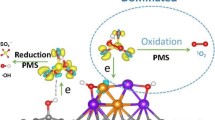
Abstract
Nonradical oxidation based on peroxydisulfate (PDS) activation has attracted increasing attention for selective degradation of organic pollutants. Herein, topological defects were introduced into biochar (BC) via removing N atoms in N-doped BC (NBC) in an attempt to improve the nonradical catalytic performance. Compared to the pristine BC and NBC, the introduction of topological defects could achieve up to 36.6- and 8.7-times catalytic activity enhancement, respectively. More importantly, it was found that the catalytic activity was dominated by topological defects, which was verified by the significant positive correlation between the pseudo-first-order rate constants and the content of topological defects. Theoretical calculations suggested that topological defects enhanced the electron-donating ability of BC by reducing the energy gap, which made the electrons transfer to PDS molecules more easily. As a result, holes were generated after the carbon defects lost electrons, and induced a nonradical oxidation process. Benefiting from the merits of nonradical oxidation, the developed BC/PDS system showed superior performance in removing electron-rich contaminants in the presence of inorganic anions and in the actual environments. This study not only provides a potential avenue for designing efficient biochar-based catalysts, but also advances the mechanism understanding of nonradical oxidation process induced by carbon defects.
Graphical Abstract

Highlights
-
Biochar full of topological defects (NBC-T) was developed as excellent PDS catalyst for tetracycline degradation.
-
The catalytic activity of NBC-T was found to be positively correlated with the content of topological defects.
-
Topological defects with narrow energy gap facilitate electron-transfer thus promoting the generation of holes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Persulfates-based advanced oxidation processes driven by metal-free carbocatalyst have attracted extensive attention to deal with environmental pollution caused by organic contaminants in water and soil (Chen et al. 2023; Tang et al. 2023; Wang et al. 2022b; Yu et al. 2020). Biochar (BC) is a porous carbon material with high specific surface area that could be prepared from waste biomass (Ashraf et al. 2022). Due to its low cost, widely available, and environmental friendliness, BC was considered as a potential candidate for activating persulfates [peroxymonosulfate (PMS) or peroxydisulfate (PDS)] (Fang et al. 2015; Liu et al. 2015; Shi et al. 2022; Wan et al. 2021). Generally, the system with biochar/persulfates could degrade contaminants through radical or nonradical process (Duan et al. 2015, 2018). The radical reactive oxygen species (ROSs) possess the merits of higher mineralization efficiency, while suffering from the disadvantages of instability and consumption by the background matrix (Qian et al. 2021). On the contrary, the nonradical ROSs with moderate oxidative potential were generally considered resistant to the inorganic ions and could selectively degrade electron-rich contaminants in the actual environments (Gao et al. 2020; Mi et al. 2021; Shao et al. 2018). Therefore, regulating the nonradical catalytic performance of BC is of significant importance for its practical application in environmental remediation.
Previous mechanistic studies have suggested that the electronic structure of BC plays an important role during persulfate activation (Wang et al. 2019a; Zhu et al. 2018). Several strategies such as heteroatoms doping (Dou et al. 2022; Liu et al. 2020; Zhu et al. 2018) or regulating oxygen functional groups (OFGs) (Sun et al. 2020) were explored to optimize the electronic environment in order to promote the nonradical activity of BC. However, the heavy doping of heteroatoms may reduce the electronic conductivity, and the introduction of OFGs would generate free radicals (Shi et al. 2022; Zhao et al. 2015). So far, there is still a lack of electron modulation strategies to promote the nonradical catalytic activity of BC with high reactivity and selectivity.
Intrinsic defects have recently emerged to modulate the electron-transfer efficiency of metal-free carbonaceous materials (Jia et al. 2019; Wang et al. 2019b). Compared with the extrinsic defects induced by heteroatom doping, intrinsic defects are the lattice imperfection caused by the arrangement of carbon atoms themselves (Zhu and Mu 2020). Due to the high energy requirement of directly removing lattice carbon atoms, the commonly used strategy of regulating intrinsic defects is removing the doped heteroatoms in the carbon framework (Dong et al. 2020). Previous studies suggested that the removal of oxygen and sulfur could result in the formation of edge and vacancy and promote the generation of surface confined reactive species or singlet oxygen, thus enhancing the nonradical activity (Liu et al. 2022; Miao et al. 2022). However, the large atomic radii of O and S normally allow them load to the edge of carbon framework at low content (Xiao et al. 2023). As a result, the catalytic activities of the obtained materials after removing O and S were usually poor. In contrast, removing the high loading content of N atoms in carbon framework provided an effective route for deliberately creating defects. Recent studies suggested that removing N atoms could generate topological defects in carbon framework (Jia et al. 2019; Li et al. 2020). These nonhexagonal topological defects exhibit quite different electronic and geometric structures from the regular hexagonal carbocycles and could affect the electrocatalytic performance of carbonaceous materials (Zhu and Mu 2020). For instance, topological defects were introduced into graphene by removing N atoms, and the obtained materials presented excellent activity for hydrogen evolution, oxygen reduction, and oxygen evolution (Jia et al. 2016). Another work suggested that the reaction activity of porous carbon particles rich in pentagon and octagon toward electrocatalytic CO2 reduction was significantly promoted owing to the local charge redistribution induced by topological defects (Dong et al. 2020). Therefore, it is expected that the introduction of topological defects into BC could facilitate electron transfer efficiency, thus satisfying the demands for nonradical activation of persulfate with high reactivity and selectivity.
Herein, biochars with abundant topological defects were synthesized via removing N from N-doped biochar and their catalytic performance in PDS activating were explored. PDS was selected as the model oxidant due to its low cost and higher intrinsic redox potential (Ren et al. 2020; Zhu et al. 2018). Tetracycline (TC) as a common antibiotic, which is widely detected in water and soil and poses a serious threat to the environment and human health, was selected as the model pollutant to investigate the catalytic performance of the material. Quantitative structure–activity relationship and theoretical calculations were used to reveal the potential activation sites. The ROSs generated during PDS activation were detailed investigated by electron paramagnetic resonance (EPR), quenching experiments, Raman spectra, PDS consumption monitoring, and electrochemical measurements. This work provides a promising strategy to design BC-based catalysts with outstanding nonradical catalytic activity.
2 Materials and methods
2.1 Chemicals
The spin trapping agents 5,5-dimethyl-pyrroline-oxide (DMPO) and 2,2,6,6,-tetramethyl-4-piperidinol (TEMP) were obtained from Dojindo Laboratories in Shanghai, China, and their purity were higher than 99%. Sulfamethoxazole (SMX, 98%) was purchased from TCI Development Co., Ltd. in Shanghai, China. 2,2,6,6-tetramethylpi-peridine-1-oxyl (TEMPO, 98%), TC (96%), bisphenol A (BPA, AR), 4-chlorophenol (4-CP, AR), rhodamine B (Rhb, AR), benzoic acid (BA, > 99.0%), and deuterium oxide (D2O, 99% atom D) were purchased from Aladdin Co. Ltd., China. Other chemicals used in this work were at least of analytic grade.
2.2 Materials
Topological defect-rich biochars were synthesized by a N-removal method (Jia et al. 2016). Firstly, the N-doped biochar (NBC) was prepared to improve the N content of biochar. Pine sawdust was washed with deionized water three times and then dried at 80 °C. 5.0 g of dried sawdust was mixed with 25.0 g of urea and ground in an agate mortar. The mixture was heated to 700 °C (5 °C min−1) for 2 h under nitrogen protection. After cooling naturally, the pyrolysis residue was collected, ground to pass through an 80-mesh sieve for usage. Then, the obtained NBC was further annealed at 700 °C, 850 °C, 1000 °C, and 1150 °C for 2 h to remove the doped N atoms and produce topological defects. The obtained materials were denoted as NBC-T, where T represented the annealed temperature.
For comparison, BC was synthesized with the similar method to NBC without adding urea. To investigate the influence of N-precursors, dicyandiamide and melamine were used to replace urea to prepare N-doped biochar (denoted as NBCD and NBCM, respectively), and then annealed at 1000 °C under nitrogen atmosphere (denoted as NBCD-1000 and NBCM-1000, respectively).
2.3 Characterization
The surface elemental contents and category were analyzed by X-ray photoelectron spectroscopy (XPS, ESCALab 250Xi, Thermo Fisher) with Al Kα radiation at 1486.6 eV. The total N content on the surface and in the bulk of samples was quantified by XPS full spectrum analysis and Element analyzer (Elenemtar Analysensysteme GmbH, Vario Micro), respectively. The powder X-ray diffraction (XRD) patterns were carried out on an X-ray diffractometer (German Bruker D8 Advance Davinci) with Cu Kα radiation source (λ = 0.154 nm). The surface morphologies of biochars were recorded on a field emission scanning electron microscope (FE-SEM, Zeiss GEMINI 300). Aberration-corrected high-resolution transmission electron microscopy (AC-HRTEM) images were acquired by aberration-corrected transmission electron microscopy (FEI Titan G2 60-300) with an acceleration voltage of 80 kV. Raman spectroscopy was collected from a confocal LabRAM HR Evolution (Horiba, France) spectrometer with a laser at 532 nm. The Brunaure–Emmett–Teller (BET) surface area was measured by N2 adsorption/desorption isotherms at liquid nitrogen temperature (77 K) on a physisorption instrument (ASAP2460, Micromeritics). Thermogravimetry-mass spectrometry (TG-MS, Perkin Elmer Pyris Diamond TG/DTA coupled with Omnistar) was used to investigate gas products during annealing at a heating rate of 5 °C min−1 under nitrogen atmosphere.
2.4 Catalytic degradation experiments
TC degradation experiments were performed in a conical flask at room temperature. Typically, 10 mg of the catalyst was first added into 100 mL TC solution (0.1 mM) and stirred for 60 min to reach adsorption equilibrium (the pre-adsorption experiment is shown in Additional file 1: Fig. S1). After that, the degradation reaction was started with addition of PDS stock solution (final concentration of PDS is 1.0 mM). At predetermined time intervals, 1.0 mL reaction solution was withdrawn, filtered through a 0.22 μm millipore filter, and immediately quenched with 0.5 mL methanol. The pH value of the reaction solution was not adjusted (pH = 4.2) except in the experiments investigating the influence of pH (adjusted by diluted HCl and NaOH). Other factors, such as TC concentration, PDS dosage, and naturally occurring substances on the performance, were also investigated. To investigate the reusability of catalyst, NBC-1000 was collected from the solution after reaction, washed with water for 3 times, and dried in an oven at 80 °C overnight for next cycle. Moreover, the catalytic activity was further evaluated by degradation of SMX, BPA, 4-CP, Rhb, and BA.
2.5 Analytic methods
UV–vis spectrophotometer and high-performance liquid chromatography were adopted to analyze the concentration of the contaminants (i.e., TC, BPA, SMX, 4-CP, Rhb, BA). The detecting parameters are listed in Additional file 1: Table S1. A modified spectrophotometric method was adopted to measure the concentration of PDS at 352 nm (Liang et al. 2008). EPR measurements (Bruker A300 EPR spectrometer, Germany) were carried out to investigate the ROSs during the reaction (see Additional file 1: Text S1 for details). Electrochemical tests were performed on an electrochemical working station (CHI-760E, China). The operational methods are provided in Additional file 1: Text S2.
2.6 Computational methods
The electronic structure of hexagon and pentagon was investigated by Density functional theory (DFT) calculations. All-electron DFT calculations have been carried out by the latest version of ORCA quantum chemistry software (Version 5.0.1) (Frank 2018). The BLYP functional and def2-SVP basis set were adopted for all geometry optimization calculations (Becke 1993). The singlet point energy calculations were performed with B3LYP functional and a larger basis set def2-TZVP basis set4. The SMD implicit solvation model9 was used to account for the solvation effect of water. The DFT-D3 with BJ-damping (Grimme et al. 2011) was used to correct the weak interaction to improve the calculation accuracy. Orbital energy level analysis was performed by Multiwfn software (Lu and Chen 2012). The visualization of the orbitals was achieved using VESTA software.
3 Results and discussion
3.1 Evaluation of catalytic activity
The catalytic activity of annealed N-doped BCs for PDS activation was investigated the degradation of TC. As shown in Fig. 1a, PDS alone and BC/PDS system exhibited a very limited degradation of TC (4.6% and 11.0%, respectively). 32.4% of TC could be degraded in 120 min with NBC as a catalyst, suggesting that N-doping could slightly enhance the catalytic activity of biochar. Notably, the catalytic performance of N-doped BC was greatly improved after annealing. For instance, 94.6% of TC could be degraded in the NBC-1000/PDS system. The TC degradation kinetics were further studied by fitting the results to pseudo-first-order model to compare the catalytic activities more clearly. As shown in Fig. 1b, the obtained rate constant (kobs) could be enhanced by increasing the thermal temperature from 700 to 1000 °C. For NBC-1000, the kobs (0.0658 min−1) was nearly 36.6 and 8.7 times higher than that of BC (0.0018 min−1) and NBC (0.0076 min−1), respectively. The slightly lower activity of NBC-1150 could be due to the recombination of topological defects, thus resulting in a decrease of active sites (Miao et al. 2022). Considering the influence of reaction conditions, the catalytic performance of the annealed BCs was further evaluated by a normalized kinetic (k-value) (Bingqing Wang et al. 2022a). And it could be found that the k-value of NBC-1000 overpassed all of other reported metal-free biochars for PDS activation, even comparable to the single-atom catalysts (Additional file 1: Table S2). In addition, dicyanamide and melamine were used instead of urea to investigate the influence of nitrogen source. As shown in Additional file 1: Fig. S2, the kobs of samples annealed at 1000 °C could still improve by 17.3 and 27.2 times relative to BC, suggesting that annealing is a versatile strategy to enhance catalytic activity for N-doped BC prepared with various nitrogen sources.
3.2 Topological defects dominated catalytic performance
To explore the factors that influenced the catalytic performance, quantitative structure–activity relationship was conducted. The characteristic peaks at 2θ = 25° and 43° on the XRD spectra of NBC and NBC-T (Additional file 1: Fig. S3) could be attributed to (002) and (111) reflections of graphitic carbon, respectively (Shao et al. 2020; Wang et al. 2019b). Previous studies reported that specific surface area, graphitization degree, C=O content, and C–O content was beneficial to electron transfer in PDS activation (Ge 2022; Ke et al. 2022; Shao et al. 2020). However, the correlation coefficient of kobs and these factors were relatively poor, which are shown in Additional file 1: Figs. S4–S6. Moreover, it could be observed from Additional file 1: Fig. S7a and Table 1 that the C=O and C–O contents of NBC-1000 were increased after reaction. These results indicated that these factors played a limited role in PDS activation.
N-doping significantly enhanced the N content of BC (from 0.11 wt% in BC to 19.65 wt% in NBC), and the N atoms could be removed during annealing. It could be seen from Additional file 1: Fig. S8a that NBC exhibited good thermal stability before 700 °C, while the mass gradually decreased as the temperature further increased due to the decomposition of N-containing functional groups. As shown in Fig. 2a and Table 1, the total N content both on the surface and in the bulk of materials showed a gradually decreasing trend as the annealing temperature increased, suggested that a higher annealing temperature would result in a significant decrease of N content. The N 1s spectra of materials in Fig. 2b showed four peaks at 398.3, 400.0, 401.1, and 403.3 eV, assigned to the pyridinic N, pyrrolic N, graphitic N, and oxidized N, respectively (Guo et al. 2016). Interestingly, the proportion of pyridinic N and pyrrolic N (edge N) was substantially decreased as the annealing temperature increased, while most of the graphitic N remained. For instance, compared to the original NBC, 91.8% pyridinic N and 99.6% pyrrolic N were selectively removed under thermal annealing at 1000 °C (Table 1), which accounted for 82.2% of the total removed N species. In comparison, 67.5% graphitic N still reserved in NBC-1000 due to its higher thermal stability (Dong et al. 2020; Wang et al. 2020b). In addition, it could be found that there was a well fitted negative correlation between kobs and the nitrogen content (Fig. 2c). Similar phenomenon was also observed in other biochar/persulfate systems (Cai et al. 2021; Wan et al. 2021), i.e., the less the nitrogen content, the better the catalytic performance. The higher correlation coefficients of pyridinic N and pyrrolic N than graphitic N may be due to the higher thermal stability of graphitic N. This may also be due to the fact that graphite N serve as active site during PDS activation, which was identified by the decrease of the content of graphitic N after reaction (Additional file 1: Fig. S7b and Table 1). However, considering the lower performance of NBC with higher graphitic N content, its role was limited. The N species were usually acknowledged as active sites to activate peroxymonosulfate to generate ROSs in the previous work (He et al. 2022a; Miao et al. 2020). However, it was found that the N species were inert for activating PDS. The carbon framework, rather than N configuration, was suggested to be the real active sites in PDS activation by N-doped carbonaceous materials (Ren et al. 2020). Therefore, the negative linearity correlation of kobs and N species suggested new active sites were generated on carbon network after removing N atoms.
TG-MS was carried out to explore the form of N removal during thermal annealing. Two possible products, NH3 and HCN, were detected, which represented that N atoms were removed as individual atoms or as bonded carbon atoms, respectively. As shown in Additional file 1: Fig. S8b, there was almost no signal of HCN during the annealing process, while a significant signal at m/z = 17 representing NH3 could be observed. These results suggested that the removal of doped N atoms would not take away adjacent C atoms, which was consistent with previous studies about N-doped graphene (Wang et al. 2020b). As a result, N vacancies or topological defects would generate in the carbon framework. Previous studies revealed that the decomposition of nitrogen dopant could form nitrogen vacancies in the carbon plane and thus improve catalytic performance (Wan et al. 2021). However, the characteristic peak intensity of nitrogen vacancies for NBC and NBC-T continuously decreased as the annealing temperature increased (Additional file 1: Fig. S9), which was contrary to the tendency of catalytic performance (Fig. 1a).
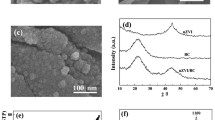
Besides, removing edge N may lead to the reconstruction of carbon atoms in carbon framework, which could create topological defects with the form of pentagon (Dong et al. 2020; Wang et al. 2020b). As shown in Fig. 3a, the SEM image of NBC showed a loose and porous morphology. In comparison, NBC-1000 showed a distinctly compact and rough structure (Fig. 3b), which may be attributed to the local structure reconstruction caused by the rearrangement of carbon atoms after nitrogen removal (Guan et al. 2020). More importantly, with the aid of AC-HRTEM, pentagonal rings were directly observed in NBC-1000 (Fig. 3c, d), demonstrating conclusively the formation of pentagonal defect with the removal of edge N.
Raman spectroscopy was carried out to quantitatively characterize the topological defect content in catalysts (Fig. 4a). AD/AG obtained by calculating the area of D band (ca. 1350 cm−1) and G band (ca. 1590 cm−1) was employed as an index of defect content. Previous studies suggested that N heteroatoms serving as extrinsic defects could influence the results of Raman spectroscopy (Niu et al. 2017; Wan et al. 2021; Zhang et al. 2017). Therefore, the spectra were deconvoluted into four characteristic peaks to deduct the influence of N dopants on AD/AG. There was a positive linear correlation between the density of defects and the content of removed edge N (Additional file 1: Fig. S10), implied that the generation of pentagonal defects is mainly due to the selective removal of edge N dopants. Moreover, the positive correlation between kobs and AD/AG shows a very significant linearity change (R2 = 0.99) in Fig. 4b, demonstrating conclusively that the improvement of catalytic activity was induced by the introduction of topological defects.
To further understand the intrinsic catalytic performance of topological defects, DFT calculations were conducted. The optimized configurations are displayed in Fig. 4c. The pentagonal ring (C5), identified in AC-HRTEM images, was selected as the representative of topological defects in DFT calculations. The intact hexagonal ring (C6) was used as a control model for comparison. The value of highest occupied molecular orbital (HOMO) could reflect the potential of electron-donating (Pan et al. 2023). As shown in Fig. 4c, the pentagon possessed a higher HOMO value than hexagon, suggesting that the introduction of pentagon could make it more easier for BC to donate electrons. Moreover, the pentagon displayed a much smaller energy gap of HOMO-lowest unoccupied molecular orbital (LUMO) compared to that of the intact hexagon, further indicating the high reactivity of pentagon than hexagon in the process of electron transfer (Zhu et al. 2019). The densities of states (DOS) of these two models are presented in Fig. 4d. Compared with C6 (0.40 eV), the Fermi level (Ef) of C5 shifted upward, suggesting that pentagon could modulate the electron distribution of carbon network and thus lead to a higher electron-transfer efficiency of catalysts with PDS (Shao et al. 2020). Therefore, the introduction of pentagon in biochar was beneficial for enhancing catalytic performance owing to the reduced energy barrier and optimized electronic structure. Furthermore, it has been reported that the introduction of defects could enhance the adsorption energy between materials and persulfate, leading to a higher oxidant adsorption capacity (Yang et al. 2021). As shown in Additional file 1: Fig. S11, NBC-1000 exhibited a much higher PDS adsorption capacity than NBC, suggested that the pentagon could promote the adsorption of PDS.
In sum, thermal annealing treatment selectively removed edge N from the C networks and created abundant pentagon defects at the edge of carbon plane, which enhanced the electron mobility because of the smaller energy gap than the intact hexagon in the catalyst, thus boosting the catalytic activity of biochar.
3.3 Identification of reactive species during PDS activated by topological defects
NBC-1000 was selected as the model catalyst to explore the ROSs generated from PDS catalyzed by topological defects. As exhibited in Fig. 5a, with the addition of DMPO (the trapping agent of SO4.− or HO·), no characteristic peaks of radicals were observed, indicating the absence of free radicals. This was also confirmed by the quenching experiments (Fig. 5b). High concentrations of methanol (MeOH) and tert-butanol (TBA) (radical scavengers for SO4.− or HO·, respectively) displayed negligible inhibitory effect on the TC degradation (Gao et al. 2021). The role of surface-bound radical was also excluded due to the very little inhibition effect of dimethyl sulfoxide (DMSO) on TC degradation (Miao et al. 2022). The role of singlet oxygen (1O2) was explored by EPR measurements and solvent substitution experiments. In brief, no signal of TEMP-1O2 was observed (Fig. 5a), and the replacement of reaction solvent had no significant effect on TC oxidation (Additional file 1: Fig. S12), implying the minor role of 1O2 (Gao et al. 2020; Wang et al. 2021a).
EPR spectra of NBC-1000/PDS with (a) DMPO, TEMP, and (f) TEMPO as trapping agents, effect of scavengers on TC degradation in NBC-1000/PDS system (b), the influence of premixing NBC-1000 and PDS on TC degradation (c), the decomposition of PDS under different conditions (d), and in situ Raman spectra of NBC-1000/PDS (e). Experimental conditions: catalyst dose = 0.1 g L−1, [PDS]0 = 1 mM, [TC]0 = 0.1 mM, [DMPO]0 = [TEMP]0 = 100 mM, [TEMPO]0 = 0.05 mM, [MeOH]0 = [TBA]0 = 100 mM, [DMSO]0 = 10 mM
Considering the limited role of radicals and 1O2, the electron transfer pathway was investigated through electrochemical measurements. Electrochemical impedance spectroscopy (EIS) of the products is shown in Additional file 1: Fig. S13a. NBC-1000 exhibited a smaller semicircle than that of NBC, indicating that topological defects could improve the charge transfer capability of the catalysts. The linear sweep voltammetry (LSV) showed a jump increase of current when PDS was added into the system (Additional file 1: Fig. S13b). Another current increase could be observed with the injection of TC, which confirmed the existence of electron transfer among catalyst surface, PDS and TC (Wang et al. 2019a). Moreover, the direction of electron transfer was revealed by chronoamperometry tests (Additional file 1: Fig. S13C). Adding PDS generated a negative current, whereas the addition of TC gave rise to a positive current which showed a gradually rising tendency. These results suggested that the TC degradation was induced by the electron transfer from TC to PDS molecules, in which the catalyst acted as the electron mediator (Shao et al. 2020).
The electron transfer pathway in the system of NBC5-1000/PDS was also confirmed by the premixing experiment of NBC5-1000 and PDS (He et al. 2022b). Compared with free radicals and singlet oxygen, the ROSs of electron transfer normally possess much longer lifetime. Therefore, the premixing of catalyst and oxidant usually does not inhibit the catalytic performance (Miao et al. 2022). As shown in Fig. 5c, no significant inhibition on the TC degradation performance could be observed in the NBC5-1000/PDS system, even if the premixing time reached 30 min. Moreover, the addition of TC led to a higher PDS consumption of the NBC-1000/PDS system (Fig. 5d), further indicating the system was dominated by the electron transfer pathway (Sun et al. 2020).
In situ Raman analysis was further conducted to investigate the generation of surface activated PDS molecules, which previously suggested to trigger electron transfer (Ren et al. 2022). However, no new characteristic peak could be observed in Raman spectra (Fig. 5e), except for a small peak representing sulfate ions, indicating the electron transfer pathway was induced by other reactive species (Dong et al. 2022; Miao et al. 2022). A significant upshift of the D and G bands of NBC-1000 could be observed in Raman spectra, suggesting that PDS could act as an electron acceptor attracting electrons from topological defects and could result in the formation of holes on NBC-1000 (Wang et al. 2021b). The increase of O content of NBC-1000 after reacting PDS also indicated that NBC-1000 could be oxidized by PDS (Additional file 1: Fig. S7a and Table 1). EPR with TEMPO as the spin label agent was carried out to detect the generation of holes in NBC-1000/PDS system. As previous studies reported, the TEMPO could react with h+ and lead to a decrease of the signal intensity (Liu et al. 2019; Wang et al. 2022c). It can be observed from Fig. 5f that the signal intensity of TEMPO-h+ was significantly decreased with the addition of PDS. And it became more weaker as time increased, suggesting that holes were generated during the PDS activation by NBC-1000.
To further confirm the generation of holes, ethylenediamine tetraacetic acid disodium (EDTA-2Na, scavenger for hole) was added into the system of NBC-1000/PDS (Wang et al. 2022b). It can be seen from Fig. 5b that adding EDTA-2Na resulted in a sharply decrease on TC degradation, and the inhibitory effect would be further enhanced with the increasing amount of EDTA-2Na. Moreover, when TC or EDTA-2Na (electron donors) was added into the system, a noticeable increase of PDS decomposition could be observed since holes could further grab electrons from electron-rich substances (Fig. 5d). The higher PDS consumption rate of EDTA-2Na than that of TC could be attributed to its higher electron-donating capabilities (Wang et al. 2022b). In addition, the generation of holes could be analyzed by the frontier molecular orbital theory. In the electron transfer pathway, electrons localized at the HOMO of catalyst could be injected into the LUMO of PDS molecules (ELUMO (PDS) = 2.81 eV) owing to the potential energy difference (Dou et al. 2022; Shao et al. 2020). The energy gap of pentagon between HOMO of pentagon and LUMO of PDS was − 7.61 eV, higher than that of hexagon (− 8.02 eV). The narrower energy gap could make the electrons located in the HOMO of materials transfer to the LUMO of PDS more easily, which was favorable for the generation of holes. Therefore, it could be concluded that holes were main ROSs in NBC-1000/PDS system, inducing the degradation of contaminants via electron transfer.
A plausible mechanism of activating PDS by topological defects is proposed in Fig. 6 according to the above observations. Defects could optimize the electronic structure of carbon network and promote the adsorption of PDS by biochar. These adsorbed PDS molecules could attract electrons from the defect sites and decompose into sulfate ions without releasing free radicals. Holes were generated in the carbon framework after BC lost electrons. At the same time, holes could gain electrons from the preadsorbed TC molecules due to the energy potential difference. During the electron transfer process, TC serving as electron donors could transfer electrons to PDS through defect sites on biochar, realizing the nonradical oxidation. Thermal annealing removed N atoms at the edge of the carbon framework, introducing abundant pentagonal defects on the biochar. The pentagon reduced the gap between HOMO of BC and LUMO of PDS, and more free-flowing π electrons of BC could be injected into PDS, thereby greatly improving the catalytic performance.
3.4 Application potential of topological defect-rich BC
The nonradical pathway normally possessed high selectivity and degradation efficiency towards electron-rich substances such as antibiotics, phenol/chlorophenols, and dyes. On the other hand, when the pollutant was functioned with electron withdrawn groups, it normally exhibited poor degradation performance (Duan et al. 2018). In this study, electron-rich contaminants (e.g., TC, BPA, SMX, 4-CP, and RhB) and pollutants functioned with electron withdrawn groups (e.g., BA) were used to investigate the selectivity of NBC-1000/PDS system. As shown in Fig. 7a, NBC-1000/PDS system exhibited a good catalytic performance to remove TC, BPA, SMX, 4-CP, and RhB. However, almost no BA could be degraded by NBC-1000/PDS system. This was consistent with previous reports about nonradical pathway, indicating the high selectivity of the NBC-1000/PDS system towards different organic contaminants (Dou et al. 2022; Shao et al. 2020).
Degradation of various pollutants by the NBC-1000/PDS system (a), the influence of TC concentration (b) and PDS concentration (c) on the degradation performance, the reusability of NBC-1000 (d), and effect of initial pH value (e) and naturally occurring substances (f) on TC degradation in NBC-1000/PDS system. Experimental conditions: catalyst dose = 0.1 g L−1, [PDS]0 = 1 mM, [TC]0 = [BPA]0 = [SMX]0 = [4-CP]0 = [Rhb]0 = [BA]0 = 0.1 mM, [Cl−]0 = [NO3−]0 = [HCO3−]0 = [HPO42−]0 = 10 mM
As shown in Additional file 1: Fig. S14, the system exhibited much better catalytic performance with the TC concentration decreasing. For instance, it only took 20 min to remove more than 90% of TC when the concentration was 0.02 mM, while 80 min was needed when the initial concentration of TC was 0.1 mM. And the kobs of NBC-1000/PDS system was gradually increased as the TC concentration decreased (Fig. 7b), indicating that the materials could exhibit satisfactory performance towards the degradation of different concentration range of pollutants. It can be observed from Fig. 7c that the TC degradation performance of NBC-1000/PDS system remained almost unchanged as the PDS concentration reduced. The slight decrease of removal efficiency after 60 min maybe caused by the high concentration of pollutants, which could be verified by the degradation experiments at lower TC concentration (Additional file 1: Fig. S15). These results suggested that PDS concentration played a limited role on the catalytic activity of NBC-1000. Therefore, the impact of sulfate ion could be avoided by adjusting the dosage of PDS due to the low concentration of pollutants in the actual environments.
The reusability test of NBC-1000 was carried out (Fig. 7d). The degradation efficiency of TC decreased from 94.6% to 69.9% after 3 cycles, which may be due to the covering of active sites by degradation intermediates produced under moderate redox potential in nonradical system (Miao et al. 2022; Wang et al. 2020a). After thermal treatment at 400 °C, the adsorbed intermediates were nearly removed completely and 92.3% TC removal was achieved with the recycled catalyst, suggesting that the catalytic performance of NBC-1000 could be recovered through annealing.
In addition, it can be seen from Fig. 7e that the system of NBC-1000/PDS maintained over 92.0% TC degradation in a broad initial pH range from 4.2 to 9.8. With the pH value further increased to 11.0, a decrease of TC degradation efficiency could be observed. High alkalinity usually leads to a negatively charged surface of the carbonaceous materials, resulting in a high electrostatic repulsion between the materials and PDS, thus exhibiting a reduced TC degradation efficiency (Xiong et al. 2022). Moreover, the inorganic anions commonly present in the environment exhibited little influence on TC degradation (Fig. 7f). Even in the actual environment media, 94.2% of TC could be degraded by the system of NBC-1000/PDS after 120 min reaction. These results suggested that the topological defect-rich BC could be a potential candidate for the environmental remediation through PDS activation.
4 Conclusion
In this work, biochar full of topological defects was prepared via selectively removing edge N from carbon networks. Pentagonal rings were created by the restructure of C atoms at the edge of the carbon layer, which was verified through AC-HRTEM measurements. The content of the pentagon defect could be regulated by changing the annealing temperature. The as-prepared NBC-1000 exhibited excellent activity in PDS activation for the degradation of TC, which could be up to 36.6 times and 8.7 times performance improvement than the pristine BC and N-doped BC, respectively. DFT calculation found the pentagon defect possesses smaller energy gap of HOMO–LUMO compared to the intact hexagon. Together with correlation analysis, the pentagon defect was determined to be the active site in PDS activating. The unique electronic band structure endowed the pentagonal defects with advantages in delivering electrons to PDS molecules more easily. EPR test and quenching experiment confirmed that radicals are not the primary ROSs. In contrast, the electron transfer induced by hole was identified as the dominant pathway for NBC-1000/PDS system. Owing to the merits of nonradical oxidation, the developed system exhibited good pH adaptability and inorganic ion tolerance, making it maintain superior degradation efficiency even in the actual environments. This work shed light on the crucial role of topological defects, especially the pentagonal defects in the nonradical oxidization, which was of important implications for designing highly efficient metal-free catalyst.
Data availability
The datasets used or analyzed in this work are available for reasonable reason.
References
Ashraf I, Li R, Chen B, Al-Ansari N, Rizwan Aslam M, Altaf AR, Elbeltagi A (2022) Nanoarchitectonics and kinetics insights into fluoride removal from drinking water using magnetic tea biochar. Int J Environ Res Public Health 19:13092. https://doi.org/10.3390/ijerph192013092
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652. https://doi.org/10.1063/1.464913
Cai S, Zhang Q, Wang Z, Hua S, Ding D, Cai T, Zhang R (2021) Pyrrolic N-rich biochar without exogenous nitrogen doping as a functional material for bisphenol A removal: performance and mechanism. Appl Catal B Environ 291:120093. https://doi.org/10.1016/j.apcatb.2021.120093
Chen Y, Cheng M, Lai C, Wei Z, Zhang G, Li L, Tang C, Du L, Wang G, Liu H (2023) The collision between g-C3N4 and QDs in the fields of energy and environment: synergistic effects for efficient photocatalysis. Small 19:2205902. https://doi.org/10.1002/smll.202205902
Dong Y, Zhang Q, Tian Z, Li B, Yan W, Wang S, Jiang K, Su J, Oloman CW, Gyenge EL, Ge R, Lu Z, Ji X, Chen L (2020) Ammonia thermal treatment toward topological defects in porous carbon for enhanced carbon dioxide electroreduction. Adv Mater 32:2001300. https://doi.org/10.1002/adma.202001300
Dong X, Chen Z, Tang A, Dionysiou DD, Yang H (2022) Mineral modulated single atom catalyst for effective water treatment. Adv Funct Mater 32:2111565. https://doi.org/10.1002/adfm.202111565
Dou J, Cheng J, Lu Z, Tian Z, Xu J, He Y (2022) Biochar co-doped with nitrogen and boron switching the free radical based peroxydisulfate activation into the electron-transfer dominated nonradical process. Appl Catal B Environ 301:120832. https://doi.org/10.1016/j.apcatb.2021.120832
Duan X, Sun H, Wang Y, Kang J, Wang S (2015) N-doping-induced nonradical reaction on single-walled carbon nanotubes for catalytic phenol oxidation. ACS Catal 5:553–559. https://doi.org/10.1021/cs5017613
Duan X, Sun H, Shao Z, Wang S (2018) Nonradical reactions in environmental remediation processes: uncertainty and challenges. Appl Catal B Environ 224:973–982. https://doi.org/10.1016/j.apcatb.2017.11.051
Fang G, Liu C, Gao J, Dionysiou DD, Zhou D (2015) Manipulation of persistent free radicals in biochar to activate persulfate for contaminant degradation. Environ Sci Technol 49:5645–5653. https://doi.org/10.1021/es5061512
Frank N (2018) Software update: the ORCA program system, version 4.0. Wires Comput Mol Sci 8(1):e1327. https://doi.org/10.1002/wcms.1327
Gao Y, Chen Z, Zhu Y, Li T, Hu C (2020) New insights into the generation of singlet oxygen in the metal-free peroxymonosulfate activation process: important role of electron-deficient carbon atoms. Environ Sci Technol 54:1232–1241. https://doi.org/10.1021/acs.est.9b05856
Gao Y, Wu T, Yang C, Ma C, Zhao Z, Wu Z, Cao S, Geng W, Wang Y, Yao Y, Zhang Y, Cheng C (2021) Activity trends and mechanisms in peroxymonosulfate-assisted catalytic production of singlet oxygen over atomic metal-N-C catalysts. Angew Chem Int Ed 60:22513–22521. https://doi.org/10.1002/anie.202109530
Ge Y (2022) Electro-activating persulfate via biochar catalytic cathode for sulfamethazine degradation: performance and mechanism insight. J Environ Chem Eng 10:109020. https://doi.org/10.1016/j.jece.2022.109020
Grimme S, Ehrlich S, Goerigk L (2011) Effect of the damping function in dispersion corrected density functional theory. J Comp Chem 32:1456–1465. https://doi.org/10.1002/jcc.21759
Guan L, Hu H, Li L, Pan Y, Zhu Y, Li Q, Guo H, Wang K, Huang Y, Zhang M, Yan Y, Li Z, Teng X, Yang J, Xiao J, Zhang Y, Wang X, Wu M (2020) Intrinsic defect-rich hierarchically porous carbon architectures enabling enhanced capture and catalytic conversion of polysulfides. ACS Nano 14:6222–6231. https://doi.org/10.1021/acsnano.0c02294
Guo D, Shibuya R, Akiba C, Saji S, Kondo T, Nakamura J (2016) Active sites of nitrogen-doped carbon materials for oxygen reduction reaction clarified using model catalysts. Science 351:361–365. https://doi.org/10.1126/science.aad0832
He M, Zhao P, Duan R, Xu S, Cheng G, Li M, Ma S (2022a) Insights on the electron transfer pathway of phenolic pollutant degradation by endogenous N-doped carbonaceous materials and peroxymonosulfate system. J Hazard Mater 424:127568. https://doi.org/10.1016/j.jhazmat.2021.127568
He Y-L, He C-S, Lai L-D, Zhou P, Zhang H, Li L-L, Xiong Z-K, Mu Y, Pan Z-C, Yao G, Lai B (2022b) Activating peroxymonosulfate by N and O co-doped porous carbon for efficient BPA degradation: a re-visit to the removal mechanism and the effects of surface unpaired electrons. Appl Catal B Environ 314:121390. https://doi.org/10.1016/j.apcatb.2022.121390
Jia Y, Zhang L, Du A, Gao G, Chen J, Yan X, Brown CL, Yao X (2016) Defect graphene as a trifunctional catalyst for electrochemical reactions. Adv Mater 28:9532–9538. https://doi.org/10.1002/adma.201602912
Jia Y, Zhang L, Zhuang L, Liu H, Yan X, Wang X, Liu J, Wang J, Zheng Y, Xiao Z, Taran E, Chen J, Yang D, Zhu Z, Wang S, Dai L, Yao X (2019) Identification of active sites for acidic oxygen reduction on carbon catalysts with and without nitrogen doping. Nat Catal 2:688–695. https://doi.org/10.1038/s41929-019-0297-4
Ke J, Ge Y, Yang Q, Liu Y, Show P-L, Guo R, Chen J (2022) Degradation of sulfamethazine using sludge-derived photocatalysts from dyeing industry and livestock farm: preparation and mechanism. J Hazard Mater 440:129837. https://doi.org/10.1016/j.jhazmat.2022.129837
Li W, Wang D, Zhang Y, Tao L, Wang T, Zou Y, Wang Y, Chen R, Wang S (2020) Defect engineering for fuel-cell electrocatalysts. Adv Mater 32:1907879. https://doi.org/10.1002/adma.201907879
Liang C, Huang C-F, Mohanty N, Kurakalva RM (2008) A rapid spectrophotometric determination of persulfate anion in ISCO. Chemosphere 73:1540–1543. https://doi.org/10.1016/j.chemosphere.2008.08.043
Liu W-J, Jiang H, Yu H-Q (2015) Development of biochar-based functional materials: toward a sustainable platform carbon material. Chem Rev 115:12251–12285. https://doi.org/10.1021/acs.chemrev.5b00195
Liu Q, Shen J, Yu X, Yang X, Liu W, Yang J, Tang H, Xu H, Li H, Li Y, Xu J (2019) Unveiling the origin of boosted photocatalytic hydrogen evolution in simultaneously (S, P, O)-Codoped and exfoliated ultrathin g-C3N4 nanosheets. Appl Catal B Environ 248:84–94. https://doi.org/10.1016/j.apcatb.2019.02.020
Liu B, Guo W, Wang H, Si Q, Zhao Q, Luo H, Ren N (2020) B-doped graphitic porous biochar with enhanced surface affinity and electron transfer for efficient peroxydisulfate activation. Chem Eng J 396:125119. https://doi.org/10.1016/j.cej.2020.125119
Liu S, Lai C, Zhou X, Zhang C, Chen L, Yan H, Qin L, Huang D, Ye H, Chen W, Li L, Zhang M, Tang L, Xu F, Ma D (2022) Peroxydisulfate activation by sulfur-doped ordered mesoporous carbon: insight into the intrinsic relationship between defects and 1O2 generation. Water Res 221:118797. https://doi.org/10.1016/j.watres.2022.118797
Lu T, Chen F (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem 33:580–592. https://doi.org/10.1002/jcc.22885
Mi X, Wang P, Xu S, Su L, Zhong H, Wang H, Li Y, Zhan S (2021) Almost 100 % peroxymonosulfate conversion to singlet oxygen on single-atom CoN2+2 sites. Angew Chem Int Ed 60:4588–4593. https://doi.org/10.1002/anie.202014472
Miao J, Geng W, Alvarez PJJ, Long M (2020) 2D N-doped porous carbon derived from polydopamine-coated graphitic carbon nitride for efficient nonradical activation of peroxymonosulfate. Environ Sci Technol 54:8473–8481. https://doi.org/10.1021/acs.est.0c03207
Miao X, Chen X, Wu W, Lin D, Yang K (2022) Intrinsic defects enhanced biochar/peroxydisulfate oxidation capacity through electron-transfer regime. Chem Eng J 438:135606. https://doi.org/10.1016/j.cej.2022.135606
Niu J, Shao R, Liang J, Dou M, Li Z, Huang Y, Wang F (2017) Biomass-derived mesopore-dominant porous carbons with large specific surface area and high defect density as high performance electrode materials for Li-ion batteries and supercapacitors. Nano Energy 36:322–330. https://doi.org/10.1016/j.nanoen.2017.04.042
Pan G, Wei J, Xu M, Li J, Wang L, Li Y, Cui N, Li J, Wang Z (2023) Insight into boron-doped biochar as efficient metal-free catalyst for peroxymonosulfate activation: important role of -O-B-O- moieties. J Hazard Mater 445:130479. https://doi.org/10.1016/j.jhazmat.2022.130479
Qian K, Chen H, Li W, Ao Z, Wu Y, Guan X (2021) Single-atom Fe catalyst outperforms its homogeneous counterpart for activating peroxymonosulfate to achieve effective degradation of organic contaminants. Environ Sci Technol 55:7034–7043. https://doi.org/10.1021/acs.est.0c08805
Ren W, Nie G, Zhou P, Zhang H, Duan X, Wang S (2020) The intrinsic nature of persulfate activation and N-doping in carbocatalysis. Environ Sci Technol 54:6438–6447. https://doi.org/10.1021/acs.est.0c01161
Ren W, Cheng C, Shao P, Luo X, Zhang H, Wang S, Duan X (2022) Origins of electron-transfer regime in persulfate-based nonradical oxidation processes. Environ Sci Technol 56:78–97. https://doi.org/10.1021/acs.est.1c05374
Shao P, Tian J, Yang F, Duan X, Gao S, Shi W, Luo X, Cui F, Luo S, Wang S (2018) Identification and regulation of active sites on nanodiamonds: establishing a highly efficient catalytic system for oxidation of organic contaminants. Adv Funct Mater 28:1705295. https://doi.org/10.1002/adfm.201705295
Shao P, Yu S, Duan X, Yang L, Shi H, Ding L, Tian J, Yang L, Luo X, Wang S (2020) Potential difference driving electron transfer via defective carbon nanotubes toward selective oxidation of organic micropollutants. Environ Sci Technol 54:8464–8472. https://doi.org/10.1021/acs.est.0c02645
Shi Q, Deng S, Zheng Y, Du Y, Li L, Yang S, Zhang G, Du L, Wang G, Cheng M, Liu Y (2022) The application of transition metal-modified biochar in sulfate radical based advanced oxidation processes. Environ Res 212:113340. https://doi.org/10.1016/j.envres.2022.113340
Sun C, Chen T, Huang Q, Zhan M, Li X, Yan J (2020) Activation of persulfate by CO2-activated biochar for improved phenolic pollutant degradation: performance and mechanism. Chem Eng J 380:122519. https://doi.org/10.1016/j.cej.2019.122519
Tang C, Cheng M, Lai C, Li L, Yang X, Du L, Zhang G, Wang G, Yang L (2023) Recent progress in the applications of non-metal modified graphitic carbon nitride in photocatalysis. Coordin Chem Rev 474:214846. https://doi.org/10.1016/j.ccr.2022.214846
Wan Z, Xu Z, Sun Y, He M, Hou D, Cao X, Tsang DCW (2021) Critical impact of nitrogen vacancies in nonradical carbocatalysis on nitrogen-doped graphitic biochar. Environ Sci Technol 55:7004–7014. https://doi.org/10.1021/acs.est.0c08531
Wang H, Guo W, Liu B, Wu Q, Luo H, Zhao Q, Si Q, Sseguya F, Ren N (2019a) Edge-nitrogenated biochar for efficient peroxydisulfate activation: an electron transfer mechanism. Water Res 160:405–414. https://doi.org/10.1016/j.watres.2019.05.059
Wang W, Shang L, Chang G, Yan C, Shi R, Zhao Y, Waterhouse GIN, Yang D, Zhang T (2019b) Intrinsic carbon-defect-driven electrocatalytic reduction of carbon dioxide. Adv Mater 31:1808276. https://doi.org/10.1002/adma.201808276
Wang J, Duan X, Gao J, Shen Y, Feng X, Yu Z, Tan X, Liu S, Wang S (2020a) Roles of structure defect, oxygen groups and heteroatom doping on carbon in nonradical oxidation of water contaminants. Water Res 185:116244. https://doi.org/10.1016/j.watres.2020.116244
Wang X, Jia Y, Mao X, Zhang L, Liu D, Song L, Yan X, Chen J, Yang D, Zhou J, Wang K, Du A, Yao X (2020b) A directional synthesis for topological defect in carbon. Chem 6:2009–2023. https://doi.org/10.1016/j.chempr.2020.05.010
Wang J, Li B, Li Y, Fan X, Zhang F, Zhang G, Peng W (2021a) Facile synthesis of atomic Fe-N-C materials and dual roles investigation of Fe-N4 sites in Fenton-like reactions. Adv Sci 8:2101824. https://doi.org/10.1002/advs.202101824
Wang J, Yu J, Fu Q, Yang H, Tong Q, Hao Z, Ouyang G (2021b) Unprecedented nonphotomediated hole (h+) oxidation system constructed from defective carbon nanotubes and superoxides. ACS Cent Sci 7:355–364. https://doi.org/10.1021/acscentsci.0c01600
Wang B, Cheng C, Jin M, He J, Zhang H, Ren W, Li J, Wang D, Li Y (2022a) A site distance effect induced by reactant molecule matchup in single-atom catalysts for Fenton-like reactions. Angew Chem Int Ed 61:e202207268. https://doi.org/10.1002/anie.202207268
Wang J, Fu Q, Yu J, Yang H, Hao Z, Zhu F, Ouyang G (2022b) An ultrafast and facile nondestructive strategy to convert various inefficient commercial nanocarbons to highly active Fenton-like catalysts. Proc Natl Acad Sci USA 119:e2114138119. https://doi.org/10.1073/pnas.2114138119
Wang J, Wang K, He Z-H, Zhang R-R, Guo P, Wang W, Yang Y, Liu Z-T (2022c) Constructing of ultrathin Bi2WO6/BiOCl nanosheets with oxygen vacancies for photocatalytic oxidation of cyclohexane with air in solvent-free. Appl Surf Sci 584:152606. https://doi.org/10.1016/j.apsusc.2022.152606
Xiao W, Cheng M, Liu Y, Wang J, Zhang G, Wei Z, Li L, Du L, Wang G, Liu H (2023) Functional metal/carbon composites derived from metal–organic frameworks: insight into structures, properties, performances, and mechanisms. ACS Catal 13:1759–1790. https://doi.org/10.1021/acscatal.2c04807
Xiong Y, Li H, Liu C, Zheng L, Liu C, Wang J, Liu S, Han Y, Gu L, Qian J, Wang D (2022) Single-atom Fe catalysts for Fenton-like reactions: roles of different N species. Adv Mater 34:2110653. https://doi.org/10.1002/adma.202110653
Yang S, Xu S, Tong J, Ding D, Wang G, Chen R, Jin P, Wang XC (2021) Overlooked role of nitrogen dopant in carbon catalysts for peroxymonosulfate activation: intrinsic defects or extrinsic defects? Appl Catal B Environ 295:120291. https://doi.org/10.1016/j.apcatb.2021.120291
Yu J, Feng H, Tang L, Pang Y, Zeng G, Lu Y, Dong H, Wang J, Liu Y, Feng C, Wang J, Peng B, Ye S (2020) Metal-free carbon materials for persulfate-based advanced oxidation process: microstructure, property and tailoring. Prog Mater Sci 111:100654. https://doi.org/10.1016/j.pmatsci.2020.100654
Zhang H, Hwang S, Wang M, Feng Z, Karakalos S, Luo L, Qiao Z, Xie X, Wang C, Su D, Shao Y, Wu G (2017) Single atomic iron catalysts for oxygen reduction in acidic media: particle size control and thermal activation. J Am Chem Soc 139:14143–14149. https://doi.org/10.1021/jacs.7b06514
Zhao H, Sun C, Jin Z, Wang D-W, Yan X, Chen Z, Zhu G, Yao X (2015) Carbon for the oxygen reduction reaction: a defect mechanism. J Mater Chem A 3:11736–11739. https://doi.org/10.1039/C5TA02229K
Zhu J, Mu S (2020) Defect engineering in carbon-based electrocatalysts: insight into intrinsic carbon defects. Adv Funct Mater 30:2001097. https://doi.org/10.1002/adfm.202001097
Zhu S, Huang X, Ma F, Wang L, Duan X, Wang S (2018) Catalytic removal of aqueous contaminants on N-doped graphitic biochars: inherent roles of adsorption and nonradical mechanisms. Environ Sci Technol 52:8649–8658. https://doi.org/10.1021/acs.est.8b01817
Zhu J, Huang Y, Mei W, Zhao C, Zhang C, Zhang J, Amiinu IS, Mu S (2019) Effects of intrinsic pentagon defects on electrochemical reactivity of carbon nanomaterials. Angew Chem Int Ed 58:3859–3864. https://doi.org/10.1002/anie.201813805
Acknowledgements
The authors appreciate the School of Earth Sciences of Zhejiang University for providing the detection instruments and Dr. Yangfan Lu (School of Materials Science and Engineering) for her help on XPS measurement and analysis.
Funding
This work was funded by the National Key Research and Development Program of China (2021YFC1809204), the National Natural Science Foundation of China (42192573, 21621005, and U21A20163), the Key Research and Development Program of Zhejiang Province, China (2021C0167), and the Fundamental Research Funds for the Central Universities (226-2022-00212).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation was performed by XM. Data collection and analysis were performed by XM and XC. The first draft of the manuscript was written by XM, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Handling editor: Baoshan Xing.
Supplementary Information
Additional file 1: Text S1.
EPR measurement. Text S2. Electrochemical Measurements. Figure S1. Adsorption of TC by NBC, NBC-700, NBC-850, NBC-1000, and NBC-1150 within 120 min. Experimental conditions: catalyst dose = 0.1 g L−1, [TC]0 = 0.1 mM. Figure S2. Degradation curves (a) and the pseudo-first-order kinetics constant (b) for the oxidative removal of TC by PDS with BC, NBCD, NBCM, NBCD-1000, and NBCM-1000 as catalysts. Experimental conditions: catalyst dose = 0.1 g L−1, [PDS]0 = 1 mM, [TC]0 = 0.1 mM. Figure S3. XRD patterns of NBC, NBC-700, NBC-850, NBC-1000, and NBC-1150. Figure S4. N2 adsorption–desorption isotherms (a) and the correlations of kobs with BET surface area (b) of NBC, NBC-700, NBC-850, NBC-1000, and NBC-1150. Figure S5. XPS C1s spectra (a) and the correlations of kobs with graphitization degree (b) of NBC, NBC-700, NBC-850, NBC-1000, and NBC-1150. Figure S6. XPS O1s spectra (a) and the correlations of kobs with C=O content (b) and C–O content (c) of NBC, NBC-700, NBC-850, NBC-1000, and NBC-1150. Figure S7. XPS O1s spectra (a) and N1s spectra (b) of NBC-1000 collected from NBC-1000/PDS system with or without TC after 120 min reaction. Figure S8. TG analysis of NBC (a) and TG-MS analysis of gases evolved from the annealed NBC (b). Figure S9. EPR spectra of NBC, NBC-700, NBC-850, NBC-1000, and NBC-1150. Figure S10. Correlations of AD/AG with the removal content of pyridinic N and Pyrrolic N of NBC, NBC-700, NBC-850, NBC-1000, and NBC-1150. Figure S11. Adsorption of PDS by NBC and NBC-1000 within 120 min. Experimental conditions: catalyst dose = 0.1 g L−1, [PDS]0 = 1.0 mM. Figure S12. TC degradation by NBC-1000/PDS system in H2O and D2O. Experimental conditions: catalyst dose = 0.1 g L−1, [PDS]0 = 1 mM, [TC]0 = 0.1 mM. Figure S13. EIS Nyquist plots of NBC and NBC-1000 (a), LSV curves under different conditions (b), and CA curves upon the addition of PDS and TC using NBC-1000 as the working electrodes (c). Figure S14. The influence of TC concentration on the degradation performance of NBC-1000/PDS system. Experimental conditions: catalyst dose = 0.1 g L−1, [PDS]0 = 1.0 mM, [TC]0 = 0.1, 0.06, 0.04, or 0.02 mM. Figure S15. The influence of PDS concentration on TC degradation in NBC-1000/PDS system. Experimental conditions: catalyst dose = 0.1 g L−1, [PDS]0 = 1.0, 0.7, or 0.4 mM, [TC]0 = 0.02 mM. Table S1. Analytical methods for selected pollutants. Table S2. Comparison of reported catalysts for persulfate activation in the degradation of organic contaminants.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Miao, X., Chen, X., Wu, W. et al. Topological defects strengthened nonradical oxidation performance of biochar catalyzed peroxydisulfate system. Biochar 5, 40 (2023). https://doi.org/10.1007/s42773-023-00243-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42773-023-00243-9