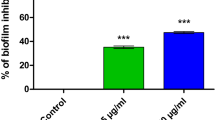
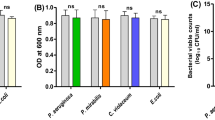
Abstract
Background
Serratia marcescens becomes an apparent nosocomial pathogen and causes a variety of infections. S. marcescens possess various virulence factors that are regulated by intercellular communication system quorum sensing (QS). Targeting bacterial virulence is a proposed strategy to overcome bacterial resistance. Sitagliptin anti-QS activity has been demonstrated previously and we aimed in this study to investigate the effects of antidiabetic drugs vildagliptin and metformin compared to sitagliptin on S. marcescens pathogenesis.
Methods
We assessed the effects of tested drugs in subinhibitory concentrations phenotypically on the virulence factors and genotypically on the virulence encoding genes' expressions. The protection of tested drugs on S. marcescens pathogenesis was performed in vivo. Molecular docking study has been conducted to evaluate the interference capabilities of tested drugs to the SmaR QS receptor.
Results
Vildagliptin reduced the expression of virulence encoding genes but did not show in vitro or in vivo anti-virulence activities. Metformin reduced the expression of virulence encoding genes and inhibited bacterial virulence in vitro but did not show in vivo protection. Sitagliptin significantly inhibited virulence factors in vitro, reduced the expression of virulence factors and protected mice from S. marcescens. Docking study revealed that sitagliptin is more active than metformin and fully binds to SmaR receptor, whereas vildagliptin had single interaction to SmaR.
Conclusion
The downregulation of virulence genes was not enough to show anti-virulence activities. Hindering of QS receptors may play a crucial role in diminishing bacterial virulence.





Similar content being viewed by others
Change history
26 March 2021
A Correction to this paper has been published: https://doi.org/10.1007/s42770-021-00468-5
References
Su LH, Ou JT, Leu HS, Chiang PC, Chiu YP, Chia JH, Kuo AJ, Chiu CH, Chu C, Wu TL, Sun CF, Riley TV, Chang BJ, Infection Control G (2003) Extended epidemic of nosocomial urinary tract infections caused by Serratia marcescens. J Clin Microbiol 41(10):4726–4732. https://doi.org/10.1128/jcm.41.10.4726-4732.2003
Van Houdt R, Givskov M, Michiels CW (2007) Quorum sensing in Serratia. FEMS Microbiol Rev 31(4):407–424. https://doi.org/10.1111/j.1574-6976.2007.00071.x
Cristina ML, Sartini M, Spagnolo AM (2019) Serratia marcescens Infections in Neonatal Intensive Care Units (NICUs). Int J Environ Res Public Health 16(4). https://doi.org/10.3390/ijerph16040610
Jones RN (2010) Microbial etiologies of hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia. Clinical infectious diseases 51(Suppl 1):S81–S87. https://doi.org/10.1086/653053
Davies DG, Parsek MR, Pearson JP, Iglewski BH, Costerton JW, Greenberg EP (1998) The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science 280(5361):295–298. https://doi.org/10.1126/science.280.5361.295
Coulthurst SJ, Williamson NR, Harris AK, Spring DR, Salmond GP (2006) Metabolic and regulatory engineering of Serratia marcescens: mimicking phage-mediated horizontal acquisition of antibiotic biosynthesis and quorum-sensing capacities. Microbiology 152(Pt 7):1899–1911. https://doi.org/10.1099/mic.0.28803-0
Horng YT, Deng SC, Daykin M, Soo PC, Wei JR, Luh KT, Ho SW, Swift S, Lai HC, Williams P (2002) The LuxR family protein SpnR functions as a negative regulator of N-acylhomoserine lactone-dependent quorum sensing in Serratia marcescens. Mol Microbiol 45(6):1655–1671. https://doi.org/10.1046/j.1365-2958.2002.03117.x
Van Houdt R, Moons P, Aertsen A, Jansen A, Vanoirbeek K, Daykin M, Williams P, Michiels CW (2007) Characterization of a luxI/luxR-type quorum sensing system and N-acyl-homoserine lactone-dependent regulation of exo-enzyme and antibacterial component production in Serratia plymuthica RVH1. Res Microbiol 158(2):150–158. https://doi.org/10.1016/j.resmic.2006.11.008
Stock I, Burak S, Sherwood KJ, Gruger T, Wiedemann B (2003) Natural antimicrobial susceptibilities of strains of ‘unusual’ Serratia species: S. ficaria, S. fonticola, S. odorifera, S. plymuthica and S. rubidaea. J Antimicrob Chemother 51(4):865–885. https://doi.org/10.1093/jac/dkg156
Traub WH (2000) Antibiotic susceptibility of Serratia marcescens and Serratia liquefaciens. Chemotherapy 46(5):315–321. https://doi.org/10.1159/000007304
Adonizio A, Kong KF, Mathee K (2008) Inhibition of quorum sensing-controlled virulence factor production in Pseudomonas aeruginosa by South Florida plant extracts. Antimicrob Agents Chemother 52(1):198–203. https://doi.org/10.1128/AAC.00612-07
Kalia VC, Purohit HJ (2011) Quenching the quorum sensing system: potential antibacterial drug targets. Crit Rev Microbiol 37(2):121–140. https://doi.org/10.3109/1040841X.2010.532479
Rutherford ST, Bassler BL (2012) Bacterial quorum sensing: its role in virulence and possibilities for its control. Cold Spring Harbor Perspect Med 2(11). https://doi.org/10.1101/cshperspect.a012427
Hentzer M, Wu H, Andersen JB, Riedel K, Rasmussen TB, Bagge N, Kumar N, Schembri MA, Song Z, Kristoffersen P, Manefield M, Costerton JW, Molin S, Eberl L, Steinberg P, Kjelleberg S, Hoiby N, Givskov M (2003) Attenuation of Pseudomonas aeruginosa virulence by quorum sensing inhibitors. EMBO J 22(15):3803–3815. https://doi.org/10.1093/emboj/cdg366
Pushpakom S, Iorio F, Eyers PA, Escott KJ, Hopper S, Wells A, Doig A, Guilliams T, Latimer J, McNamee C, Norris A, Sanseau P, Cavalla D, Pirmohamed M (2019) Drug repurposing: progress, challenges and recommendations. Nat Rev Drug Discov 18(1):41–58. https://doi.org/10.1038/nrd.2018.168
Abbas HA, Shaldam MA, Eldamasi D (2020) Curtailing Quorum Sensing in Pseudomonas aeruginosa by Sitagliptin. Curr Microbiol 77:1051–1060. https://doi.org/10.1007/s00284-020-01909-4
Abbas HA, Hegazy WAH (2020) Repurposing anti-diabetic drug “Sitagliptin” as a novel virulence attenuating agent in Serratia marcescens. PLoS One 15(4):e0231625. https://doi.org/10.1371/journal.pone.0231625
Hegazy WAH, Khayat MT, Ibrahim TS, Nassar MS, Bakhrebah MA, Abdulaal WH, Alhakamy NA, Bendary MM (2020) Repurposing anti-diabetic drugs to cripple quorum sensing in pseudomonas aeruginosa. Microorganisms 8(9). https://doi.org/10.3390/microorganisms8091285
Cegelski L, Marshall GR, Eldridge GR, Hultgren SJ (2008) The biology and future prospects of antivirulence therapies. Nat Rev Microbiol 6(1):17–27. https://doi.org/10.1038/nrmicro1818
Rasko DA, Sperandio V (2010) Anti-virulence strategies to combat bacteria-mediated disease. Nat Rev Drug Discov 9(2):117–128. https://doi.org/10.1038/nrd3013
Papaioannou E, Utari PD, Quax WJ (2013) Choosing an appropriate infection model to study quorum sensing inhibition in Pseudomonas infections. Int J Mol Sci 14(9):19309–19340. https://doi.org/10.3390/ijms140919309
Abbas HA, Hegazy WAH (2017) Targeting the virulence factors of Serratia marcescens by ambroxol. Roum Arch Microbiol Immunol 76(2):27–32
Nalca Y, Jansch L, Bredenbruch F, Geffers R, Buer J, Haussler S (2006) Quorum-sensing antagonistic activities of azithromycin in Pseudomonas aeruginosa PAO1: a global approach. Antimicrob Agents Chemother 50(5):1680–1688. https://doi.org/10.1128/AAC.50.5.1680-1688.2006
Issac Abraham SV, Palani A, Ramaswamy BR, Shunmugiah KP, Arumugam VR (2011) Antiquorum sensing and antibiofilm potential of Capparis spinosa. Arch Med Res 42(8):658–668. https://doi.org/10.1016/j.arcmed.2011.12.002
Hegazy WAH (2016) Diclofenac inhibits virulence of Proteus mirabilis isolated from diabetic foot ulcer. Afr J Microbiol Res 10(21):733–743. https://doi.org/10.5897/AJMR2016.8043
Matsuyama T, Kaneda K, Nakagawa Y, Isa K, Hara-Hotta H, Yano I (1992) A novel extracellular cyclic lipopeptide which promotes flagellum-dependent and -independent spreading growth of Serratia marcescens. J Bacteriol 174(6):1769–1776. https://doi.org/10.1128/jb.174.6.1769-1776.1992
Krishnan T, Yin WF, Chan KG (2012) Inhibition of quorum sensing-controlled virulence factor production in Pseudomonas aeruginosa PAO1 by Ayurveda spice clove (Syzygium aromaticum) bud extract. Sensors 12(4):4016–4030. https://doi.org/10.3390/s120404016
Abdelmoteleb A, Troncoso-Rojas R, Gonzalez-Soto T, Gonzalez-Mendoza D (2017) Antifungical activity of autochthonous Bacillus subtilis isolated from Prosopis juliflora against Phytopathogenic fungi. Mycobiology 45(4):385–391. https://doi.org/10.5941/MYCO.2017.45.4.385
Rossignol G, Merieau A, Guerillon J, Veron W, Lesouhaitier O, Feuilloley MG, Orange N (2008) Involvement of a phospholipase C in the hemolytic activity of a clinical strain of Pseudomonas fluorescens. BMC Microbiol 8:189. https://doi.org/10.1186/1471-2180-8-189
Slater H, Crow M, Everson L, Salmond GP (2003) Phosphate availability regulates biosynthesis of two antibiotics, prodigiosin and carbapenem, in Serratia via both quorum-sensing-dependent and -independent pathways. Mol Microbiol 47(2):303–320. https://doi.org/10.1046/j.1365-2958.2003.03295.x
Guelfi M, Pantalone A, Cambiaso Daniel J, Vanni D, Guelfi MG, Salini V (2015) Arthrodesis of proximal inter-phalangeal joint for hammertoe: intramedullary device options. J Orthopaed Traumatol 16(4):269–273. https://doi.org/10.1007/s10195-015-0360-0
Ramanathan S, Ravindran D, Arunachalam K, Arumugam VR (2018) Inhibition of quorum sensing-dependent biofilm and virulence genes expression in environmental pathogen Serratia marcescens by petroselinic acid. Antonie Van Leeuwenhoek 111(4):501–515. https://doi.org/10.1007/s10482-017-0971-y
Srinivasan R, Mohankumar R, Kannappan A, Karthick Raja V, Archunan G, Karutha Pandian S, Ruckmani K, Veera Ravi A (2017) Exploring the anti-quorum sensing and antibiofilm efficacy of phytol against Serratia marcescens associated acute pyelonephritis infection in wistar rats. Front Cell Infect Microbiol 7:498. https://doi.org/10.3389/fcimb.2017.00498
Kim HS, Lee SH, Byun Y, Park HD (2015) 6-Gingerol reduces Pseudomonas aeruginosa biofilm formation and virulence via quorum sensing inhibition. Sci Rep 5:8656. https://doi.org/10.1038/srep08656
Elfaky MA, El-Halawany AM, Koshak AE, Alshali KZ, El-Araby ME, Khayat MT, Abdallah HM (2020) Bioassay guided isolation and docking studies of a potential beta-lactamase inhibitor from Clutia myricoides. Molecules 25(11). https://doi.org/10.3390/molecules25112566
Memariani H, Memariani M, Ghasemian A (2019) An overview on anti-biofilm properties of quercetin against bacterial pathogens. World J Microbiol Biotechnol 35(9):143. https://doi.org/10.1007/s11274-019-2719-5
Gebreyohannes G, Nyerere A, Bii C, Sbhatu DB (2019) Challenges of intervention, treatment, and antibiotic resistance of biofilm-forming microorganisms. Heliyon 5(8):e02192. https://doi.org/10.1016/j.heliyon.2019.e02192
Hema M, Vasudevan S, Balamurugan P, Adline Princy S (2017) Modulating the Global Response Regulator, LuxO of V. cholerae quorum sensing system using a pyrazine dicarboxylic acid derivative (PDCA(py)): An Antivirulence Approach. Front Cell Infect Microbiol 7:441. https://doi.org/10.3389/fcimb.2017.00441
Fu O, Pukin AV, Quarles van Ufford HC, Kemmink J, de Mol NJ, Pieters RJ (2015) Functionalization of a Rigid Divalent Ligand for LecA, a Bacterial Adhesion Lectin. ChemistryOpen 4(4):463–470. https://doi.org/10.1002/open.201402171
Senwar KR, Sharma P, Reddy TS, Jeengar MK, Nayak VL, Naidu VG, Kamal A, Shankaraiah N (2015) Spirooxindole-derived morpholine-fused-1,2,3-triazoles: Design, synthesis, cytotoxicity and apoptosis inducing studies. Eur J Med Chem 102:413–424. https://doi.org/10.1016/j.ejmech.2015.08.017
Hansen MR, Jakobsen TH, Bang CG, Cohrt AE, Hansen CL, Clausen JW, Le Quement ST, Tolker-Nielsen T, Givskov M, Nielsen TE (2015) Triazole-containing N-acyl homoserine lactones targeting the quorum sensing system in Pseudomonas aeruginosa. Bioorg Med Chem 23(7):1638–1650. https://doi.org/10.1016/j.bmc.2015.01.038
Shanks RM, Stella NA, Brothers KM, Polaski DM (2016) Exploitation of a “hockey-puck” phenotype to identify pilus and biofilm regulators in Serratia marcescens through genetic analysis. Can J Microbiol 62(1):83–93. https://doi.org/10.1139/cjm-2015-0566
Shanks RM, Stella NA, Kalivoda EJ, Doe MR, O'Dee DM, Lathrop KL, Guo FL, Nau GJ (2007) A Serratia marcescens OxyR homolog mediates surface attachment and biofilm formation. J Bacteriol 189(20):7262–7272. https://doi.org/10.1128/JB.00859-07
Iguchi A, Nagaya Y, Pradel E, Ooka T, Ogura Y, Katsura K, Kurokawa K, Oshima K, Hattori M, Parkhill J, Sebaihia M, Coulthurst SJ, Gotoh N, Thomson NR, Ewbank JJ, Hayashi T (2014) Genome evolution and plasticity of Serratia marcescens, an important multidrug-resistant nosocomial pathogen. Genom Biol Evolut 6(8):2096–2110. https://doi.org/10.1093/gbe/evu160
Nicholson WL, Leonard MT, Fajardo-Cavazos P, Panayotova N, Farmerie WG, Triplett EW, Schuerger AC (2013) Complete Genome Sequence of Serratia liquefaciens Strain ATCC 27592. Genom Announc 1(4). https://doi.org/10.1128/genomeA.00548-13
Ang S, Horng YT, Shu JC, Soo PC, Liu JH, Yi WC, Lai HC, Luh KT, Ho SW, Swift S (2001) The role of RsmA in the regulation of swarming motility in Serratia marcescens. J Biomed Sci 8(2):160–169. https://doi.org/10.1007/bf02256408
Liaw SJ, Lai HC, Ho SW, Luh KT, Wang WB (2003) Role of RsmA in the regulation of swarming motility and virulence factor expression in Proteus mirabilis. J Med Microbiol 52(Pt 1):19–28. https://doi.org/10.1099/jmm.0.05024-0
Wei CF, Tsai YH, Tsai SH, Lin CS, Chang CJ, Lu CC, Huang HC, Lai HC (2017) Cross-talk between bacterial two-component systems drives stepwise regulation of flagellar biosynthesis in swarming development. Biochem Biophys Res Commun 489(1):70–75. https://doi.org/10.1016/j.bbrc.2017.05.077
Lin CS, Tsai YH, Chang CJ, Tseng SF, Wu TR, Lu CC, Wu TS, Lu JJ, Horng JT, Martel J, Ojcius DM, Lai HC, Young JD (2016) An iron detection system determines bacterial swarming initiation and biofilm formation. Sci Rep 6:36747. https://doi.org/10.1038/srep36747
Brothers KM, Stella NA, Romanowski EG, Kowalski RP, Shanks RM (2015) EepR Mediates Secreted-Protein Production, Desiccation Survival, and Proliferation in a Corneal Infection Model. Infect Immun 83(11):4373–4382. https://doi.org/10.1128/IAI.00466-15
Stella NA, Fender JE, Lahr RM, Kalivoda EJ, Shanks RM (2012) The LysR transcription factor, HexS, is required for glucose inhibition of prodigiosin production by serratia marcescens. Adv Microbiol 2(4):511–517. https://doi.org/10.4236/aim.2012.24065
Uphoff TS, Welch RA (1990) Nucleotide sequencing of the Proteus mirabilis calcium-independent hemolysin genes (hpmA and hpmB) reveals sequence similarity with the Serratia marcescens hemolysin genes (shlA and shlB). J Bacteriol 172(3):1206–1216. https://doi.org/10.1128/jb.172.3.1206-1216.1990
Reboud E, Bouillot S, Patot S, Beganton B, Attree I, Huber P (2017) Pseudomonas aeruginosa ExlA and Serratia marcescens ShlA trigger cadherin cleavage by promoting calcium influx and ADAM10 activation. PLoS Pathog 13(8):e1006579. https://doi.org/10.1371/journal.ppat.1006579
Lazzaro M, Krapf D, Garcia Vescovi E (2019) Selective blockage of Serratia marcescens ShlA by nickel inhibits the pore-forming toxin-mediated phenotypes in eukaryotic cells. Cell Microbiol 21(9):e13045. https://doi.org/10.1111/cmi.13045
Pramanik A, Konninger U, Selvam A, Braun V (2014) Secretion and activation of the Serratia marcescens hemolysin by structurally defined ShlB mutants. Int J Med Microbiol 304(3-4):351–359. https://doi.org/10.1016/j.ijmm.2013.11.021
Eligar VS, Bain SC (2013) A review of sitagliptin with special emphasis on its use in moderate to severe renal impairment. Drug Des Develop Therapy 7:893–903. https://doi.org/10.2147/DDDT.S32331
Fishman MR, Giglio K, Fay D, Filiatrault MJ (2018) Physiological and genetic characterization of calcium phosphate precipitation by Pseudomonas species. Sci Rep 8(1):10156. https://doi.org/10.1038/s41598-018-28525-4
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
This article does not include any studies with human participants. Animal experiments have been approved by Institutional Review Board (ethical committee) at the Faculty of Pharmacy, Zagazig University approved. The procedures were performed in compliance with the ARRIVE guidelines, in agreement with the U.K. Animals (Scientific Procedures) Act, 1986, and related guidelines (ECAHZU, 22 April 2015).
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Cristiano Gallina Moreira
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hegazy, W.A., Khayat, M.T., Ibrahim, T.S. et al. Repurposing of antidiabetics as Serratia marcescens virulence inhibitors. Braz J Microbiol 52, 627–638 (2021). https://doi.org/10.1007/s42770-021-00465-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-021-00465-8