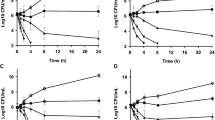
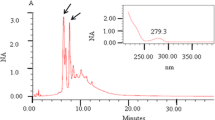
Abstract
The aquatic plant Salvinia auriculata has been shown to possess promising properties for the treatment of Staphylococcus aureus bovine mastitis. The disease affects cattle health and compromises dairy cattle productivity, resulting in reduced milk production and higher mortality rates. The aim of this study was to evaluate the antimicrobial activity, antibiofilm activity, and toxicity of S. auriculata root extracts using bovine mammary epithelial cells (MAC-T); determine the chemical composition of the most active extract; and develop an S. auriculata antiseptic solution for pre- and post-milking teat disinfection. Plants were collected during the four seasons of the year. The most active hexane extract was subjected to bioguided fractionation, which resulted in the isolation of six known compounds, stigmast-22-ene-3,6-dione, stigmasterol, friedelinol, β-sitosterol, octadecyl alcohol, and octadecanoic acid. The antimicrobial and antibiofilm activities of the most active extract and isolated compounds were determined against nine S. aureus strains isolated from cows with mastitis. The efficacy of the S. auriculata teat dip formulation was tested using an excised teat model (ex vivo), and promising results were obtained. The S. auriculata extract formulation proved to be as effective as commercial antimicrobials in reducing log counts in excised teats.






Similar content being viewed by others
References
Sabbag OJ, Costa SMAL (2015) Análise de custos de produção de leite: aplicação do método de Monte Carlo. Rev Ext Rural 22:125–145. https://doi.org/10.5902/2318179614153
Silva LCA, Pessoa DAN, Silva LSA, Aquino SS, Macedo MMS, Mattos RAT, Junior FG (2015) Avaliação in vitro da sensibilidade de estirpes de Staphylococcus spp. isoladas de mastite caprina frente a desinfetantes comerciais. Arq Inst Biol 82:1–4. https://doi.org/10.1590/1808-1657000262013
Nunes SF, Bexiga R, Cavaco LM, Vilela CL (2007) Technical Note: Antimicrobial Susceptibility of Portuguese Isolates of Staphylococcus aureus and Staphylococcus epidermidis in Subclinical Bovine Mastitis. J Dairy Sci 7:3242–3246. https://doi.org/10.3168/jds.2006-739
Machado TRO, Correa MGE, Marin JM (2008) Susceptibilidade antimicrobiana de cepas de Staphylococci coagulase-negativa isoladas de leite de bovinos com mastite no Brasil. Braz Arch Vet Med Zootech 60:278–282. https://doi.org/10.1590/S0102-09352008000100041
Meléndez PA, Capriles VA (2006) Antibacterial properties of tropical plants from Puerto Rico. Phytomedicine 13:272–276. https://doi.org/10.1016/j.phymed.2004.11.009
Hu JFE, Garo MG, Goering M, Pasmore HD, Yoo T, Esser J, Sestrich PA, Cremin GH, Hough P, Perrone YSL, Lee NT, Ngoc-Tram L, O’Neil-Johnson M, Costerton JW, Eldridge GR (2006) Bacterial biofilm inhibitors from Diospyrosdendo. J Nat Prod 69:118–120. https://doi.org/10.1021/np049600s
Vattem DA, Mihalik K, Crixell SH, McLean RJ (2007) Dietary phytochemicals as quorum sensing inhibitors. Fitoterapia 78:279–332. https://doi.org/10.1016/j.fitote.2007.03.009
Özbay H, Alim A (2009) Antimicrobial activity of some water plants from the northeastern anatolian region of Turkey. Molecules 14:321–328. https://doi.org/10.3390/molecules14010321
Lolis SF, Thomaz SM (2011) Monitoramento da composição específica da comunidade de macrófitas aquáticas no reservatório Luís Eduardo Magalhães. Plant Daninha 29:247–258. https://doi.org/10.1590/S0100-83582011000200002
Wolff G, Assis LR, Pereira GC, Carvalho JG, Castro EM (2009) Effects of zinc toxicity on leaves of Salvinia auriculata cultivated in nutrient solution. Plant Daninha 26:315–325. https://doi.org/10.1590/S0100-83582009000100017
Toledo JJ, Penha J (2011) Performance of Azolla caroliniana Willd. and Salvinia auriculata Aubl. on fish farming effluent. Braz J Biol 71:37–45. https://doi.org/10.1590/S1519-69842011000100007
Rossi CC, Aguilar AP, Diaz MAN, Ribon AOB (2011) Aquatic plants as potential sources of antimicrobial compounds active against bovine mastitis pathogens. Afr J Biotechnol 10:8023–8030. https://doi.org/10.5897/AJB11.440
Lima S, Diaz G, Diaz MAN (2013) Antibacterial chemical constituent and antiseptic herbal soap from Salvinia auriculate Aubl. Evid Based Complement Altern Med 480509. https://doi.org/10.1155/2013/480509
Shirsath SR, Sonawane SH, Gogate PR (2012) Intensification of extraction of natural products using ultrasonic irradiations - a review of current status. Chem Eng Process 53:10–23. https://doi.org/10.1016/j.cep.2012.01.003
Nascimento FR, Albuquerque KRS, Oliveira MR, Pizziolo VR, Brasileiro BG, Diaz G, Diaz MAN (2017) Antibiotic activity of Plectranthus ornatus Codd., a Traditional Medicinal Plant. An Acad Bras Cienc 89:2461–2469. https://doi.org/10.1590/0001-3765201720170068
Georges P, Sylvestre M, Ruegger H, Bourgeois P (2006) Ketosteroids and hydroxyketosteroids, minor metabolites of sugarcane wax. Steroids 71:647–652. https://doi.org/10.1016/j.steroids.2006.01.016
Radulović NS, Dordević ND (2011) Steroids from poison hemlock (Conium maculatum L.): a GC-MS analysis. J Serbian Chem Soc 76:1471–1483. https://doi.org/10.2298/JSC110206128R
Edilu A, Adane L, Woyessa D (2015) In vitro antibacterial activities of compounds isolated from roots of Caylusea abyssinica. Ann Clin Microbiol Antimicrob 14:15. https://doi.org/10.1186/s12941-015-0072-6
Jue SG, Dawson GW, Brogden RN (1985) Ciclopirox olamine 1% cream; a preliminary review of its antimicrobial activity and therapeutic use. Drugs 29:330–341. https://doi.org/10.2165/00003495-198529040-00002
Clinical and Laboratory Standards Institute (2009) Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard eighth edition
Klein RC, Fabres-Klein MH, de Oliveira LL, Feio RN, Malouin F, Ribon ADOB (2015) A C-type lectin from Bothrops jararacussu venom disrupts Staphylococcal biofilms. PLoS ONE 10(3):e0120514. https://doi.org/10.1371/journal.pone.0120514
Čabarkapa I, Čolović R, Đuragić O, Popović S, Kokić B, Milanov D, Pezo L (2019) Anti-biofilm activities of essential oils rich in carvacrol and thymol against Salmonella Enteritidis. Biofouling 35:361–3751. https://doi.org/10.1080/08927014.2019.1610169
Carvalho MG, Velandia JR, Oliveira LF, Bezerra FB (1998) Triterpenos isolados de Eschweilera longipes miers (lecythidaceae). Quim Nova 21:740–743. https://doi.org/10.1590/S0100-40421998000600014
Fabry W, Okemo PO, Ansorg R (1998) Antibacterial activity of East African medicinal plants. J Ethnopharmcol 60:79–84. https://doi.org/10.1016/s0378-8741(97)00128-1
Bussmann RW, Malca-García G, Glenn D, Sharon G, Chait D, Díaz K, Pourmand B, Jonat A, Somogy S, Guardado G, Aguirre C, Chan R, Meyer K, Kuhlman A, Townesmith A, Effio-Carbajal J, Frías-Fernandez F, Benito M (2010) Minimum inhibitory concentrations of medicinal plants used in Northern Peru as antibacterial remedies. J Ethnopharmacol 132:101–108. https://doi.org/10.1016/j.jep.2010.07.048
Aligiannis N, Kalpoutzakis E, Mitaku S, Chinou IB (2001) Composition and antimicrobial activity of the essential oils of two Origanum species. J Agric Food Chem 49:4168–4170. https://doi.org/10.1021/jf001494m
Costerton JW, Stewart PS, Greenberg EP (1999) Bacterial biofilms: a common cause of persistent infections. Science 284:1318–1322. https://doi.org/10.1126/science.284.5418.1318
Knothe G (2006) 1H-NMR spectroscopy of fatty acids and their derivatives. AOCS Lipid Library
Jain PS, Bari SB (2010) Isolation of lupeol, stigmasterol e campesterol from petroleum ether extract od woody stem of Wrightia tinctoria. Asian J Plant Sci 9:163–167. https://doi.org/10.3923/ajps.2010.163.167
Chaturvedula VSP, Prakash I (2012) Isolation of Stigmasterol and β-Sitosterol from the dichloromethane extract of Rubus suavissimus. Int Curr Pharm J 1:239–242. https://doi.org/10.3329/icpj.v1i9.11613
Kondaveeti S, Chejara DR, Siddhanta AK (2014) Synthesis of self-assembly of agarose-fatty acid ester nanoparticles. Ind J Chem B 53A:679–687. http://nopr.niscair.res.in/handle/123456789/28856
Felisbino JKRP (2019) Identificação de substâncias produzidas pelos fungos Cercospora brachiata. Thesis, Universidade Federal de Uberlância, Beauveria bassiana e Verticillium sp e avaliação da atividade antibacteriana
Rutherford ST, Bassler BL (2012) Bacterial quorum sensing: its role in virulence and possibilities for its control. Cold Spring Harb Perspect Med 2:1–26. https://doi.org/10.1101/cshperspect.a012427
Duarte MCT, Figueira GM, Pereira B, Magalhães PM, Delarmelina C (2004) Atividade antimicrobiana de extratos hidroalcólicos de espécies da coleção de plantas medicinais. Braz J Pharmacog 14:06–08. https://doi.org/10.1590/S0102-695X2004000300003
Virtuoso S, Davet A, Dias JFG, Cunico MM, Miguel MD, Oliveira AB, Miguel OG (2005) Estudo preliminar da atividade antibacteriana das cascas de Erythrina velutina Willd., Fabaceae (Leguminosae). Braz J Pharmacog 15:137–142. https://doi.org/10.1590/S0102-695X2005000200012
Castillo MG, Morosini MI, Valverde A, Almaraz F, Baquero F, Cantón R, Campo R (2007) Differences in biofilm development and antibiotic susceptibility among Streptococcus pneumonia isolates from cystic fibrosis samples and blood cultures. J Antimicrob Chemother 59:301–304. https://doi.org/10.1093/jac/dkl482
Nostro A, Roccaro AS, Bisignano G, Marino A, Cannatelli MA, Pizzimenti FC, Cioni PL, Procópio F, Blanco AR (2007) Effects of oregano, carvacrol and thymol on Staphylococcus aureus and Staphylococcus epidermidis biofilms. J Med Microbiol 56:519–523. https://doi.org/10.1099/jmm.0.46804-0
Dolabela MF, Oliveira SG, Nascimento JM, Peres JM, Wagner H, Póvoa MM, de Oliveira AB (2008) In vitro antiplasmodial activity of extract and constituents from Esenbeckia febrifuga, a plant traditionally used to treat malaria in the Brazilian Amazon. Phytomedicine 15:367–72. https://doi.org/10.1016/j.phymed.2008.02.001
Piotrowska-Tomala KK, Bah MM, Jankowska K, Lukasik K, Warmowski P, Galvao AM, Skarzynski DJ (2015) Lipopolysaccharides, cytokines, and nitric oxide affect secretion of prostaglandins and leukotrienes by bovine mammary gland during experimentally induced mastitis in vivo and in vitro. Domest Anim Endocrinol 52:90–99. https://doi.org/10.1016/j.domaniend.2015.03.001
Philpot WN, Boddie RL, Pankey JW (1978) Hygiene in the prevention of udder infections. IV. Evaluation of teat dips with excised cows’ teats. J Dairy Sci 61:950–955. https://doi.org/10.3168/jds.S0022-0302(78)83672-8
Acknowledgements
We would like to thank the Coordination for the Improvement of Higher Education Personnel (CAPES), Brazil, for the scholarships awarded to J.V.P.B. B., G.A.P., and S.L. We would also like to thank Embrapa/CNPGL, Juiz de Fora, Minas Gerais, for kindly providing the bacterial strains.
Funding
This work was supported by a grant (APQ-00454–11) from the Minas Gerais Research Foundation (Fapemig).
Author information
Authors and Affiliations
Contributions
G.A. Purgato, S. Lima, and J.V.P.B. Baeta performed the data acquisition and interpretation of data. M.A.N. Diaz, V.R. Pizziolo, G.N. Souza, and G. Diaz-Muñoz performed data analysis, interpretation of data and elaborated the manuscript. All authors performed a critical review and approved the final approval of the version to be published.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Plant study
This plant was registered in SisGen in compliance with Law no. 13,123/2015 and its regulations. Registration number A99795C.
Animal study
The teat experiments were carried out with dairy cows used for human consumption and provided by a slaughterhouse certified by the Department of Inspection of Products of Animal Origin (DIPOA). We declare that no animals were killed to be used in this research.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Jorge Luiz Mello Sampaio
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Purgato, G.A., Lima, S., Baeta, J.V.P.B. et al. Salvinia auriculata: chemical profile and biological activity against Staphylococcus aureus isolated from bovine mastitis. Braz J Microbiol 52, 2401–2411 (2021). https://doi.org/10.1007/s42770-021-00595-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-021-00595-z