Abstract
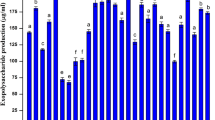
Inoculants with beneficial microorganisms comprise both selected strains and carriers that ensure a favorable microenvironment for cell survival and stability. Formulations of inoculants using synthetic polymers as carriers are common. However, only a few studies are available in the literature regarding the formulation of inoculants using natural biomolecules as carriers. Exopolysaccharides (EPS) are biomolecules produced by a vast array of microbial species, including symbiotic nitrogen-fixing bacteria, commonly known as rhizobia. EPS perform several functions, such as the protection against the deleterious effects of diverse environmental soil stresses. Two Rhizobium tropici strains and one Paraburkholderia strain were selected after semiquantitative analysis by scanning electron microscopy (SEM) of their EPS production in liquid YMA medium. Their EPS were characterized through a series of analytical techniques, aiming at their use in the formulation of plant inoculants. In addition, the effect of the carbon source on EPS yield was evaluated. Multi-stage fragmentation analysis showed the presence of xylose, glucose, galactose, galacturonic acid, and glucuronic acid in EPS chemical composition, which was confirmed by FT-IR spectra and 13C NMR spectroscopy. Thermal stability (thermogravimetric) was close to 270 °C and viscosity ranged from 120 to 1053.3 mPa.s. Surface morphology (SEM) was rough and irregular, with a cross-linked spongy matrix, which, together with the hydrophilic functional groups, confers water holding capacity. The present study showed that the three EPS have potential as microorganism carriers for formulation of microbial inoculants to be applied in plants.







Similar content being viewed by others
Data availability
Available upon reasonable request.
Code availability
Not applicable.
References
Bashan Y, de-Bashan LE, Prabhu SR, Hernandez J-P (2014) Advances in plant growth-promoting bacterial inoculant technology: formulations and practical perspectives (1998–2013). Plant Soil 378(1–2):1–33. https://doi.org/10.1007/s11104-013-1956-x
Keswani C, Prakash O, Bharti N et al (2019) Re-addressing the biosafety issues of plant growth promoting rhizobacteria. Sci Total Environ 690:841–852. https://doi.org/10.1016/j.scitotenv.2019.07.046
Ruíz-Valdiviezo VM, Canseco LMCV, Suárez LAC, Gutiérrez-Miceli FA, Dendooven L, Rincón-Rosales R (2015) Symbiotic potential and survival of native rhizobia kept on different carriers. Braz J Microbiol 46(3):735–742. https://doi.org/10.1590/S1517-838246320140541
Farias TP, Trochmann A, Soares BL, Moreira FMS (2016) Rhizobia inoculation and liming increase cowpea productivity in Maranhão State. Acta Sci Agron 38(3):387. https://doi.org/10.4025/actasciagron.v38i3.28630
de Oliveira-Longatti SM, Marra LM, de Carvalho TS, de Moreira FM, S, (2020) The culture medium volume and the inoculation method should be considered in semi-quantitative screening of calcium phosphate solubilization by bacteria. Acta Sci Agron 42:e44332. https://doi.org/10.4025/actasciagron.v42i1.44332
Lee S-K, Lur H-S, Lo K-J et al (2016) Evaluation of the effects of different liquid inoculant formulations on the survival and plant-growth-promoting efficiency of Rhodopseudomonas palustris strain PS3. Appl Microbiol Biotechnol 100(18):7977–7987. https://doi.org/10.1007/s00253-016-7582-9
Rivera D, Obando M, Barbosa H, Rojas Tapias D, Bonilla Buitrago R (2014) Evaluation of polymers for the liquid rhizobial formulation and their influence in the Rhizobium-Cowpea interaction. Univ Sci 19(3):265–275. https://doi.org/10.11144/Javeriana.SC19-3.eplr
Schoebitz M, López MD, Roldán A (2013) Bioencapsulation of microbial inoculants for better soil–plant fertilization. A review Agron Sustain Dev 33(4):751–765. https://doi.org/10.1007/s13593-013-0142-0
Marcelino PRF, Milani KML, Mali S, dos Santos OJAP, de Oliveira ALM (2016) Formulations of polymeric biodegradable low-cost foam by melt extrusion to deliver plant growth-promoting bacteria in agricultural systems. Appl Microbiol Biotechnol 100(16):7323–7338. https://doi.org/10.1007/s00253-016-7566-9
Flemming H-C, Wingender J (2010) The biofilm matrix. Nat Rev Microbiol 8(9):623–633. https://doi.org/10.1038/nrmicro2415
Cieśla J, Kopycińska M, Łukowska M, Bieganowski A, Janczarek M (2016) Surface properties of wild-type Rhizobium leguminosarum bv. trifolii strain 24.2 and its derivatives with different extracellular polysaccharide content Martinez-Abarca F, ed. PLOS ONE 11(10):e0165080. https://doi.org/10.1371/journal.pone.0165080
Kawaharada Y, Kelly S, Nielsen MW et al (2015) Receptor-mediated exopolysaccharide perception controls bacterial infection. Nature 523(7560):308–312. https://doi.org/10.1038/nature14611
Bomfeti CA, Florentino LA, Guimarães AP, Cardoso PG, Guerreiro MC, de Moreira FMS (2011) Exopolysaccharides produced by the symbiotic nitrogen-fixing bacteria of leguminosae. Rev Bras Ciênc Solo 35(3):657–671. https://doi.org/10.1590/S0100-06832011000300001
Castellane TCL, Persona MR, Campanharo JC, de Macedo Lemos EG (2015) Production of exopolysaccharide from rhizobia with potential biotechnological and bioremediation applications. Int J Biol Macromol 74:515–522. https://doi.org/10.1016/j.ijbiomac.2015.01.007
Fred EB, Waksman SA (1928) Laboratory manual of general microbiology with special reference to the microorganisms of the soil. McGraw-Hill Book Co., Published online, p 145
Vincent JM (1970) A manual for the practical study of root-nodule bacteria. Vol 15. 15th ed. International Biological Programme.
Araújo KS, Carvalho F de, Moreira FM de S (2017) Bukholderia strains promote Mimosa spp. growth but not Macroptilium atropurpureum. Rev Ciênc Agronômica 48. https://doi.org/10.5935/1806-6690.20170005
de Rangel WM, de Oliveira Longatti SM, Ferreira PAA et al (2017) Leguminosae native nodulating bacteria from a gold mine As-contaminated soil: multi-resistance to trace elements, and possible role in plant growth and mineral nutrition. Int J Phytoremediation 19(10):925–936. https://doi.org/10.1080/15226514.2017.1303812
Graham PH, Draeger KJ, Ferrey ML et al (1994) Acid pH tolerance in strains of Rhizobium and Bradyrhizobium, and initial studies on the basis for acid tolerance of Rhizobium tropici UMR1899. Can J Microbiol 40(3):198–207. https://doi.org/10.1139/m94-033
Castellane TCL, Otoboni AMMB, de Lemos EGM (2015) Characterization of exopolysaccharides produced by rhizobia species. Rev Bras Ciênc Solo 39(6):1566–1575. https://doi.org/10.1590/01000683rbcs20150084
Staudt AK, Wolfe LG, Shrout JD (2012) Variations in exopolysaccharide production by Rhizobium tropici. Arch Microbiol 194(3):197–206. https://doi.org/10.1007/s00203-011-0742-5
Castellane TCL, Lemos MVF, de Lemos EGM (2014) Evaluation of the biotechnological potential of Rhizobium tropici strains for exopolysaccharide production. Carbohydr Polym 111:191–197. https://doi.org/10.1016/j.carbpol.2014.04.066
Castellane TCL, de Lemos EGM (2007) Composição de exopolissacarídeos produzidos por estirpes de rizóbios cultivados em diferentes fontes de carbono. Pesqui Agropecuária Bras 42(10):1503–1506. https://doi.org/10.1590/S0100-204X2007001000019
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1–2):248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Moretto C, Castellane TCL, Lopes EM, Omori WP, Sacco LP, de Lemos EGM (2015) Chemical and rheological properties of exopolysaccharides produced by four isolates of rhizobia. Int J Biol Macromol 81:291–298. https://doi.org/10.1016/j.ijbiomac.2015.07.056
Donot F, Fontana A, Baccou JC, Schorr-Galindo S (2012) Microbial exopolysaccharides: main examples of synthesis, excretion, genetics and extraction. Carbohydr Polym 87(2):951–962. https://doi.org/10.1016/j.carbpol.2011.08.083
Polak-Berecka M, Choma A, Waśko A, Górska S, Gamian A, Cybulska J (2015) Physicochemical characterization of exopolysaccharides produced by Lactobacillus rhamnosus on various carbon sources. Carbohydr Polym 117:501–509. https://doi.org/10.1016/j.carbpol.2014.10.006checardoi
Monteiro NK, Aranda-Selverio G, Exposti DTD et al (2012) Caracterização química dos géis produzidos pelas bactérias diazotróficas Rhizobium tropici e Mesorhizobium sp. Quím Nova 35(4):705–708. https://doi.org/10.1590/S0100-40422012000400009
Faria S, de Oliveira Petkowicz CL, de Morais SAL et al (2011) Characterization of xanthan gum produced from sugar cane broth. Carbohydr Polym 86(2):469–476. https://doi.org/10.1016/j.carbpol.2011.04.063
Kanamarlapudi SLRK, Muddada S (2017) Characterization of exopolysaccharide produced by Streptococcus thermophilus CC30. BioMed Res Int 2017:1–11. https://doi.org/10.1155/2017/4201809
Pereira PAA, Oliver A, Bliss FA, Crowe L, Crowe J (2002) Preservation of rhizobia by lyophilization with trehalose. Pesqui Agropecuária Bras 37(6):831–839. https://doi.org/10.1590/S0100-204X2002000600012
Gil-Serrano A, del Junco AS, Tejero-Mateo P, Megias M, Caviedes MA (1990) Structure of the extracellular polysaccharide secreted by Rhizobium leguminosarum var. phaseoli CIAT 899. Carbohydr Res. 204:103–107. https://doi.org/10.1016/0008-6215(90)84025-P
Miao M, Ma Y, Huang C, Jiang B, Cui SW, Zhang T (2015) Physicochemical properties of a water soluble extracellular homopolysaccharide from Lactobacillus reuteri SK24.003. Carbohydr Polym 131:377–383. https://doi.org/10.1016/j.carbpol.2015.05.066
Dertli E, Toker OS, Durak MZ et al (2016) Development of a fermented ice-cream as influenced by in situ exopolysaccharide production: rheological, molecular, microstructural and sensory characterization. Carbohydr Polym 136:427–440. https://doi.org/10.1016/j.carbpol.2015.08.047
Han Y, Liu E, Liu L et al (2015) Rheological, emulsifying and thermostability properties of two exopolysaccharides produced by Bacillus amyloliquefaciens LPL061. Carbohydr Polym 115:230–237. https://doi.org/10.1016/j.carbpol.2014.08.044
Kaith BS, Sharma R, Kalia S (2015) Guar gum based biodegradable, antibacterial and electrically conductive hydrogels. Int J Biol Macromol 75:266–275. https://doi.org/10.1016/j.ijbiomac.2015.01.046
Rasulov B, Rozi P, Pattaeva M, Yili A, Aisa H (2016) Exopolysaccharide-based bioflocculant matrix of Azotobacter chroococcum XU1 for synthesis of AgCl nanoparticles and its application as a novel biocidal nanobiomaterial. Materials 9(7):528. https://doi.org/10.3390/ma9070528
Ballesteros LF, Cerqueira MA, Teixeira JA, Mussatto SI (2015) Characterization of polysaccharides extracted from spent coffee grounds by alkali pretreatment. Carbohydr Polym 127:347–354. https://doi.org/10.1016/j.carbpol.2015.03.047
Chen T, Wang Y, Li J et al (2016) Phthaloyl modification of a polysaccharide from Lachnum YM262 and immunomodulatory activity. Process Biochem 51(10):1599–1609. https://doi.org/10.1016/j.procbio.2016.08.004
Wang K, Li W, Rui X et al (2015) Chemical modification, characterization and bioactivity of a released exopolysaccharide (r-EPS1) from Lactobacillus plantarum 70810. Glycoconj J 32(1–2):17–27. https://doi.org/10.1007/s10719-014-9567-1
Mishra A, Kavita K, Jha B (2011) Characterization of extracellular polymeric substances produced by micro-algae Dunaliella salina. Carbohydr Polym 83(2):852–857. https://doi.org/10.1016/j.carbpol.2010.08.067
Prasanna PHP, Bell A, Grandison AS, Charalampopoulos D (2012) Emulsifying, rheological and physicochemical properties of exopolysaccharide produced by Bifidobacterium longum subsp. infantis CCUG 52486 and Bifidobacterium infantis NCIMB 702205. Carbohydr Polym. 90(1):533–540. https://doi.org/10.1016/j.carbpol.2012.05.075
Priyanka P, Arun AB, Ashwini P, Rekha PD (2015) Versatile properties of an exopolysaccharide R-PS18 produced by Rhizobium sp. PRIM-18. Carbohydr Polym 126:215–221. https://doi.org/10.1016/j.carbpol.2015.03.017
Janczarek M (2011) Environmental signals and regulatory pathways that influence exopolysaccharide production in rhizobia. Int J Mol Sci 12(12):7898–7933. https://doi.org/10.3390/ijms12117898
Saravanan C, Shetty PKH (2016) Isolation and characterization of exopolysaccharide from Leuconostoc lactis KC117496 isolated from idli batter. Int J Biol Macromol 90:100–106. https://doi.org/10.1016/j.ijbiomac.2015.02.007
Liu Y, Gu Q, Ofosu FK, Yu X (2016) Production, structural characterization and gel forming property of a new exopolysaccharide produced by Agrobacterium HX1126 using glycerol or d-mannitol as substrate. Carbohydr Polym 136:917–922. https://doi.org/10.1016/j.carbpol.2015.09.107
Mudgil D, Barak S, Khatkar BS (2014) Guar gum: processing, properties and food applications – a Review. J Food Sci Technol 51(3):409–418. https://doi.org/10.1007/s13197-011-0522-x
Ahmed Z, Wang Y, Anjum N, Ahmad A, Khan ST (2013) Characterization of exopolysaccharide produced by Lactobacillus kefiranofaciens ZW3 isolated from Tibet kefir – Part II. Food Hydrocolloids 30(1):343–350. https://doi.org/10.1016/j.foodhyd.06.009
Farias TP, Soares BL, D´eca CSB, Moreira FMSM (2022) Polymeric formulations of liquid inoculants with rhizobia exopolysaccharides increase the survival and symbiotic efficiency of elite Bradyrhizobium strains. Arch Microbiol 204:177–186. https://doi.org/10.1007/s00203-022-02779-z
Acknowledgements
We thank the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) (CAPES/PROEX AUXPE 593/2018), the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (process: 304527/2016-5; process: 431504/2016-4), and the Fundação de Amparo à Pesquisa do estado de Minas Gerais (Fapemig) (CAG-RED-00330-16) for financial support and for granting scholarships. This research is associated with the Brazilian National Institute of Science and Technology (Soil Biodiversity/INCT-CNPq). We also thank the Program for the Qualification of Public Servants of IFMA (PROQUALIS) for granting a PhD scholarship to the first author.
Funding
Financial support and for granting scholarships by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES/PROEX AUXPE 593/2018), the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (process: 304527/2016–5; process: 431504/2016–4), and the Fundação de Amparo e Pesquisa de Minas Gerais (Fapemig) (CAG- RED-00330–16).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
We warrant that all of the authors have contributed substantially to the manuscript and approved the final submission.
Consent for publication
We warrant that all of the authors have contributed substantially to the manuscript and approved the final submission to BJM.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Responsible Editor: Luiz Henrique Rosa
Highlights.
• Multi-technique-analyzed/characterized rhizobia EPS.
• Attractive EPS production from three rhizobia strains.
• Presence of xylose, glucose, and galacturonic acid determined.
• Interesting WHC and thermal stability close to 270 °C.
• Potential for use as biodegradable inoculant carriers.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Palhares Farias, T., de Melo Castro, E., Marucci Pereira Tangerina, M. et al. Rhizobia exopolysaccharides: promising biopolymers for use in the formulation of plant inoculants. Braz J Microbiol 53, 1843–1856 (2022). https://doi.org/10.1007/s42770-022-00824-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-022-00824-z