson M (2023) Magnesium activation affects the properties and phosphate sorption capacity of poultry litter biochar. Biochar 5:64. https://doi.org/10.1007/s42773-023-00263-5
Palansooriya KN, Kim S, Igalavithana AD, Hashimoto Y, Choi Y, Mukhopadhyay R, Sarkar B, Ok YS (2021) Fe(III) loaded chitosan-biochar composite fibers for the removal of phosphate from water. J Hazard Mater 415:125464. https://doi.org/10.1016/j.jhazmat.2021.125464
Pitakteeratham N, Hafuka A, Satoh H, Watanabe Y (2013) High efficiency removal of phosphate from water by zirconium sulfate-surfactant micelle mesostructure immobilized on polymer matrix. Water Res 47:3583–3590. https://doi.org/10.1016/j.watres.2013.04.006
Reguyal F, Sarmah A, Gao W (2017) Synthesis of magnetic biochar from pine sawdust via oxidative hydrolysis of FeCl2 for the removal sulfamethoxazole from aqueous solution. J Hazard Mater 321:868–878. https://doi.org/10.1016/j.jhazmat.2016.10.006
Shan X, Zhao Y, Bo S, Yang L, Xiao Z, An Q, Zhai S (2021) Magnetic aminated lignin/CeO2/Fe3O4 composites with tailored interfacial chemistry and affinity for selective phosphate removal. Sci Total Environ 796:148984. https://doi.org/10.1016/j.scitotenv.2021.148984
Shepherd JG, Joseph S, Sohi SP, Heal KV (2017) Biochar and enhanced phosphate capture: mapping mechanisms to functional properties. Chemosphere 179:57–74. https://doi.org/10.1016/j.chemosphere.2017.02.123
Singh J, Kumar P, Eid EM, Taher MA, El-Morsy MHE, Osman HEM, Al-Bakre DA, Kumar V (2023) Phytoremediation of nitrogen and phosphorus pollutants from glass industry effluent by using water hyacinth (Eichhornia crassipes (Mart.) Solms): application of RSM and ANN techniques for experimental optimization. Environ Sci Pollut R 30:20590–20600. https://doi.org/10.1007/s11356-022-23601-9
Smit AL, Bindraban PS, Conijn JG, van der Meer HG (2009) Phosphorus in agriculture: global resources, trends and developments. Plant Research International Bv, Wageningen, p 282
Tang X, Lei Y, Yu C, Wang C, Zhang P, Lu H (2023) Highly-efficient degradation of organic pollutants by oxalic acid modified sludge biochar: mechanism and pathways. Chemosphere 325:138409. https://doi.org/10.1016/j.chemosphere.2023.138409
Tran HN, You S, Hosseini-Bandegharaei A, Chao H (2017) Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: a critical review. Water Res 120:88–116. https://doi.org/10.1016/j.watres.2017.04.014
Tu Y, Peng Z, Huang J, Wu X, Kong L, Liang Z, Yang L, Lin Z (2020) Preparation and characterization of magnetic biochar nanocomposites via a modified solvothermal method and their use as efficient heterogeneous Fenton-like catalysts. Ind Eng Chem Res 59:1809–1821. https://doi.org/10.1021/acs.iecr.9b04590
Villanueva ME, Salinas A, Copello GJ, Díaz LE (2014) Point of zero charge as a factor to control biofilm formation of Pseudomonas aeruginosa in sol-gel derivatized aluminum alloy plates. Surf Coat Technol 254:145–150. https://doi.org/10.1016/j.surfcoat.2014.05.074
Wang H, Xiao K, Yang J, Yu Z, Yu W, Xu Q, Wu Q, Liang S, Hu J, Hou H, Liu B (2020a) Phosphorus recovery from the liquid phase of anaerobic digestate using biochar derived from iron−rich sludge: a potential phosphorus fertilizer. Water Res 174:115629. https://doi.org/10.1016/j.watres.2020.115629
Wang Z, Hong S, Wen J, Ma C, Tang L, Jiang H, Chen J, Li S, Shen X, Yuan T (2020b) Lewis acid-facilitated deep eutectic solvent (DES) pretreatment for producing high-purity and antioxidative lignin. Acs Sustain Chem Eng 8:1050–1057. https://doi.org/10.1021/acssuschemeng.9b05846
Wang B, Hu X, Zhou D, Zhang H, Chen R, Guo W, Wang H, Zhang W, Hong Z, Lyu W (2021a) Highly selective and sustainable clean–up of phosphate from aqueous phase by eco–friendly lanthanum cross–linked polyvinyl alcohol/alginate/palygorskite composite hydrogel beads. J Clean Prod 298:126878. https://doi.org/10.1016/j.jclepro.2021.126878
Wang Z, Chen L, Liu C, Jin Y, Li F, Khan S, Liang X (2021b) Reduced colloidal phosphorus loss potential and enhanced phosphorus availability by manure-derived biochar addition to paddy soils. Geoderma 402:115348. https://doi.org/10.1016/j.geoderma.2021.115348
Wang J, Jiang Y, Xu M, Han C, Zhang L, Liu G (2023) Resin-based iron-manganese binary oxide for phosphate selective removal. Environ Sci Pollut Res 30(2):4642–4652. https://doi.org/10.1007/s11356-022-22525-8
Wu B, Wan J, Zhang Y, Pan B, Lo IMC (2020) Selective phosphate removal from water and wastewater using sorption: process fundamentals and removal mechanisms. Environ Sci Technol 54:50–66. https://doi.org/10.1021/acs.est.9b05569
Wu D, Shen C, Cheng Y, Ding J, Li W (2023) Phosphorus removal by aquatic vegetation in shallow eutrophic lakes: a laboratory study. Environ Sci Pollut R 30:16166–16177. https://doi.org/10.1007/s11356-022-23403-z
Xie F, Wu F, Liu G, Mu Y, Feng C, Wang H, Giesy JP (2014) Removal of phosphate from eutrophic lakes through adsorption by in situ formation of magnesium hydroxide from diatomite. Environ Sci Technol 48:582–590. https://doi.org/10.1021/es4037379
Xie F, Li L, Song K, Li G, Wu F, Giesy JP (2019) Characterization of phosphorus forms in a eutrophic lake, China. Sci Total Environ 659:1437–1447. https://doi.org/10.1016/j.scitotenv.2018.12.466
Xu Q, Chen Z, Wu Z, Xu F, Yang D, He Q, Li G, Chen Y (2019) Novel lanthanum doped biochars derived from lignocellulosic wastes for efficient phosphate removal and regeneration. Bioresource Technol 289:121600. https://doi.org/10.1016/j.biortech.2019.121600
Yamashita T, Hayes P (2008) Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl Surf Sci 254:2441–2449. https://doi.org/10.1016/j.apsusc.2007.09.063
Yang Q, Wang X, Luo W, Sun J, Xu Q, Chen F, Zhao J, Wang S, Yao F, Wang D, Li X, Zeng G (2018) Effectiveness and mechanisms of phosphate adsorption on iron-modified biochars derived from waste activated sludge. Bioresource Technol 247:537–544. https://doi.org/10.1016/j.biortech.2017.09.136
Yang J, Wang Z, Li R, Xu X, Liu J, Huang Y, Ye X, Wang W (2022) Effect of Fe (III) modification on the phosphorus removal behavior of Ce (III) carbonate adsorbents. ACS Omega 7(36):31767–31777. https://doi.org/10.1021/acsomega.2c02269
Yao Y, Gao B, Chen J, Yang L (2013) Engineered biochar reclaiming phosphate from aqueous solutions: Mechanisms and potential application as a slow-release fertilizer. Environ Sci Technol 47:8700–8708. https://doi.org/10.1021/es4012977
Yi Y, Tu G, Zhao D, Tsang P, Fang Z (2019) Biomass waste components significantly influence the removal of Cr(VI) using magnetic biochar derived from four types of feedstocks and steel pickling waste liquor. Chem Eng J 360:212–220. https://doi.org/10.1016/j.cej.2018.11.205
Yin Q, Ren H, Wang R, Zhao Z (2018) Evaluation of nitrate and phosphate adsorption on Al-modified biochar: influence of Al content. Sci Total Environ 631:895–903. https://doi.org/10.1016/j.scitotenv.2018.03.091
Yu C, Shi C, Ji M, Xu X, Zhang Z, Ma J, Wang G (2019) Taste and odor compounds associated with aquatic plants in Taihu lake: distribution and producing potential. Environ Sci Pollut R 26:34510–34520. https://doi.org/10.1007/s11356-019-06188-6
Yu J, Feng H, Tang L, Pang Y, Wang J, Zou J, Xie Q, Liu Y, Feng C, Wang J (2021) Insight into the key factors in fast adsorption of organic pollutants by hierarchical porous biochar. J Hazard Mater 403:123610. https://doi.org/10.1016/j.jhazmat.2020.123610
Zhang B, Wang L, Li Y (2019) Fractionation and identification of iron-phosphorus compounds in sewage sludge. Chemosphere 223:250–256. https://doi.org/10.1016/j.chemosphere.2019.02.052
Zhao D, Qiu S, Li M, Luo Y, Zhang L, Feng M, Yuan M, Zhang K, Wang F (2022) Modified biochar improves the storage capacity and adsorption affinity of organic phosphorus in soil. Environ Res 205:112455. https://doi.org/10.1016/j.envres.2021.112455
Zhou Z, Liu Y, Liu S, Liu H, Zeng G, Tan X, Yang C, Ding Y, Yan Z, Cai X (2017) Sorption performance and mechanisms of arsenic (V) removal by magnetic gelatin-modified biochar. Chem Eng J 314:223–231. https://doi.org/10.1016/j.cej.2016.12.113
Acknowledgements
We acknowledge the Key Research and Development Project of Science and Technology Department of Zhejiang Province (2023C02016; 2023C02019), the National Key Research and Development Program of China (2022YFD1700704-3), the Bingtuan Science and Technology Program (2021DB019), and the Key Laboratory of Environment Remediation and Ecological Health, Ministry of Education, College of Environmental and Resource Sciences, Zhejiang University.
Funding
This study was funded by the Key Research and Development Project of Science and Technology Department of Zhejiang Province (2023C02016; 2023C02019), the National Key Research and Development Program of China (2022YFD1700704-3), and Bingtuan Science and Technology Program (2021DB019).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by HX, JY, YL, HX, HW, KME, XZ, CL, YF, YY and XL. The first draft of the manuscript was written by HX and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Handling editor: Xiangzhou Yuan.
Supplementary Information
Additional file 1:
Fig.S1. Magnetic biochar dispersed (a) and gathered under the power of magnets (b). Fig.S2. (a) The kinetic release of P from P-laden MBs. (b) The cyclical P release of P-laden MBs at 72 hours.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xin, H., Yang, J., Lu, Y. et al. Potentials of emergent plant residue derived biochar to be alternative carbon-based phosphorus fertilizer by Fe(II)/Fe(III) magnetic modification. Biochar 6, 15 (2024). https://doi.org/10.1007/s42773-024-00300-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42773-024-00300-x