Abstract
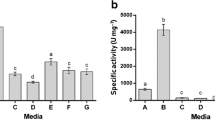
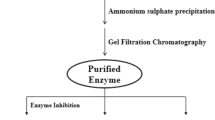
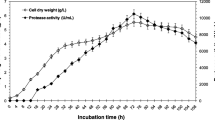
Aspergillus awamori was cultivated in a modified Breccia medium, and the extracellular fraction was obtained, which presented 260 ± 15 µg of protein/mg and specific protease activity of 3.87 ± 0.52 mM.min−1.mg of protein−1 using Nα-p-tosyl-L-arginine methyl ester hydrochloride (L-TAME) as substrate. This fraction showed major proteins about 104 and 44 kDa and maximal protease activity at pH 5.5, 6.5, and 9.0, suggesting that A. awamori secretes acidic, neutral, and alkaline proteases with expressive thermal stability, however, aspartic protease was the most important activity. When yeast extract was supplemented to a modified Breccia medium, A. awamori protein secretion and protease activity were maximal and the affinity chromatography on pepstatin-agarose was employed to isolate the aspartic protease activity, which was called ASPA, with approximately 75 kDa. ASPA maximal activity was obtained at pH 4.5 and 6.5, and 50 °C. Pepstatin inhibited about 80% of ASPA activity, with IC50 and Ki values of 0.154 and 0.072 μM, respectively. ASPA cleaved protein and peptides substrates with the highest activity against gelatin (95 U/mg) and good peptidase activity with KM 0.0589 mM and Vmax 1.909 mM.min−1.mg protein−1, using L-TAME as substrate. A. awamori extracellular fraction is a source of proteases with important activity, and the supplementation of modified Breccia medium increased the aspartic protease production. This enzyme presented different biochemical characteristics from the previously reported A. awamori aspartic proteases. Therefore, ASPA is an excellent candidate for biotechnological application due to its important activity and thermostability.










Similar content being viewed by others
References
Wiltschi B, Cernava T, Dennig A, Galindo Casas M, Geier M, Gruber S, Haberbauer M, Heidinger P et al (2020) Enzymes revolutionize the bioproduction of value-added compounds: from enzyme discovery to special applications. Biotechnol Adv 40:107520. https://doi.org/10.1016/j.biotechadv.2020.107520
Rathore DS, Singh SP (2021) Kinetics of growth and co-production of amylase and protease in novel marine actinomycete, Streptomyces lopnurensis KaM5. Folia Microbiol. https://doi.org/10.1007/s12223-020-00843-z
Deckers M, Deforce D, FraitureRoosens MANHC (2020) Genetically modified micro-organisms for industrial food enzyme production: an overview. Foods 19(3):326. https://doi.org/10.3390/foods9030326
Takazono T, Izumikawa K (2018) Recent advances in diagnosing chronic pulmonary aspergillosis. Front Microbiol 9:1810. https://doi.org/10.3389/fmicb.2018.01810
Perrone G, Stea G, Epifani F, Varga J, Frisvad JC, Samson RA (2011) Aspergillus niger contains the cryptic phylogenetic species A. awamori. Fungal Biol 115(11):1138–1150. https://doi.org/10.1016/j.funbio.2011.07.008
Li Q, Yi L, Marek P, Iverson BL (2013) Commercial proteases: present and future. FEBS Lett 587(8):1155–1163. https://doi.org/10.1016/j.febslet.2012.12.019
Silva-López RE, Gonçalves RN (2019) Therapeutic proteases from plants: biopharmaceuticals with multiple applications. J Appl Biotechnol Bioeng 6(2):101–109. https://doi.org/10.15406/jabb.2019.06.00180
Singh S, Gaur S (2021) Fungal byproducts in food technology. In: Dai X., Sharma M., Chen J. (eds) Fungi in sustainable food production. Fungal Biology. Springer, Cham. https://doi.org/10.1007/978-3-030-64406-2_1
Lopez M, Edens L (2005) Effective prevention of chill-haze in beer using an acid proline-specific endoprotease from Aspergillus niger. J Agric Food Chem 53:7944–7949. https://doi.org/10.1021/jf0506535
Abrunhosa L, Santos L, Venâncio A (2006) Degradation of ochratoxin A by proteases and by a crude enzyme of Aspergillus niger. Food Biotechnol 20(3):231–242. https://doi.org/10.1080/08905430600904369
Dong Z, Yang S, Lee B (2021) Bioinformatic mapping of a more precise Aspergillus niger degradome. Sci Rep 11:693. https://doi.org/10.1038/s41598-020-80028-3
Gao X, Yin Y, Yan J, Zhang J, Ma H, Zhou C (2019) Separation, biochemical characterization and salt-tolerant mechanisms of alkaline protease from Aspergillus oryzae. J Sci Food Agric 99(7):3359–3366. https://doi.org/10.1002/jsfa.9553
Ao XL, Yu X, Wu DT, Li C, Zhang T, Liu SL, Chen SJ, He L, Zhou K, Zou LK (2018) Purification and characterization of neutral protease from Aspergillus oryzae Y1 isolated from naturally fermented broad beans. AMB Express 8(1):96. https://doi.org/10.1186/s13568-018-0611-6
Yang Y, Iwamoto A, Kumrungsee T, Okazaki Y, Kuroda M, Yamaguchi S, Kato N (2017) Consumption of an acid protease derived from Aspergillus oryzae causes bifidogenic effect in rats. Nut Res 44:60–60466. https://doi.org/10.1016/j.nutres.2017.06.004
Abarca ML, Accensi F, Cano J, Cabañes FJ (2004) Taxonomy and significance of black aspergilli. Antonie Van Leeuwenhoek 86(1):33–49. https://doi.org/10.1023/B:ANTO.0000024907.85688.05
Gottschalk LMF, Paredes RS, Teixeira RSS, Silva AS, Bon EPS (2013) Efficient production of lignocellulolytic enzymes xylanase, β-xylosidase, ferulic acid esterase and β-glucosidase by the mutant strain Aspergillus awamori 2B.361 U2/1. Braz J Microbiol 44:569–576. https://doi.org/10.1590/S1517-83822013000200037
Valkonen M, Ward M, Wang H, Penttila M, Saloheimo M (2003) Improvement of foreign protein production in Aspergillus niger var. awamori by constitutive induction of the unfolded-protein response. Appl Env Microbiol 69(12):6979–6986. https://doi.org/10.1128/AEM.69.12.6979-5886986.2003
Conesa C, Calvo M, Sánchez L (2010) Recombinant human lactoferrin: a valuable protein for pharmaceutical products and functional foods. Biotechnol Adv 28(6):831–838. https://doi.org/10.1016/j.biotechadv.2010.07.002
Singh B, Kaur A (2015) Antidiabetic potential of a peptide isolated from an endophytic Aspergillus awamori. J Appl Microbiol 120(2):301–311. https://doi.org/10.1111/jam.12998
Moralejo FJ, Cardoza RE, Gutiérrez S, Sisniega H, Faus I, Martín JF (2000) Overexpression and lack of degradation of thaumatin in an aspergillopepsin A-defective mutant of Aspergillus awamori containing an insertion in the pepA gene. Appl Microbiol Biotechnol 54:772–777. https://doi.org/10.1007/s002530000463
Moralejo FJ, Cardoza RE, Gutierrez S, Lombraña M, Fierro F, Martín JF (2002) Silencing of the aspergillopepsin B (pepB) gene of Aspergillus awamori by antisense RNA expression or protease removal by gene disruption results in a large increase in thaumatin production. Applied Env Microbiol 68(7):3550–3559. https://doi.org/10.1128/AEM.68.7.3550-3559.2002
Cardoza RE, Gutiérrez S, Ortega N, Colina A, Casqueiro J, Martín JF (2003) Expression of a synthetic copy of the bovine chymosin gene in Aspergillus awamori from constitutive and pH-regulated promoters and secretion using two different pre-pro sequences. Biotechnol Bioenginee 83(3):249–259. https://doi.org/10.1002/bit.10666
Marangon M, Van Sluyter SC, Robinson EMC, Muhlack RA, Holt HE, Haynes PA, Godden PW, Smith PA, Waters EJ (2012) Degradation of white wine haze proteins by Aspergillopepsin I and II during juice flash pasteurization. Food Chem 135:1157–1165. https://doi.org/10.1016/j.foodchem.2012.05.042
Berka RM, Ward M, Wilson LJ, Hayenga KJ, Kodama KH, Carlomagno LP, Thompson SA (1990) Molecular cloning and deletion of the gene encoding aspergillopepsin A from Aspergillus awamori. Gene 86:153–162. https://doi.org/10.1016/0378-1119(90)90274-u
Kour D, Rana KL, Thakur S, Sharma S, Yadav N, Rastegari AA, Yadav AN, Saxena AK (2019) Chapter 3 - disruption of protease genes in microbes for production of heterologous proteins. New and Future Developments in Microbial Biotechnology and Bioengineering Microbial Genes Biochemistry and Applications 35–75. https://doi.org/10.1016/B978-0-444-63503-7.00003-6
Breccia JD, Castro GR, Baigorí MD, Siñeriz F (1995) Screening of xylanolytic bacteria using a colour plate method. J Appl Bacteriol 78(5):469–472. https://doi.org/10.1111/j.13652672.1995.tb03086.x
Kulkarni A, Rao M (2007) Biochemical characterization of an aspartic protease from Vignaradiata: kinetic interactions with the classical inhibitor pepstatin implicating a tight binding mechanism. Biochim Biophys Acta 1774(5):619–627. https://doi.org/10.1016/j.bbapap.2007.03.014
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(12):248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head bacteriophage T4. Nature 227:680–685. https://doi.org/10.1038/227680a0
Silva-López RE, Morgado-Díaz JA, dos Santos PT, Giovanni-De-Simone S (2008) Purification and subcellular localization of a secreted 75 kDa Trypanosoma cruzi serine oligopeptidase. Acta Trop 107(2):159–167. https://doi.org/10.1016/j.actatropica.2008.05.016
Pacheco JS, Silva-López RE (2012) Study of the proteolytic activity of the tropical legume Crotalaria spectabilis. Z Naturforsch 67:495–509. https://doi.org/10.5560/znc.2012
Gonçalves RN, Kalume DE, Ferrara MA, Silva-López RE (2021) A novel cucumisin-like serine protease from leaf of legume Canavaliaensiformis. J Plant Biochem Biotechnol 30:147–159. https://doi.org/10.1007/s13562-020-00578-5
Gonçalves RN, Barbosa SDG, Silva-López RE (2016) Proteases from Canavaliaensiformis: active and thermostable enzymes with potential of application in biotechnology. Biotechnol Res Int 2016:3427098. https://doi.org/10.1155/2016/3427098
Vries RP, Visser J (2001) Aspergillus enzymes involved in degradation of plant cell wall polysaccharides. Microbiol Mol Biol Rev 65(4):497–522. https://doi.org/10.1128/MMBR.65.4.497-522.2001
Teixeira RSS, Siqueira FG, Souza MV, Filho EX, Bon EP (2010) Purification and characterization studies of a thermostable beta-xylanase from Aspergillus awamori. J Ind Microbiol Biotechnol 37(10):1041–1051. https://doi.org/10.1007/s10295-010-0751-4
Rawlings ND, Bateman A (2021) How to use the MEROPS database and website to help understand peptidase specificity. Protein Sci 30(1):83–92. https://doi.org/10.1002/pro.3948
Silva-López RE (2010) Proteases de Leishmania: novos alvos para o desenvolvimento racional de fármacos. Quim Nova 33(7):1541–1548. https://doi.org/10.1590/S010040422010000700022
Negi S, Banerjee R (2009) Characterization of amylase and protease produced by Aspergillus awamori in a single bioreactor. Food Res Int 42(4):443–448. https://doi.org/10.1016/j.foodres.2009.01.004
Kunert J, Kopecek P (2000) Multiple forms of the serine protease Alp of Aspergillus fumigatus. Mycoses 43(9–10):339–347. https://doi.org/10.1046/j.1439-0507.2000.00586.x
Souza PM, Aliakbarian B, Ferreira-Filho EX, Magalhães PO, Pessoa-Junior A, Converti A, Perego P (2015) Kinetic and thermodynamic studies of a novel acid protease from Aspergillus foetidus. Int J Biol Macromol 81:17–21. https://doi.org/10.1016/j.ijbiomac.2015.07.043
Sethi BK, Jana A, Nanda PK, Mohapatra PK, Sahoo SL (2016) Thermostable acidic protease production in Aspergillus terreus NCFT 4269.10 using chickling vetch peels. J Taibah Univ Sci 10(4):571–583. https://doi.org/10.1016/j.jtusci.2015.11.001
Purushothaman K, Bhat SK, Singh SA, Marathe GK, Appu RA (2019) Aspartic protease from Aspergillus niger: molecular characterization and interaction with pepstatin A. Int J Biol Macromol 139:199–212. https://doi.org/10.1016/j.ijbiomac.2019.07.133
Daniel RM, Danson MJ (2013) Temperature and the catalytic activity of enzymes: a fresh understanding. FEBS Lett 587:2738–2743. https://doi.org/10.1016/j.febslet.2013.06.027
Umezawa H, Aoyagi T, Morishima H, Matsuzaki M, Hamada M (1970) Pepstatin, a new pepsin inhibitor produced by Actinomycetes. J Antibiot 23(5):259–262. https://doi.org/10.7164/antibiotics.23.259
Reichard U, Léchenne B, Asif AR, Streit F, Grouzmann E, Jousson O, Monod M (2006) Sedolisins, a new class of secreted proteases from Aspergillus fumigatus with endoprotease or tripeptidyl-peptidase activity at acidic pHs. Appl Environ Microbiol 72(3):1739–1748. https://doi.org/10.1128/AEM.72.3.1739-1748.2006
Soares da Silva O, Almeida EM, Melo AHF, Porto TS (2018) Purification and characterization of a novel extracellular serine-protease with collagenolytic activity from Aspergillus tamarii URM4634. Int J Biol Macromol 117:1081–1088. https://doi.org/10.1016/j.ijbiomac.2018.06.002
Nelson DL, Cox MM (2017) Lehninger principles of biochemistry. W.H. Freeman, New York. ISBN-13: 978-1464187964
Alkan N, Espeso EA, Prusky D (2013) Virulence regulation of phytopathogenic fungi by pH. Antioxid Redox Signal 19(9):1012–1025. https://doi.org/10.1089/ars.2012.5062
Wheeler KA, Hurdman BF, Pitt JI (1991) Influence of pH on the growth of some toxigenic species of Aspergillus, Penicillium and Fusarium. Int J Food Microbiol 12(2–3):141–149. https://doi.org/10.1016/0168-1605(91)90063-U
Krappmann S, Braus GH (2005) Nitrogen metabolism of Aspergillus and its role in pathogenicity. Med Mycol 43:31–40. https://doi.org/10.1080/13693780400024271
Lin S, Zhu Q, Wen L, Yang B, Jiang G, Gao H, Chen F, Jiang Y (2014) Production of quercetin, kaempferol and their glycosidic derivatives from the aqueous-organic extracted residue of litchi pericarp with Aspergillus awamori. Food Chem 145:220–227. https://doi.org/10.1016/j.foodchem.2013.08.048
Hazra S, Guha R, Jongkey G, Palui H, Mishra A, Geeta K, Vemuganti, Basak SK, Mandal TK, Konar A (2012) Modulation of matrix metalloproteinase activity by EDTA prevents posterior capsular opacification. Mol Vision 18: 1701–1711. http://www.molvis.org/molvis/v18/a175.
Kuş C, Özer E, Korkmaz Y, Yurtcu E, Dağalp R (2018) Benzamide and benzamidine compounds as new inhibitors of urokinasetype plasminogen activators. Mini Rev Med Chem 18(20):1753–1758. https://doi.org/10.2174/1389557518666180816110740
Sabotic J, Kos J (2012) Microbial and fungal protease inhibitors - current and potential applications. Appl Microbiol Biotechnol 93:1351–1375. https://doi.org/10.1007/s00253-011-3834-x
Navale R, Atul AAD, Sijwali PS (2014) Characterization of the autophagy marker protein Atg8 reveals atypical features of autophagy in Plasmodium falciparum. PLoS One 9(11):e113220. https://doi.org/10.1371/journal.pone.0113220
Prescott M, Peek K, Daniel RM (1995) Characterization of a thermostable pepstatin-insensitive acid proteinase from a Bacillus sp. Int J Biochem Cell Biol 27(7):729–739. https://doi.org/10.1016/1357-2725(95)00032-K
Aoki W, Kitahara N, Miura N, Morisaka H, Yamamoto Y et al (2012) Candida albicans possesses Sap7 as a pepstatin A-insensitive secreted aspartic protease. PLoS One 7(2):e32513. https://doi.org/10.1371/journal.pone.0032513
Cairns TC, Nai C, Meyer V (2018) How a fungus shapes biotechnology: 100 years of Aspergillus niger research. Fungal Biol Biotechnol 5:13. https://doi.org/10.1186/s40694-018-0054-5
Segel IH (1976) Enzyme kinetics, behavior and analysis of rapid equilibrium and steady-state enzyme systems. Wiley-Interscience, New York. https://doi.org/10.1016/0307-4412(76)90018-2
Silva-López RE, Pinto Coelho MG, De Simone SG (2005) Characterization of an extracellular serine protease of Leishmania (Leishmania) amazonensis. Parasitol 131:85–96. https://doi.org/10.1017/S003118200400667
Acknowledgements
The authors are thankful to Sharon de Queiroz Silva from Bioethanol Laboratory for assistance with the fungus cultivation. This paper was revised by Ms. Patrícia Fernandes Ferreira and Dr. Maria Antonieta Ferrara, researchers at the Department of Natural Products from FIOCRUZ.
Funding
This study was supported by National Council for Scientific and Technological Development (CNPq-grant number 490029/2009–4) and the Oswaldo Cruz Foundation (FIOCRUZ, PROEP CNPq/ FARMANGUINHOS-407839/2017–8).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
da Silva-López, R.E., de Araujo, T.A.A., Monteiro, H.J.J. et al. Study of protease activity from Aspergillus awamori INCQS2B.361U2/1 extracellular fraction and modification of culture medium composition to isolate a novel aspartic protease. Braz J Microbiol 53, 1599–1611 (2022). https://doi.org/10.1007/s42770-022-00750-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-022-00750-0