Abstract
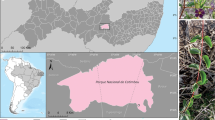
L-asparaginase is used as one of the prime chemotherapeutic agents to treat acute lymphoblastic leukemia. The present work aimed to study the endophytic fungal diversity of Grewia hirsuta and their ability to produce L-asparaginase. A total of 1575 culturable fungal endophytes belonging to four classes, Agaricomycetes, Dothideomycetes, Eurotiomycetes, and Sordariomycetes, were isolated. The isolates were grouped into twenty-one morphotypes based on their morphological characteristics. Representative species from each group were identified based on their microscopic characteristics and evaluation of the ITS and LSU rDNA sequences. Most of the fungal endophytes were recovered from the leaves compared to other plant parts. Diaporthe sp. was the predominant genus with a colonization frequency of 8.62%. Shannon-Wiener index for diversity ranged from 2.74 to 2.88. All the plant parts showed similar Simpson’s index values, indicating a uniform species diversity. Among the sixty-three fungal endophytes screened, thirty-two were identified as L-asparaginase-producing isolates. The enzyme activities of fungal endophytes estimated by the nesslerization method were found to be in the range of 4.65–0.27 IU/mL with Fusarium foetens showing maximum enzyme activity of 4.65 IU/mL. This study for the first time advocates the production of L-asparaginase from Fusarium foetens along with the endophytic fungal community composition of Grewia hirsuta. The results indicate that the fungal endophyte Fusarium foetens isolated in the present study could be a potent source of L-asparaginase.
Graphical Abstract





Similar content being viewed by others
References
Pieters R, Carroll WL (2008) Biology and treatment of acute lymphoblastic leukemia. Pediatr Clin North Am. 55(1):1–20
Pavlovic S, Kotur N, Stankovic B, Zukic B, Gasic V, Dokmanovic L (2019) Pharmacogenomic and pharmacotranscriptomic profiling of childhood acute lymphoblastic leukemia: paving the way to personalized treatment. Genes (Basel) 10(3):191
Beard MEJ, Crowther D, Galton DAG et al (1970) L-asparaginase in treatment of acute leukaemia and lymphosarcoma. Br Med J 1(5690):191–195
Kidd JG (1953) Regression of transplanted lymphomas induced in vivo by means of normal guinea pig serum: I. Course of transplanted cancers of various kinds in mice and rats given guinea pig serum, horse serum, or rabbit serum. J Exp Med 98(6):565–582
Broome JD (1963) Evidence that the L-asparaginase of guinea pig serum is responsible for its antilymphoma effects: I. Properties of the L-asparaginase of guinea pig serum in relation to those of the antilymphoma substance. J Exp Med 118:99–120
Mashburn LT, Wriston JC (1964) Tumor inhibitory effect of l-asparaginase from Escherichia coli. Arch Biochem Biophys 105(2):450–452
Keating MJ, Holmes R, Lerner S, Ho DH (1993) L-asparaginase and PEG asparaginase—past, present, and future. Leuk Lymphoma 10(sup1):153–157
Chi H, Xia B, Shen J et al (2022) Characterization of a novel and glutaminase-free type II L-asparaginase from Corynebacterium glutamicum and its acrylamide alleviation efficiency in potato chips. Int J Biol Macromol 221:1384–1393
Muneer F, Siddique MH, Azeem F et al (2020) Microbial L-asparaginase: purification, characterization and applications. Arch Microbiol 202(5):967–981
Alam S, Pranaw K, Tiwari R, Khare SK (2019) Recent development in the uses of asparaginase as food enzyme. In: Green Bio-Processes. Springer, pp 55–81
Kornbrust BA, Stringer MA, Lange NEK, Hendriksen HV, Whitehurst R, Oort MV (2009) Asparaginase–an enzyme for acrylamide reduction in food products. Enzymes Food Technol 2:59–87
Kumar K, Kaur J, Walia S, Pathak T, Aggarwal D (2014) L-asparaginase: an effective agent in the treatment of acute lymphoblastic leukemia. Leuk Lymphoma 55(2):256–262
Thakur M, Lincoln L, Niyonzima FN, Sunil SM (2014) Biotransformation isolation, purification and characterization of fungal extracellular L-asparaginase from Mucor hiemalis. J Biocatal Biotransformation 2:1–9
Tosa T, Sano R, Yamamoto K, Nakamura M, Chibata I (1972) L-Asparaginase form Proteus vulgaris. Purification, crystallization, and enzymic properties. Biochemistry 11(2):217–222
Mesas JM, Gil JA, Martn JF (1990) Characterization and partial purification of L-asparaginase from Corynebacterium glutamicum. Microbiology (N Y) 136(3):515–519
Pritsa AA, Kyriakidis DA (2001) L-Asparaginase of Thermus thermophilus: Purification, properties and identificaation of essential amino acids for its catalytic activity. Mol Cell Biochem 216(1):93–101
Scotti C, Sommi P, Pasquetto MV et al (2010) Cell-cycle inhibition by Helicobacter pylori L-asparaginase. PLoS One 5(11):e13892
El-Bessoumy AA, Sarhan M, Mansour J (2004) Production, isolation, and purification of L-asparaginase from Pseudomonas aeruginosa 50071 using solid-state fermentation. BMB Rep 37(4):387–393
Aishwarya SS, Selvarajan E, Iyappan S, Rajnish KN (2019) Recombinant l-asparaginase II from Lactobacillus casei subsp. casei ATCC 393 and its anticancer activity. Indian. J Microbiol 59(3):313–320
Saeed H, Hemida A, El-Nikhely N et al (2020) Highly efficient Pyrococcus furiosus recombinant L-asparaginase with no glutaminase activity: Expression, purification, functional characterization, and cytotoxicity on THP-1, A549 and Caco-2 cell lines. Int J Biol Macromol 156:812–828
Scheetz RW, Whelan HA, Wriston JC (1971) Purification and properties of an L-asparaginase from Fusarium tricinctum. Arch Biochem Biophys 142(1):184–189
Mishra A (2006) Production of L-asparaginase, an anticancer agent, from Aspergillus niger using agricultural waste in solid state fermentation. Appl Biochem Biotechnol 135(1):33–42
Shrivastava A, Khan AA, Shrivastav A, Jain SK, Singhal PK (2012) Kinetic studies of L-asparaginase from Penicillium digitatum. Prep Biochem Biotechnol 42(6):574–581
Monica T, Lincoln L, Niyonzima FN, Sunil SM (2013) Isolation, purification and characterization of fungal extracellular L-asparaginase from Mucor Hiemalis. J Biocatal Biotransformation 2(2):12–14
Farag AM, Hassan SW, Beltagy EA, El-Shenawy MA (2015) Optimization of production of anti-tumor l-asparaginase by free and immobilized marine Aspergillus terreus. Egypt J Aquat Res 41(4):295–302
Meghavarnam AK, Janakiraman S (2017) Solid state fermentation: an effective fermentation strategy for the production of L-asparaginase by Fusarium culmorum (ASP-87). Biocatal Agric Biotechnol 11:124–130
El-Gendy MMAA, Awad MF, El-Shenawy FS, El-Bondkly AMA (2021) Production, purification, characterization, antioxidant and antiproliferative activities of extracellular L-asparaginase produced by Fusarium equiseti AHMF4. Saudi J Biol Sci 28(4):2540–2548
Mostafa SA, Ali OKA (1983) L-asparaginase activity in cell-free extracts of Thermoactinomyces vulgaris 13 MES. Zentralbl Mikrobiol 138(5):397–404
Narayana KJP, Kumar KG, Vijayalakshmi M (2008) L-asparaginase production by Streptomyces albidoflavus. Indian J Microbiol 48(3):331–336
Amena S, Vishalakshi N, Prabhakar M, Dayanand A, Lingappa K (2010) Production, purification and characterization of L-asparaginase from Streptomyces gulbargensis. Braz J Microbiol 41:173–178
Deshpande N, Choubey P, Agashe M (2014) Studies on optimization of growth parameters for L-asparaginase production by Streptomyces ginsengisoli. Sci World J 2014:6
El-Naggar NEA, El-Shweihy NM (2020) Bioprocess development for L-asparaginase production by Streptomyces rochei, purification and in-vitro efficacy against various human carcinoma cell lines. Sci Rep 10(1):1–21
Paul JH (1982) Isolation and characterization of a Chlamydomonas L-asparaginase. Biochem 203(1):109–115
Ebrahiminezhad A, Rasoul-Amini S, Ghoshoon MB, Ghasemi Y (2014) Chlorella vulgaris, a novel microalgal source for L-asparaginase production. Biocatal Agric Biotechnol 3(2):214–217
Castro D, Marques ASC, Almeida MR et al (2021) L-asparaginase production review: Bioprocess design and biochemical characteristics. Appl Microbiol Biotechnol 105(11):4515–4534
Land VJ, Sutow WW, Fernbach DJ, Lane DM, Williams TE (1972) Toxicity of L-asparaginase in children with advanced leukemia. Cancer 30(2):339–347
Schein PS, Rakieten N, Gordon BM, Davis RD, Rall DP (1969) The toxicity of Escherichia coli L-asparaginase. Cancer Res 29(2):426–434
Fonseca MHG, da Silva FT, de Morais SB, Trevizani R (2021) Circumventing the side effects of L-asparaginase. Biomed Pharmacother 139:111616
Ali U, Naveed M, Ullah A et al (2016) L-asparaginase as a critical component to combat Acute Lymphoblastic Leukaemia (ALL): A novel approach to target ALL. Eur J Pharmacol 771:199–210
Souza PM, de Freitas MM, Cardoso SL, Pessoa A, Guerra ENS, Magalhaes PO (2017) Optimization and purification of L-asparaginase from fungi: A systematic review. Crit Rev Oncol Hematol 120:194–202
Rajesh PS, Rai VR (2013) Hydrolytic enzymes and quorum sensing inhibitors from endophytic fungi of Ventilago madraspatana Gaertn. Biocatal Agric Biotechnol 2(2):120–124
Terhonen E, Sipari N, Asiegbu FO (2016) Inhibition of phytopathogens by fungal root endophytes of Norway spruce. Biol Control 99:53–63
Khan AL, Hussain J, Al-Harrasi A, Al-Rawahi A, Lee IJ (2015) Endophytic fungi: resource for gibberellins and crop abiotic stress resistance. Crit Rev Biotechnol 35(1):62–74
Manganyi MC, Ateba CN (2020) Untapped potentials of endophytic fungi: A review of novel bioactive compounds with biological applications. Microorganisms 8(12):1934
Zhao J, Zhou L, Wang J et al (2010) Endophytic fungi for producing bioactive compounds originally from their host plants. Curr Res, Technol Educ Trop Appl Microbiol Microbial Biotechnol 1:567–576
Puri SC, Verma V, Amna T, Qazi GN, Spiteller M (2005) An endophytic fungus from Nothapodytes f oetida that produces Camptothecin. J Nat Prod 68(12):1717–1719
Kour A, Shawl AS, Rehman S et al (2008) Isolation and identification of an endophytic strain of Fusarium oxysporum producing podophyllotoxin from Juniperus recurva. World J Microbiol Biotechnol 24(7):1115–1121
Manasa C, Nalini MS (2014) L-Asparaginase activity of fungal endophytes from Tabernaemontana heyneana Wall.(Apocynaceae), endemic to the Western Ghats (India). Int Sch Res Notices 2014:7
Chow Y, Ting ASY (2015) Endophytic L-asparaginase-producing fungi from plants associated with anticancer properties. J Adv Res 6(6):869–876
Krishnapura PR, Belur PD (2016) Partial purification and characterization of L-asparaginase from an endophytic Talaromyces pinophilus isolated from the rhizomes of Curcuma amada. J Mol Catal B Enzym 124:83–91
Birudu RB, Padmavathi P (2018) Secondary metabolites of ethanolic leaf extract of Grewia hirsuta. European J Biomed 5(1):868–870
Thammanna NRK, Nagaraju N (1990) Medicinal plants of Tirumala. TTD publication, Tirupati, p 55
Ema A, Kumar MS, Rebecca LJ et al (2013) Evaluation of Antiproliferative effect of Grewia hirsuta on HepG2 cell lines. J Acad Ind Res 2(1):1
Rakshith D, Santosh P, Satish S (2013) Isolation and characterization of antimicrobial metabolite producing endophytic Phomopsis sp. from Ficus pumila Linn.(Moraceae). Int J Chem Anal Sci 4(3):156–160
Ainsworth GC, Sussman AS, Sparrow FK (1973) The Fungi: An Advanced Treatise. A Taxonomic Review with Keys: Ascomycetes and Fungi Imperfecti. Volume 4A. Academic Press
Barnett HL, Hunter BB (1998) Illustrated genera of imperfect fungi, 4th edn. The American Phytopathological Society Minnesota USA
Kim JS, Seo SG, Jun BK, Kim JW, Kim SH (2010) Simple and reliable DNA extraction method for the dark pigmented fungus, Cercospora sojina. Plant Pathol J 26(3):289–292
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR protocols: a guide to methods and applications 18(1):315–322
Rehner SA, Samuels GJ (1994) Taxonomy and phylogeny of Gliocladium analysed from nuclear large subunit ribosomal DNA sequences. Mycol Res 98(6):625–634
Kumar DSS, Hyde KD (2004) Biodiversity and tissue-recurrence of endophytic fungi in Tripterygium wilfordii. Fungal Divers
Arora P, Wani ZA, Ahmad T, Sultan P, Gupta S, Riyaz-Ul-Hassan S (2019) Community structure, spatial distribution, diversity and functional characterization of culturable endophytic fungi associated with Glycyrrhiza glabra L. Fungal Biol 123(5):373–383
Polley HW, Wilsey BJ, Derner JD (2003) Do species evenness and plant density influence the magnitude of selection and complementarity effects in annual plant species mixtures? Ecol Lett 6(3):248–256
Kusari S, Hertweck C, Spiteller M (2012) Chemical ecology of endophytic fungi: origins of secondary metabolites. Chem Biol 19(7):792–798
Jin Z, Gao L, Zhang L et al (2017) Antimicrobial activity of saponins produced by two novel endophytic fungi from Panax notoginseng. Nat Prod Res 31(22):2700–2703
Theantana T, Hyde KD, Lumyong S (2009) Asparaginase production by endophytic fungi from Thai medicinal plants: cytotoxicity properties. Int J Integr Biol 7(1):1–8
Kumar R, Sedolkar VK, Triveni AG, Kumar MS, Shivannavar CT, Gaddad SM (2016) Isolation, screening and characterization of L-asparaginase producing fungi from medicinal plants. Int J Pharm Pharm Sci 8(1):281–283
Gond SK, Mishra A, Sharma VK et al (2012) Diversity and antimicrobial activity of endophytic fungi isolated from Nyctanthes arbor-tristis, a well-known medicinal plant of India. Mycoscience 53(2):113–121
Bezerra JDP, Nascimento CCF, Barbosa RDN et al (2015) Endophytic fungi from medicinal plant Bauhinia forficata: Diversity and biotechnological potential. Braz J Microbiol 46:49–57
Zhao X, Hu Z, Hou D, Xu H, Song P (2020) Biodiversity and antifungal potential of endophytic fungi from the medicinal plant Cornus officinalis. Symbiosis 81(3):223–233
Shi Y, Xie H, Cao L et al (2017) Effects of Cd-and Pb-resistant endophytic fungi on growth and phytoextraction of Brassica napus in metal-contaminated soils. Environ Sci Pollut Res 24(1):417–426
Zheng YK, Miao CP, Chen HH et al (2017) Endophytic fungi harbored in Panax notoginseng: diversity and potential as biological control agents against host plant pathogens of root-rot disease. J Ginseng Res 41(3):353–360
Schoch CL, Sung GH, López-Giráldez F et al (2009) The Ascomycota tree of life: a phylum-wide phylogeny clarifies the origin and evolution of fundamental reproductive and ecological traits. Syst Biol 58(2):224–239
He X, Han G, Lin Y et al (2012) Diversity and decomposition potential of endophytes in leaves of a Cinnamomum camphora plantation in China. Ecol Res 27(2):273–284
Chen J, Hu KX, Hou XQ, Guo SX (2011) Endophytic fungi assemblages from 10 Dendrobium medicinal plants (Orchidaceae). World J Microbiol Biotechnol 27(5):1009–1016
Shipunov A, Newcombe G, Raghavendra AKH, Anderson CL (2008) Hidden diversity of endophytic fungi in an invasive plant. Am J Bot 95(9):1096–1108
Crozier J, Thomas SE, Aime MC, Evans HC, Holmes KA (2006) Molecular characterization of fungal endophytic morphospecies isolated from stems and pods of Theobroma cacao. Plant Pathol 55(6):783–791
Huang WY, Cai YZ, Hyde KD, Corke H, Sun M (2008) Biodiversity of endophytic fungi associated with 29 traditional Chinese medicinal plants. Fungal Divers
Ma B, Lv X, Warren A, Gong J (2013) Shifts in diversity and community structure of endophytic bacteria and archaea across root, stem and leaf tissues in the common reed, Phragmites australis, along a salinity gradient in a marine tidal wetland of northern China. Antonie van Leeuwenhoek 104(5):759–768
Cui XX, Wang L, Fang HY, Zheng YG, Su CY (2022) The cultivable endophytic fungal community of Scutellaria baicalensis: diversity and relevance to flavonoid production by the host. Plant Signal Behav 17(1):2068834
Verma VC, Gond SK, Kumar A, Kharwar RN, Strobel G (2007) The endophytic mycoflora of bark, leaf, and stem tissues of Azadirachta indica A. Juss (Neem) from Varanasi (India). Microb Ecol 54(1):119–125
Juybari HZ, Tajick Ghanbary MA, Rahimian H, Karimi K, Arzanlou M (2019) Seasonal, tissue and age influences on frequency and biodiversity of endophytic fungi of Citrus sinensis in Iran. For Pathol 49(6):e12559
Gautam AK, Kant M, Thakur Y (2013) Isolation of endophytic fungi from Cannabis sativa and study their antifungal potential. Arch Phytopathol Plant Prot 46(6):627–635
Chareprasert S, Piapukiew J, Thienhirun S, Whalley AJS, Sihanonth P (2006) Endophytic fungi of teak leaves Tectona grandis L. and rain tree leaves Samanea saman Merr. World J Microbiol Biotechnol 22(5):481–486
Harrison JG, Griffin EA (2020) The diversity and distribution of endophytes across biomes, plant phylogeny and host tissues: how far have we come and where do we go from here? Environ Microbiol 22(6):2107–2123
Nascimento TL, Oki Y, Lima DMM, Almeida-Cortez JS, Fernandes GW, Souza-Motta CM (2015) Biodiversity of endophytic fungi in different leaf ages of Calotropis procera and their antimicrobial activity. Fungal Ecol 14:79–86
Arfi Y, Buee M, Marchand C, Levasseur A, Record E (2012) Multiple markers pyrosequencing reveals highly diverse and host-specific fungal communities on the mangrove trees a vicennia marina and R hizophora stylosa. FEMS Microbiol Ecol 79(2):433–444
Hardoim PR, Van Overbeek LS, Berg G et al (2015) The hidden world within plants: ecological and evolutionary considerations for defining functioning of microbial endophytes. Microbiol Mol Biol Rev 79(3):293–320
Dos Santos GD, Gomes RR, Gonçalves R et al (2021) Molecular identification and antimicrobial activity of foliar endophytic fungi on the Brazilian pepper tree (Schinus terebinthifolius) reveal new species of Diaporthe. Curr Microbiol 78(8):3218–3229
Del Frari G, Gobbi A, Aggerbeck MR, Oliveira H, Hansen LH, Ferreira RB (2019) Characterization of the wood mycobiome of Vitis vinifera in a vineyard affected by esca. Spatial distribution of fungal communities and their putative relation with leaf symptoms. Front Plant Sci 10:910
de Siqueira VM, Conti R, de Araújo JM, Souza-Motta CM (2011) Endophytic fungi from the medicinal plant Lippia sidoides Cham. and their antimicrobial activity. Symbiosis 53(2):89–95
Cannon PF, Simmons CM (2002) Diversity and host preference of leaf endophytic fungi in the Iwokrama Forest Reserve, Guyana. Mycologia 94(2):210–220
Yuan ZL, Chen YC, Yang Y (2009) Diverse non-mycorrhizal fungal endophytes inhabiting an epiphytic, medicinal orchid (Dendrobium nobile): Estimation and characterization. World J Microbiol Biotechnol 25(2):295–303
Li P, Wu Z, Liu T, Wang Y (2016) Biodiversity, phylogeny, and antifungal functions of endophytic fungi associated with zanthoxylum bungeanum. Int J Mol Sci 17(9):1541
Uzma F, Murthy KN, Srinivas C (2016) Optimization of physiological conditions for L-asparaginase production by endophytic fungi (Fusarium solani) isolated from Tinospora cordifolia (Willd.) Hook. F & Thomson. Eur J Exp Biol 6:37–45
Pádua APSLD, Freire KTLDS, Oliveira TGLD et al (2018) Fungal endophyte diversity in the leaves of the medicinal plant Myracrodruon urundeuva in a Brazilian dry tropical forest and their capacity to produce L-asparaginase. Acta Bot Brasilica 33:39–49
Dame ZT, Silima B, Gryzenhout M, van Ree T (2016) Bioactive compounds from the endophytic fungus Fusarium proliferatum. Nat Prod Res 30(11):1301–1304
Kumar A, Patil D, Rajamohanan PR, Ahmad A (2013) Isolation, purification and characterization of vinblastine and vincristine from endophytic fungus Fusarium oxysporum isolated from Catharanthus roseus. PLoS One 8(9):e71805
Ran X, Zhang G, Li S, Wang J (2017) Characterization and antitumor activity of camptothecin from endophytic fungus Fusarium solani isolated from Camptotheca acuminate. Afr Health Sci 17(2):566–574
Ito K, Matsushima K, Koyama Y (2012) Gene cloning, purification, and characterization of a novel peptidoglutaminase-asparaginase from Aspergillus sojae. Appl Environ Microbiol 78(15):5182–5188
Dias FFG, Ruiz ALTG, Della Torre A, Sato HH (2016) Purification, characterization and antiproliferative activity of L-asparaginase from Aspergillus oryzae CCT 3940 with no glutaminase activity. Asian Pac J Trop Biomed 6(9):785–794
Prihanto AA, Caisariyo IO, Pradarameswari KA (2019) Aspergillus sp. as a potential producer for L-Asparaginase from mangrove (Avicennia germinans). In: IOP Conference Series: Earth and Environmental Science, vol 230. IOP Publishing, p 12101
Moharram A, Zohri AN, Seddek N (2016) L-Asparaginase production by endophytic fungi isolated from Withania Somnifera in Egypt. SS Int J Multidiscip Res 2:30–40
Hölker U, Höfer M, Lenz J (2004) Biotechnological advantages of laboratory-scale solid-state fermentation with fungi. Appl Microbiol Biotechnol 64(2):175–186
Bhavana NS, Prakash HS, Nalini MS (2020) Fungal Endophytes from Tabernaemontana heyneana Wall.(Apocynaceae), their Molecular Characterization, L-asparaginase and Antioxidant Activities. Jordan J Biol Sci 13(4):543–550
Funding
The study was funded by CSIR (Council of Scientific and Industrial Research) under Junior Research Fellowship (File No:09/119(0218)/2019-EMR-I).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Solange I. Mussatto
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Parashiva, J., Nuthan, B.R., Rakshith, D. et al. Insights into diversity and L-asparaginase activity of fungal endophytes associated with medicinal plant Grewia hirsuta. Braz J Microbiol 54, 1573–1587 (2023). https://doi.org/10.1007/s42770-023-01045-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-023-01045-8