Abstract
Long-term antibiotic treatment results in the spread of multi-drug resistance in Pseudomonas aeruginosa that complicates treatment. Anti-virulence agents can be viewed as alternative options that cripple virulence factors of the bacteria to facilitate their elimination by the host immunity. The use of nanoparticles in the inhibition of P. aeruginosa virulence factors is a promising strategy. This study aims to study the effect of metformin (MET), metformin nano emulsions (MET-NEs), silver metformin nano emulsions (Ag-MET-NEs) and silver nanoparticles (AgNPs) on P. aeruginosa virulence factors’ expression. The phenotypic results showed that MET-NEs had the highest virulence inhibitory activity. However, concerning RT-PCR results, all tested agents significantly decreased the expression of quorum sensing regulatory genes of P. aeruginosa; lasR, lasI, pqsA, fliC, exoS and pslA, with Ag-MET-NEs being the most potent one, however, it failed to protect mice from P. aeruginosa pathogenesis. MET-NEs showed the highest protective activity against pseudomonal infection in vivo. Our findings support the promising use of nano formulations particularly Ag-MET-NEs as an alternative against multidrug resistant pseudomonal infections via inhibition of virulence factors and quorum sensing gene expression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pseudomonas aeruginosa is a frequent etiological agent of opportunistic and hospital acquired infections (Pang et al. 2019). P. aeruginosa infection may trigger severe complications in immunocompromised patients and those suffering from respiratory, urinary tract and burn infections, sepsis, cystic fibrosis, osteomyelitis and endocarditis (Moradali et al. 2017). Several virulence factors mediate the pathogenicity of P. aeruginosa such as pili, flagella, pyocyanin, pyoverdin, elastase, hemolysins, proteases, rhamnolipids, exotoxin A and biofilm formation (Lee and Zhang 2015; Veesenmeyer et al. 2009). The overuse of antibiotics has contributed to the spread of multidrug resistant P. aeruginosa (MDR) infections (Aloush et al. 2006). Synthesis of new antibiotics is time-consuming and requires high cost-effectiveness. Moreover, rapid resistance advances shorten their lifetime (Boucher et al. 2013; Fernandes and Martens 2017). Therefore, new therapeutic approaches are required to tackle the problem of MDR organisms. One useful approach is anti-virulence therapy using FDA approved drugs (drug repurposing). This approach has the advantages of disarming of pathogens without killing them, and the availability of data on their safety and pharmacokinetics. This decreases the economic costs as well as the time needed for the process of drug development (Finlay and Falkow 1997; Miró-Canturri et al. 2019; Mullard 2012; Rasko and Sperandio 2010).
The capacity of P. aeruginosa to form biofilm is a major virulence factor. The biofilm forming cells are more tolerant to the antibiotics and the host immune system (Whiteley et al. 2001). In addition, P. aeruginosa produces pyocyanin pigment that interacts with molecular oxygen forming hydrogen peroxide (H2O2) and other reactive oxygen species (ROS). This leads to altered redox balance of the host tissues leading to the injury of cells and may lead to death (Price-Whelan et al. 2006). P. aeruginosa also produces proteases that degrade the host lung elastin resulting in lung damage which occurs during respiratory tract infections, particularly in patients with chronic cystic fibrosis (Kipnis et al. 2006). P. aeruginosa shows motility in one of three forms namely; swimming, swarming in addition to twitching motility (Floyd et al. 2016). Swarming cells in P. aeruginosa contribute to biofilm formation, antibiotic resistance and overexpression of numerous virulence factors (Coleman et al. 2021).
Quorum sensing ‘intercellular signaling network’ is the main regulator of bacterial virulence. This can occur through bacterial secretion of auto-inducers or signaling molecules, their concentration is directly proportional to the bacterial cell density. After reaching a maximal concentration, genes encoding virulence factors are activated (Davies et al. 1998). P. aeruginosa has four interacting QS signaling systems; las system and rhl system which depend on the secretion and recognition of N-acyl-homoserine lactone autoinducers (AHL), in addition to the Pseudomonas quinolone signal (PQS) system, and the integrated QS (IQS) system (Lee and Zhang 2015). This network of cell to cell communication gives P. aeruginosa the capacity to produce extracellular virulence factors merely at threshold concentration (Van Delden and Iglewski 1998).
The use of nanotechnology is required to overcome the global dilemma of bacterial resistance to antimicrobials (Wang et al. 2017). Nanomaterials vary between 1 and 100 nm in size and display distinct physical and chemical properties compared to their bulk matter (Wang et al. 2017). The use of materials in nanomeric size leads to greater interaction between bacteria and compound, eases their penetration into the cell, increases absorption and improves bioavailability (Jamil and Imran 2018; Zaidi et al. 2017). Nanomaterials can be synthesized by many methods such as chemical, physical in addition to biological methods (Kaur 2018). The radiation induced synthesis of nanomaterials has several advantages over the traditional methods, because of its simplicity, no need for excess reducing agents and no excessive oxidation products. In addition, products are completely reduced and present in a highly stable pure state (Remita et al. 1996).
Metformin is regarded as one of the most common oral hypoglycemics used for treatment of patients with type 2 diabetes mellitus. Chemically, metformin belongs to the biguanide moiety of drugs that is especially useful for obese patients (Essmat et al. 2020). Metformin disrupts the membrane permeability of bacteria. In addition, it can compromise bacterial cell walls enhancing the antibacterial activity of antibiotics by increasing their intracellular accumulation. Moreover, they modify the immune response leading to increasing resistance to infection (Coates et al. 2020; Liu et al. 2020; Xiao et al. 2020). In addition, it was reported to exhibit anti-virulence activity by interfering with quorum sensing that regulates the production of virulence factors such as biofilm, proteases, pyocyanin, elastase and hemolysin production of P. aeruginosa PAO1 in a study done by Abbas et al. (2017).
Since ancient times, metals have been used as antibacterial agents. Silver is one of the most widely used. This is attributed to its powerful antimicrobial activity as well as its low toxicity (Chen and Schluesener 2008). Silver nanoparticles (AgNPs) exhibit activity against various type of microorganisms such as viruses, bacteria and fungi (Murphy et al. 2015). Silver nanoparticles can be seen as the most effective nanomaterial that can be used against MDR bacteria, however, other metallic NPs like AuNPs, CuONPs, Fe3O2NPs and TiONPs show good activity (Dakal et al. 2016; Hemeg 2017; Slavin et al. 2017). In addition, several studies reported the anti-virulence activity of nanomaterials (Li et al. 2020; Loo et al. 2016; Qais et al. 2021).
The cytotoxic effects of AgNPs, documented in vitro studies in various cell lines, are governed by factors such as size, shape, coating, dose and cell type. In addition, toxicity and biodistribution studies, in vivo, following various routes of exposure, like inhalation, instillation, oral, dermal and intravenous, have established Ag translocation, accumulation, and toxicity to various organs (Ferdous and Nemmar 2020).
The current study aimed to evaluate the possible quorum sensing inhibitory activity of metformin and to determine for the first time whether it can be more effective in nanoform than in bulk. In addition, investigate if a combination of metformin with AgNPs may help in attenuation of P. aeruginosa virulence and pathogenicity.
Materials and methods
Media and chemicals
Mueller hinton agar (MHA) and mueller hinton broth (MHB), tryptone soya agar (TSA), tryptone soya broth (TSB), and MacConkey agar were obtained from Oxoid (St. Louis, USA). Other chemicals were of pharmaceutical grade. Metformin (MET) and silver nitrate were purchased commercially from Sigma Chemical Company, St. Louis, Mo, USA.
Bacterial isolates
The standard strain P. aeruginosa ATCC 27853 was used in this study. It was provided from the stock culture collection of Microbiology and Immunology Department, Faculty of medicine, Zagazig University. Six clinical MDR P. aeruginosa isolates (PA1, PA2, PA3, PA4, PA5 and PA10) were obtained from the stock culture collection of Microbiology and Immunology Department, Faculty of pharmacy, Zagazig University. They were obtained from patients with burn, surgical wound, respiratory tract and urinary tract infections. All isolates were maintained in MHB with 10–15% glycerol and kept at − 80 °C.
Metformin nano emulsionss and silver metformin nano emulsions preparation
In order to prepare both metformin nano emulsion (MET-NEs) and silver metformin nano emulsions (Ag-MET-NEs), the modified ultra-sonication method was used as referenced (Laxmi et al. 2015; Mosallam et al. 2021a).
In synthesis of MET-NEs and Ag-MET-NEs (O/W 30/70), both coconut oil oily phase and tween 80 emulsifier were added drop wise to aqueous phase of either MET in a concentration of 100 mg/mL or MET and already prepared AgNPs (100–0.05 mg/mL) using homogenizer at 10,000 rpm for 30 min for continuous stirring. Then, the ultrasonic sonicator was used to sonicate the emulsion for 1 h. For characterization of the prepared nano emulsions, different physicochemical parameters such as particle size and distribution in addition to zeta potential were measured at the National Center for Radiation Research and Technology (NCRRT), Cairo, Egypt. The charge of the particles determines the stability of the nano emulsions. Zeta potential was used to quantify the particle charge and it is detected by using the electrophoretic motion of the particles in an electrical field. DLS Zeta Sizer Technique (PSS-NICOMP 380-ZLS, USA) was used to measure zeta potential of the optimized formulation. The particle sizes of the prepared nano emulsions were performed by Transmission Electron Microscopy (TEM) using (JEOL electron microscope JEM-100 CX) at an accelerating speed of 80 kV.
Determination of minimum inhibitory concentrations (MICs) of the tested agents
The minimum inhibitory concentrations (MICs) of MET, MET-NEs (stock solution of 100 mg/mL, each), Ag-MET-NEs (100–0.05 mg/mL) and AgNPs (0.05 mg/mL) against P. aeruginosa were assessed using the broth micro-dilution method using 96-well microtiter plate according to the clinical and laboratory standards institute (CLSI) guidelines (Wikler 2006).
Phenotypic assay of P. aeruginosa virulence factors
Bioflm inhibition assay
The capacity of the tested clinical strains to produce biofilm was quantitatively assayed according to the method previously described by Abbas et al. (2017). The standard strain P. aeruginosa ATCC 27853 was previously reported to have strong biofilm forming capacity (Casciaro et al. 2019).
To test the inhibitory activity of tested agents against biofilms, the same procdure was repeated in the presence of 1/10 MIC of them. The following formula was used in order to calculate the biofilm inhibitory percentage (%);
Pyocyanin inhbition assay
The inhibitory activities of the tested agents against pyocyanin was assessed according to the method described by Das and Manefield (2012).
Swarming motility inhbition assay
In order to test the capacity of the tested agents to block the swarming motility of P. aeruginosa isolates, Krishnan et al. method was performed (Krishnan et al. 2012).
Total proteases inhbition assay
The effect of the tested agents on inhibition of total proteases by P. aeruginosa isolates was carried out using the modified skimmed milk broth method. P. aeruginosa overnight cultures in MHB with and without 1/10 MIC of the tested agents were centrifuged to obtain the supernatants. Aliquots of 500 μL of bacterial supernatants were incubated with 1 mL skimmed milk (1.25%) for 1 h at 37 °C. The decrease in optical density of skimmed milk was estimated at 600 nm using Biotek spectrofluorometer (USA) and considered as measure of proteolytic activity. The test was performed twice (El-Mowafy et al. 2014).
Assessment of the effect of the tested agents on the expression of some virulence genes in P. aeruginosa using qRT‑PCR
The ability of the tested drugs to downregulate the expression of QS controlled genes; namely lasR, lasI, pqsA, fliC, pslA and exoS in the standard strain P. aeruginosa ATCC 27853 was assessed by qRT-PCR. The total bacterial RNA extract was purified using TRIzol Reagent (15596026, Life Technologies, USA) according to the manufacturer instructions. In order to synthesize cDNA, QuantiTect Reverse Transcription Kit was used and it was amplified by Thermo Scientific Maximas SYBR Green/Fluorescein qPCR Master Mix. The primers used are presented (Table 1). The relative expression level of the tested genes was normalized to the housekeeping gene (rpoD) using the 2−ΔΔCt method (Livak and Schmittgen 2001). The experiment was performed in triplicate.
Evaluation of the efficacy of the tested agents on pathogenicity in mice model
The effect of the tested agents on the pathogenicity of P. aeruginosa ATCC 27853 was assessed by using mice as an infection animal model. The experment was performed in compliance to the local guidelines for animal welfare approved by the committee of The Institutional Animal Care and Use, Zagazig University (ZU-IACUC), Egypt (Approval number: ZU-IACUC/3/F/114/2020). The bacterial burden in mice was detected as previously reported by Deshmukh et al. with some modifications (Deshmukh et al. 2009). Overnight cultures of P. aeruginosa in MHB with and without 1/10 MIC of the tested agents were prepared. The cultures were centrifuged and the pellets were resuspended in buffered saline (PBS) to reach cell density equal to 2.5 × 107 CFU/mL. Seven random groups of 5–6 weeks old healthy albino mice (Mus musculus) with equal weights were included in the experiment. Each group consists of five mice was used for P. aeruginosa. In group 1, untreated bacteria in sterile PBS (100 µL) were used for intraperitoneal injection of the mice. In group 2, 100 µL of bacteria treated with MET were injected in mice, group 3 was injected with 100 µL of MET-NEs-treated bacteria, while group 4, was injected with 100 µL of Ag-MET-NEs-treated bacteria and group 5 was injected with 100 µL of AgNPs-treated bacteria. Two additional groups were used as negative controls; group 6, mice were intraperitoneally injected with sterile PBS (100 µL), while in group 7, mice were uninoculated. Normal feeding and aeration were given to all groups at room temperature. After 24 h postinfection, mice were anaesthetized, sacrificed, livers and kidneys were harvested, weighed and homogenized for enumeration of live bacterial cells as colony forming unit per gram (CFU/g).
Statistical analysis
The inhibitory activity of the tested agents against virulence factors was analyzed using GraphPad Prism 8 software (One Way ANOVA followed by Dunnett’s multiple comparison tests or Bonferroni’s multiple comparison test) at P < 0.05 for signifcance.
Results
Synthesis and cheracterization of metformin nano emulsions and silver metformin nano emulsions
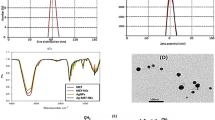
An important idiosyncratic property of nanoemulsion is its nanoscale particle size. The size distribution analysis of MET-NEs and Ag-MET-NEs was performed using DLS Zeta Sizer Technique. The illustration of the comparative particle size distribution of initially prepared AgNPs, MET-NEs and Ag-MET-NEs is shown in Fig. 1a. Figure shows size distribution with 23, 40 and 65 nm, respectively. Moreover, Fig. 1b shows the particle size distribution (DLS) of Ag-MET-NEs with 65 nm. Figure 2a shows the zeta potential at range from − 30 to 30 mV.
Figure 2b shows the TEM image of Ag-MET-NEs that confirms the circle shape of particles with average size of about 52 nm. The presence of metformin serving as capping and tween as stabilizing agents controls and prevents the aggregation and agglomeration of generated NPs.
Minimum inhibitory concentrations (MICs) of the tested agents against P. aeruginosa
Minimum inhibitory concentrations (MICs) were determined using the broth microdilution method. There was no difference between the MICs of MET and MET-NEs against the tested bacteria. However, the MICs were markedly lowered upon using the combination of MET and AgNPs (Ag-MET-NEs) compared with either MET, MET-NEs or AgNPs alone. Considering the increase in sensitivity to either MET or MET-NEs, the MICs were decreased by 16 to 128 folds, while for AgNPs (16–64) folds among the tested isolates (Table 2). The activity of the tested agents against quorum sensing and virulence of the tested isolates was evaluated at 1/10 MIC.
Phenotypic inhibition of virulence factors of P. aeruginosa by the tested agents
The tested agents inhibited biofilm formation
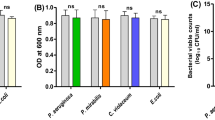
The biofilm inhibitory activities of the tested agents against P. aeruginosa were performed using crystal violet assay. The tested agents showed significant reduction in the biofilm formation compared to the control untreated isolates at (P < 0.05) as shown in (Fig. 3). A higher biofilm removal efficiency was found with MET-NEs (48.75–68.60%) and Ag-MET-NEs (22.09–54.84%) than MET (11.04–41.93%) and AgNPs (10.51–30.89%). The biofilm formation capacity of AgNPs was not significantly reduced in one isolate (PA5).
Inhibition of biofilm formation in P. aeruginosa by sub-MICs of the tested agents. A) Isolates PA1, PA2, and PA3, B) Isolates PA4, PA5, and PA27853 standard strain. Significant reduction in the biofilm formation was detected with 1/10 MIC of the tested agents against the tested isolates as compared to controls. Optical density was measured at 590 nm. The data shown represent the means ± standard errors. *Significant P < 0.05, ns non-significant
The tested agents decreased pyocyanin production
The effect of the tested agents on pyocyanin production of P. aeruginosa was estimated spectrophotometrically. The tested agents showed significant reduction in pyocyanin production compared to the untreated controls at (P < 0.05) as shown in (Fig. 4). MET-NEs showed the highest inhibitory activity against pyocyanin production (60.01–79.99%). However, the inhibitory activities of Ag-MET-NEs, MET and AgNPs were lower; 10.59–47.78%, 0.24–44.10% and 13.39–35.28%, respectively. No significant reduction in pyocyanin pigment by MET was observed in two isolates (PA1 and PA2). AgNPs showed significant increase in pyocyanin production in PA3, PA5 as well as the standard strain.
Effect of sub-MICs of the tested agents on pyocyanin production in P. aeruginosa. A) Isolates PA1, PA2, and PA3, B) Isolates PA4, PA5, and PA27853 standard strain, C) A representative image showing the effect of tested agents on pyocyanin. Pyocyanin pigment was measured at 691 nm, significant decline in production of pyocyanin pigment was observed in treated and untreated cultures. The data shown represent the means ± standard errors. *Significant P < 0.05, ns non-significant
The tested agents reduced swarming motility
The presence of sub-MICs concentration (1/10 MIC) of the tested agents significantly affected the swarming motility of all treated bacteria as compared to the untreated controls at (P < 0.05) as shown in (Fig. 5). MET-NEs showed maximum inhibition of swarming motility (88.87–94.16%) followed by MET (58.59–92.62%). Whereas, the inhibitory activities of Ag-MET-NEs (49.77–56.12%) and AgNPs (43.98–83.82%) were more or less similar.
Inhibition of swarming motility in P. aeruginosa by sub-MICs of the tested agents. A) Isolates PA1, PA2, and PA3, B) Isolates PA4, PA5, and PA27853 standard strain, C) A representative image showing the effect of tested agents on swarming motility. Significant reduction in swarming motility of all tested bacteria with 1/10 MIC as compared to controls. The data shown represent the means ± standard errors. *Significant P < 0.05
The tested agents decreased total proteases
The ability of the tested agents to inhibit proteolytic activity was measured using the modified skimmed milk broth method. It was found that the inhibitory activity of MET-NEs (77.48–99.15%) was higher than MET (35.48–66.32%). Also, Ag-MET-NEs (21.76–98.52%) exhibited higher proteolytic activity than AgNPs (17.90–48.88%). No significant inhibition of protease production was observed with either MET or AgNPs in some tested bacteria. However, AgNPs increased protease production in standard strain (49.19%) as compared to controls (Fig. 6).
Effect of 1/10 MICs of the tested agents on levels of protease. A) Isolates PA1, PA2, and PA3, B) Isolates PA4, PA5, and PA27853 standard strain. OD600 was measured after overnight culturing of bacteria in MHB with and without 1/10 MICs of the tested agents followed by incubation of supernatants with skim milk for 1 h at 37 °C. The data shown represent the means ± standard errors. *Significant P < 0.05, ns non-significant
The tested agent’s downregulated the expression of QS-regulatory genes using qRT-PCR
The influence of the tested agents on the relative expression of the genes that regulates the virulence factors’ production in the standard strain P. aeruginosa ATCC 27853 strain was assessed using qRT-PCR and the results were analyzed via the 2−ΔΔCt method. The expression levels of lasR, lasI, pqsA, fliC, exoS and pslA were significantly decreased after treatment with sub MICs of the tested agents compared to controls (Fig. 7). The expression level of lasI gene was reduced significantly; 37.58% with MET-NEs, up to 50% with either MET or AgNPs, while the highest percentage reduction was 65.45% with Ag-MET-NEs. With regards to lasR gene expression, the percentage reduction of MET was somehow comparable to that of AgNPs (34.62% and 40.38%), respectively, Ag-MET-NEs exhibited higher reduction (73.08%) than MET-NEs (56.62%). In addition, the expression level of pqsA gene was also significantly reduced; MET-NEs and Ag-MET-NEs showed higher reduction (41.56% and 55.41%), respectively than either MET or AgNPs that exhibited lower activities approximatey (27%) each. Moreover, Ag-MET-NEs showed the highest reduction in the expression of fliC gene (49.63%), however, MET-NEs (28.89%) and AgNPs (14.07%) had lower reduction. No significant reduction was observed after MET treatment. Furthermore, significant reduction in exoS gene expression was observed with MET, AgNPs and MET-NEs (41.67%, 43.75%, and 54.17%, respectively) with the highest reduction found with Ag-MET-NEs (73.84%). Concerning the relative expression of pslA, it was significantly diminished with MET-NEs and Ag-MET-NEs nearly (40%) each. However, no significant reduction was found with MET and AgNPs.
RT-qPCR showed reduced expression of A) lasI, B) lasR, C) pqsA, D) fliC, E) exoS and F) pslA with the tested agents in sub-MICs compared to untreated controls. The data shown are the means ± standard errors of three biological experiments with three technical replicates each. *Significant P < 0.05, ns non-significant
The tested agents decreased the bacterial load in liver and kidney tissues
To further study the anti-virulence activities of the tested agents, the bacterial load in livers and kidenys were estimated in the presence and absence of sub MICs of the tested agents using mice as an infection model. The live bacterial counts in the liver and kidney tissues of the mice treated groups were significantly lower than those of the untreated mice group (P < 0.05). The results were expressed as log CFU reduction in viable counts per gram of organ tissue. It was found that as compared to MET-NEs that completely eradicated bacteria in liver tissues, the mean log CFU reductions of viable counts decreased from 4.625 in untreated group to 3.090 and 3.895 in MET and AgNPs treated groups, respectively, while no significant reduction of the bacterial burden was observed with Ag-MET-NEs treated group (4.685). Similarly, MET-NEs successfully removed all live bacteria from kidney tissues, surpassing MET, Ag-MET-NEs and AgNPs with mean log CFU reductions from 6.120 in untreated group to 4.255, 5.265 and 3.080, respectively (Fig. 8).
Discussion
Antibiotic resistance has become a major health problem (Cegelski et al. 2008; Defoirdt 2018). Additionally, there has been a lack of novel antibiotic discoveries in the past decades (Ventola 2015). Antibiotic-resistant pathogens cause serious infections, this is considered as a major reason of morbidity and mortality; therefore new policies are required to tackle this problem such as drug repurosing (Prestinaci et al. 2015; Rangel-Vega et al. 2015; Thangamani et al. 2015).
Pseudomonas aeruginosa is a member of ESKAPE pathogens that include Pseudomonas aeruginosa, Klebsiella pneumoniae, Staphylococcus aureus, Enterococcus faecium, Enterobacter spp., and Acinetobacter baumannii, that are the leading cause of hospital acquired infections worldwide and possess potential drug resistance mechanisms (Santajit and Indrawattana 2016). Bacteria possess several virulence factors that enable them to infect their hosts and are quorum sensing (QS) regulated (Grandclement et al. 2016; LaSarre and Federle 2013). As a result, QS is considered as an attractive target for anti-virulence treatment.
Nanoparticles are widely used in different applications among which is treatment of bacterial infections and biofilms such as silver nanoparticles (Murphy et al. 2015). Silver nanoparticles (AgNPs) have superior bactericidal activity over Ag+ against both Gram negative and Gram positive bacteria (Kora and Arunachalam 2011; Kvítek et al. 2008; Martínez-Castañon et al. 2008). The likelihood of resistance to Ag is assumed to be low. Therefore, silver (Ag)-based compounds have seen a revival (Feng et al. 2000). Moreover, many nanomaterials were found to have anti-virulence activity against P. aeruginosa (Pham et al. 2019; Shah et al. 2019; Singh et al. 2015).
In the present study, the quorum sensing and virulence inhibitory activities of the tested agents were investigated against P. aeruginosa and using 1/10 MICs to avoid effect on bacterial growth and ensure that inhibitory effect on the tested bacteria is due to anti-virulence activity rather than killing them.
In the current study, the size of Ag-MET-NEs measured in DLS technique is larger than that of AgNPs and MET-NEs. This may be attributed to the hydrophilic properties of Ag-MET-NEs and surrounding molecules. This can be explained in the light of the fact that the particle size is not related only to the metallic NPs’ core, as all adsorbed materials on the surface of NPs such as stabilizers also have an impact on NPs’ particle size. Moreover, the particle size may be affected by the thickness of the electrical bilayer that moves along the nanoparticles (El-Batal et al. 2020).
The best approach of testing the nanoemulsion stability can be accomplished by validation of the particle size in addition to zeta potential along the time. Slight variations in these properties can be detected using an applicable technique, such as DLS.
On testing the Ag-MET-NEs, they were found to be stable. This can be obtained when the values of zeta potential are high (above − 30 mV and less than 30 mV). This ensures that high energy barriers were created to prevent coalescence of the dispersed droplets (Zhao et al. 2010). Our results showed no phase separation or other signs that denote samples′ instability. It is noteworthy that even the particle size changes found in the initially prepared or stored samples were of no significance. Moreover, the stability of the particles of nanoemulsions that are sufficiently small can be correlated with the zeta potential. As previously mentioned, high zeta potential of nanoparticles confers their stability, while low zeta potential denotes that the emulsions will break out and flocculate because of the higher attraction forces than the repulsive ones of the emulsion (Jadhav et al. 2015).
The DLS size ranges of Ag-MET-NEs were higher than measurements by TEM. This can be explained by the fact that DLS measurments are confined or restricted to the NPs′ hydrodynamic diameters. The larger size of NPs measured may be due to the encasement of amphiphilic nanoparticles within water molecules. TEM measures the actual diameters of the nanoparticles (Bendary et al. 2021; El-Batal et al. 2021).
In the current study, it was found that MET-NEs showed similar antibacterial activity to MET against P. aeruginosa. However, the combination of MET and AgNPs (Ag-MET-NEs) exhibited high synergistic activity that than that of MET-NEs or AgNPs alone (Table 2). Similar result was observed by Li et al. (2020), who compared between the antibacterial activity of biguanide-based polymetformin (PMET) and its nanoform FTP NPs which made from F-127 surfactant, tannic acid and PMET and found that both showed similar activity against the tested bacteria. In addition, Polyhexamethylene biguanide (PHMB), a cationic biocide functionalized silver nanoparticles were tested for their antimicrobial activity against E. coli, it was found that PHMB enhanced the antimicrobial properties of AgNPs of about 100 times compared to the previous reports of AgNPs (Ashraf et al. 2012). Another study done by Yi et al. reported better bactericidal effect of AgNPs-PHMB as compared to AgNPs and PHMB against S. aureus (Yi et al. 2019).
The combined action of the O/W MET-AgNEs, we recommend that, the silver nanoparticles entered into the oily phase and coated by metformin moiety through physical interaction improves release of drug into the target site, and the nano emulsion improved the antibacterial and anti-biofilm activities against different organisms (Prakash et al. 2020) and inactivated the microorganisms more than the standard (Pathania et al. 2018). The nano emulsion systems promote their interaction with the microbial cell membranes by four main routes (Mosallam et al. 2021b); (1) the augmented extent and transport through the outer plasma membrane that increases the interaction with the cytoplasmic membranes; (2) the fusion of the emulsifier droplets with the phospholipid bilayer of the cell membrane that likely promotes the targeted release of the essential oils at the required sites; (3) the sustained release over time of the essential oils from the nano emulsion droplets, driven by essential oils partition between the oil droplets and also the aqueous phase, that prolongs the activity of essential oils; and (4) the electrostatic interaction of charged nano emulsions droplets with charged microbial cell walls that increases the concentration of essential oils at the positioning of action.
Pseudomonas aeruginosa has the capability to form biofilm that confers resistance to antibiotics by up to one thousand fold more than planktonic cells which has a major role in bacterial resistance and pathogenesis (Loo et al. 2014; Mah and O’Toole 2001). The incapability of the antimicrobials to penetrate the biofilm matrix (one of the main causes of bacterial resistance) could be overcome via using nanostructures showing anti-biofilm activity (Ansari et al. 2014; Shah et al. 2013). In the present study, it was found that MET-NEs or Ag-MET-NEs demonstrated synergistic activities as they were more effective than either MET or AgNPs alone. Abbas et al. (2017) reported higher percentage reduction of PAO1 biofilm by metformin (67.9%). Metformin also enhanced gold nanoparticles′ antibacterial activities and biofilm eradication (Rasko and Sperandio 2010). A study done by Li et al. (2020) reported that FTP NPs surpassed PMET with ∼ 100-fold (∼ 2log10) greater reduction of MRSA USA300 biofilm bacterial cell counts. In addition, several studies reported antibacterial and anti-biofilm activities of Polyhexamethlene biguanide (PHMB) against a variety of bacterial species (Kamaruzzaman et al. 2017; Lefebvre et al. 2018). Moreover, the studies on another biguanide compound chlorhexidine showed contradictory results. Abdallah and Abakar (2017) reported that chlorhexidine significantly reduced S. aureus biofilm depending on the contact time and concentration used, while in another study, chlorhexidine showed no bactericidal effect on S. aureus biofilm (Vestby and Nesse 2015). Furthermore, biosynthesized AgNPs reduced the P. aeruginosa PAO1 biofilm by < 70% as reported (Hussain et al. 2019; Qais et al. 2020, 2021). On the other hand, Yang and Alvarez (2015) reported that exposure of P. aeruginosa PAO1 to non-lethal polyvinylpyrrolidone-coated silver nanoparticles concentration led to increased biofilm formation, enhanced extracellular polymer substances, lipopolysaccharide biosynthesis, and upregulation of antibiotic resistance genes.
The redox active pyocyanin pigment enables P. aeruginosa to penetrate host cell membranes and interfere with host cellular functions leading to cellular damage (Hall et al. 2016). In the current study, the lowest inhibition of pyocyanin pigment was found with MET. However, the activity was greatly enhanced using their nanoform. No significant difference between Ag-MET-NEs and AgNPs was observed. This can be attributed to some kind of chemical interaction between MET and AgNPs in the combination (Ag-MET-NEs). Surprisingly, AgNPs increased pyocyanin production in some tested bacteria. Metformin showed higher reduction in pyocyanin pigment in a study reported by Abbas et al. (2017). Several studies showed much higher inhibition by biosynthesized AgNPs than the current study such as Qais et al. (2020, 2021)However, Ellis et al. (2018) reported that P. aeruginosa can resist AgNPs by producing phenazine pigments (pyocyanin, pyoverdine, and pyochelin). Pyocyanin can reduce Ag+ to Ag0, thus it protects the bacteria from the damage caused by silver ions emitted from nanoparticles. Similarly, this result was also in good agreement with Muller and Merrett (2014).
Quorum sensing-controlled swarming motility is essential for P. aeruginosa pathogenesis as well as biofilm formation (Pamp and Tolker-Nielsen 2007). In the current study, MET-NEs was the most potent inhibitor of swarming motility as well as biofilm formation, suggesting a role of swarming motility in the biofilm interruption, which is in agreement with Shah et al. (2019). MET-NEs successfully blocked swarming motility more than MET, whereas Ag-MET-NEs and AgNPs similarly impaired bacterial swarming. Hussain et al. (2019) reported higher inhibition of P. aeruginosa PAO1 swarming motility using biosynthesized silver nanoparticle.
Proteases degrade immunoglobulins and fibrin as well as they disrupt epithelial tight junctions (Kipnis et al. 2006). In the current study, the inhibition of proteolytic activity using MET-NEs surpassed that of MET alone. Similarly, the inhibitory activity of Ag-MET-NEs was more than AgNPs alone. In a study reported by Abbas et al. (2017), metformin was capable of reducing the proteolytic activity, which was consistent with the current study. In recent studies done by Qais et al. (2020, 2021), biosynthesized AgNPs caused higher percentage reduction of P. aeruginosa PAO1 exoprotease activity than the present study.
Pseudomonas aeruginosa possesses a unipolar flagellum, which is composed of a polymer of flagellin protein subunits, encoded by the fliC gene. It is responsible for mobility and chemotaxis, in addition to helping in the attachment of the bacterium to host cells and non-living surfaces, which augments the ability to colonize and invade during the earlier stages of infections (Haiko and Westerlund-Wikstrom 2013). P. aeruginosa also produces exoS, a major cytotoxin implicated in stages of colonization, invasion and dissemination during infection (Bradbury et al. 2010). The three exopolysaccharides namely; Pel, Psl, and alginate have important roles in surface attachment, biofilm formation and stability. The pslA gene encodes psl (Billings et al. 2013; Ghafoor et al. 2011).
In the current study, the genes tested are the quorum sensing encoding genes. quorum sensing is the mechanism of cell-to-cell communication. Each cell secretes a molecule of autoinducers, so the concentration of the autoinducers is proportional to the cell density. When, the concentration of autoinducers reaches a certain threshold value, this reflects that the cell density or population reaches the quorum. At this point, the virulence genes are activated. The decrease in the expression of quorum sensing genes means that the tested agents downregulated these genes, and as a result, the production of virulence factors will be reduced. This is a confirmation of the results of the phenotypic investigation.
The expression levels of QS regulating genes lasR, lasI, pqsA, fliC, exoS and pslA were assessed by qRT-PCR. It was found that the tested inhibitors significantly downregulated the expression levels of such QS regulatory genes (Fig. 7). Ag-MET-NEs was the most potent inhibitor against QS regulatory genes followed by MET-NEs, and both were more effective than either the bulk MET or AgNPs, which had relatively similar QS inhibitory activity. This confirms that the quorum quenching activity of the nanoform surpassed that of its bulk one and also suggests some kind of synergism between MET and AgNPs in the combination (Ag-MET-NEs). Hegazy et al. (2020) reported that the expression levels of rhlI/R, lasI/R and pqsA/R was decreased using sub-MIC of metformin. Silver nanoparticles downregulated the expression of rhlI/R and lasI/R through inhibition of rhlR and lasR (Singh et al. 2015). Also, Mahnaie and Mahmoudi (2020) discovered that glutathione-stabilized silver nanoparticles exhibited antibiofilm activity in P. aeruginosa via lowering the lasI/R expression. In addition, Abdelraheem and Mohamed (2021) found that, except for the toxA gene, all biofilm and virulence genes of P. aeruginosa clinical isolates were significantly downregulated after ZnO NPs treatment. On the other hands, Costabile et al. (2015) reported that the QS inhibitory activity of the anthelmintic drug niclosamide (NCL) formulated as nanosuspensions (T80_10 or T80_10 DP) was equivalent to that of the unformulated NCL predissolved in DMSO. However, there are other systems required for P. aeruginosa virulence such as two compartment system (Francis et al. 2017).
For the in vivo study, it was found that drugs with anti-virulence activity reduced the colonization rates of invading pathogens as it aided the immune system in eradicating the infection. Regarding the log CFU reduction in viable counts, mice group treated with MET-NEs completely eliminated the living bacteria from livers and kidneys of sacrificed mice, being the most potent among the tested inhibitors; its bulk form (MET), AgNPs and even Ag-MET-NEs combination which may require further research. In addition, the formulation of metformin in nanoform has much lower accumulation than its bulk form or metal nanoparticles, thus reducing cytoxicity that occurs after exposure. Similarly, NCL formulated as nanosuspensions had lower toxicity in a rat lung infection model involving P. aeruginosa (Costabile et al. 2015).
In the current study, on the contrary to MET that caused approximately 30% clearance of P. aeruginosa ATCC 27853 infection from collected mice tissues, Hegazy et al. (2020), reported that metformin failed to protect mice from P. aeruginosa PAO1 (ATCC BAA47B1). Escárcega-González et al. (2018) reported the capability of AgNPs to reduce CFUs in a murine skin infection model in rats caused by a clinical strain of P. aeruginosa as compared to the untreated group.
Conclusion
In conclusion, targeting bacterial virulence and QS offers an alternative strategy because it curbs the bacterial ability to harm the host rather than affecting their growth, and reduces the emergence of MDR pseudomonal infections. This benefit can be maximized via repurposing of FDA approved medications. Metformin is FDA approved antidiabetic drug with QS inhibitory activity against MDR P. aeruginosa. In the present study, it was found that the formulation of metformin in nanoform was promising because it exhibited distinct physical, chemical and bioligical properties as compared to its bulk. In addition, the combination between MET and AgNPs showed synergistic antibacterial effect as well as it greatly inhibited the QS regulatory genes of P. aeruginosa.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article.
References
Abbas HA, Elsherbini AM, Shaldam MA (2017) Repurposing metformin as a quorum sensing inhibitor in Pseudomonas aeruginosa. Afr Health Sci 17:808–819
Abdallah W, Abakar M (2017) Effect of chlorhexidine and sodium hypochlorite on Staphylococcus aureus. Biofilm J Prev Infect Control https://doi.org/10.21767/2471-9668.100035
Abdelraheem WM, Mohamed ES (2021) The effect of zinc oxide nanoparticles on Pseudomonas aeruginosa biofilm formation and virulence genes expression. J Infect Dev Ctries 15:826–832
Aloush V, Navon-Venezia S, Seigman-Igra Y, Cabili S, Carmeli Y (2006) Multidrug-resistant Pseudomonas aeruginosa: risk factors and clinical impact. Antimicrob Agents Chemother 50:43–48
Ansari MA, Khan HM, Khan AA, Cameotra SS, Pal R (2014) Antibiofilm efficacy of silver nanoparticles against biofilm of extended spectrum β-lactamase isolates of Escherichia coli and Klebsiella pneumoniae. Appl Nanosci 4:859–868
Ashraf S, Akhtar N, Ghauri MA, Rajoka MI, Khalid ZM, Hussain I (2012) Polyhexamethylene biguanide functionalized cationic silver nanoparticles for enhanced antimicrobial activity. Nanoscale Res Lett 7:267
Bendary MM, Ibrahim D, Mosbah RA, Mosallam F, Hegazy WA, Awad NF, Alshareef WA, Alomar SY, Zaitone SA, El-Hamid A (2021) Thymol nanoemulsion: a new therapeutic option for extensively drug resistant foodborne pathogens. Antibiotics 10:25
Billings N, Millan M, Caldara M, Rusconi R, Tarasova Y, Stocker R, Ribbeck K (2013) The extracellular matrix component Psl provides fast-acting antibiotic defense in Pseudomonas aeruginosa biofilms. PLoS Pathog 9:e1003526
Boucher HW, Talbot GH, Benjamin DK Jr, Bradley J, Guidos RJ, Jones RN, Murray BE, Bonomo RA, Gilbert D, America IDSo (2013) 10×′20 progress—development of new drugs active against Gram-negative bacilli: an update from the infectious diseases society of America. Clin Infect Dis 56:1685–1694
Bradbury RS, Roddam LF, Merritt A, Reid DW, Champion AC (2010) Virulence gene distribution in clinical, nosocomial and environmental isolates of Pseudomonas aeruginosa. J Med Microbiol 59:881–890
Casciaro B, Lin Q, Afonin S, Loffredo MR, de Turris V, Middel V, Ulrich AS, Di YP, Mangoni ML (2019) Inhibition of Pseudomonas aeruginosa biofilm formation and expression of virulence genes by selective epimerization in the peptide Esculentin-1a (1–21) NH 2. FEBS J 286:3874–3891
Cegelski L, Marshall GR, Eldridge GR, Hultgren SJ (2008) The biology and future prospects of antivirulence therapies. Nat Rev Microbiol 6:17–27
Chen X, Schluesener HJ (2008) Nanosilver: a nanoproduct in medical application. Toxicol Lett 176:1–12
Coates AR, Hu Y, Holt J, Yeh P (2020) Antibiotic combination therapy against resistant bacterial infections: synergy, rejuvenation and resistance reduction. Expert Rev Anti Infect Ther 18:5–15
Coleman SR, Pletzer D, Hancock REW (2021) Contribution of swarming motility to dissemination in a Pseudomonas aeruginosa murine skin abscess infection model. J Infect Dis 224:726–733
Costabile G, d’Angelo I, Rampioni G, Bondì R, Pompili B, Ascenzioni F, Mitidieri E, di Villa D, Bianca R, Sorrentino R, Miro A (2015) Toward repositioning niclosamide for antivirulence therapy of Pseudomonas aeruginosa lung infections: development of inhalable formulations through nanosuspension technology. Mol Pharm 12:2604–2617
Dakal TC, Kumar A, Majumdar RS, Yadav V (2016) Mechanistic basis of antimicrobial actions of silver nanoparticles. Front Microbiol 7:1831
Das T, Manefield M (2012) Pyocyanin promotes extracellular DNA release in Pseudomonas aeruginosa. PLoS ONE 7:e46718
Davies DG, Parsek MR, Pearson JP, Iglewski BH, Costerton JW, Greenberg EP (1998) The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science 280:295–298
Defoirdt T (2018) Quorum-sensing systems as targets for antivirulence therapy. Trends Microbiol 26:313–328
Deshmukh HS, Hamburger JB, Ahn SH, McCafferty DG, Yang SR, Fowler VG Jr (2009) Critical role of NOD2 in regulating the immune response to Staphylococcus aureus. Infect Immun 77:1376–1382
El-Batal AI, Nasser HA, Mosallam FM (2020) Fabrication and characterization of cobalt hyaluronic acid nanostructure via gamma irradiation for improving biomedical applications. Int J Biol Macromol 147:1328–1342
El-Batal AI, Ragab YM, Amin MA, El-Roubi GM, Mosallam FM (2021) Investigating the antimicrobial, antioxidant and cytotoxic activities of the biological synthesized glutathione selenium nano-incorporation. Biometals 34:815–829
El-Demerdash AS, Bakry NR (2020) Evaluation of the synergistic effect of amikacin with cefotaxime against Pseudomonas aeruginosa and its biofilm genes expression. Gene expression and phenotypic traits. IntechOpen, London
Ellis DH, Maurer-Gardner EI, Sulentic CE, Hussain SM (2018) Silver nanoparticle antibacterial efficacy and resistance development in key bacterial species. Biomed Phys Eng Express 5:015013
El-Mowafy SA, Abd El Galil KH, El-Messery SM, Shaaban MI (2014) Aspirin is an efficient inhibitor of quorum sensing, virulence and toxins in Pseudomonas aeruginosa. Microb Pathog 74:25–32
Escárcega-González CE, Garza-Cervantes JA, Vazquez-Rodríguez A, Montelongo-Peralta LZ, Treviño-Gonzalez MT, Castro EDB, Saucedo-Salazar EM, Morales RC, Soto DR, González FT (2018) In vivo antimicrobial activity of silver nanoparticles produced via a green chemistry synthesis using Acacia rigidula as a reducing and capping agent. Int J Nanomed 13:2349
Essmat N, Soliman E, Mahmoud MF, Mahmoud AA (2020) Antidepressant activity of anti-hyperglycemic agents in experimental models: a review. Diabetes Metab Syndr 14(5):1179–1186
Feng QL, Wu J, Chen GQ, Cui FZ, Kim TN, Kim JO (2000) A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J Biomed Mater Res 52:662–668
Fernandes P, Martens E (2017) Antibiotics in late clinical development. Biochem Pharmacol 133:152–163
Finlay BB, Falkow S (1997) Common themes in microbial pathogenicity revisited. Microbiol Mol Biol Rev 61:136–169
Floyd M, Winn M, Cullen C, Sil P, Chassaing B, Yoo DG, Gewirtz AT, Goldberg JB, McCarter LL, Rada B (2016) Swimming motility mediates the formation of neutrophil extracellular traps induced by flagellated Pseudomonas aeruginosa. PLoS Pathog 12:e1005987
Francis VI, Stevenson EC, Porter SL (2017) Two-component systems required for virulence in Pseudomonas aeruginosa. FEMS Microbiol Lett. https://doi.org/10.1093/femsle/fnx104
Ghafoor A, Hay ID, Rehm BH (2011) Role of exopolysaccharides in Pseudomonas aeruginosa biofilm formation and architecture. Appl Environ Microbiol 77:5238–5246
Grandclement C, Tannieres M, Morera S, Dessaux Y, Faure D (2016) Quorum quenching: role in nature and applied developments. FEMS Microbiol Rev 40:86–116
Haiko J, Westerlund-Wikstrom B (2013) The role of the bacterial flagellum in adhesion and virulence. Biology 2:1242–1267
Hall S, McDermott C, Anoopkumar-Dukie S, McFarland AJ, Forbes A, Perkins AV, Davey AK, Chess-Williams R, Kiefel MJ, Arora D, Grant GD (2016) Cellular effects of pyocyanin, a secreted virulence factor of Pseudomonas aeruginosa. Toxins 8(8):236
Hegazy WAH, Khayat MT, Ibrahim TS, Nassar MS, Bakhrebah MA, Abdulaal WH, Alhakamy NA, Bendary MM (2020) Repurposing anti-diabetic drugs to cripple quorum sensing in Pseudomonas aeruginosa. Microorganisms 8(9):1285
Hemeg HA (2017) Nanomaterials for alternative antibacterial therapy. Int J Nanomed 12:8211–8225
Hussain A, Alajmi MF, Khan MA, Pervez SA, Ahmed F, Amir S, Husain FM, Khan MS, Shaik GM, Hassan I, Khan RA, Rehman MT (2019) Biosynthesized silver nanoparticle (AgNP) from pandanus odorifer leaf extract exhibits anti-metastasis and anti-biofilm potentials. Front Microbiol 10:8
Jadhav C, Kate V, Payghan SA (2015) Investigation of effect of non-ionic surfactant on preparation of griseofulvin non-aqueous nanoemulsion. Journal of Nanostructure in Chemistry 5:107–113
Jamil B, Imran M (2018) Factors pivotal for designing of nanoantimicrobials: an exposition. Crit Rev Microbiol 44:79–94
Kamaruzzaman NF, Chong SQY, Edmondson-Brown KM, Ntow-Boahene W, Bardiau M, Good L (2017) Bactericidal and anti-biofilm effects of polyhexamethylene biguanide in models of intracellular and biofilm of Staphylococcus aureus isolated from bovine mastitis. Front Microbiol 8:1518
Kaur P (2018) Biosynthesis of nanoparticles using eco-friendly factories and their role in plant pathogenicity: a review. Biotechnol Res Innov 2:63–73
Kipnis E, Sawa T, Wiener-Kronish J (2006) Targeting mechanisms of Pseudomonas aeruginosa pathogenesis. Med Et Mal Infect 36:78–91
Kora AJ, Arunachalam J (2011) Assessment of antibacterial activity of silver nanoparticles on Pseudomonas aeruginosa and its mechanism of action. World J Microbiol Biotechnol 27:1209–1216
Krishnan T, Yin W-F, Chan K-G (2012) Inhibition of quorum sensing-controlled virulence factor production in Pseudomonas aeruginosa PAO1 by ayurveda spice clove (Syzygium aromaticum) bud extract. Sensors 12:4016–4030
Kvítek L, Panáček A, Soukupova J, Kolář M, Večeřová R, Prucek R, Holecová M, Zbořil R (2008) Effect of surfactants and polymers on stability and antibacterial activity of silver nanoparticles (NPs). J Phys Chem C 112:5825–5834
LaSarre B, Federle MJ (2013) Exploiting quorum sensing to confuse bacterial pathogens. Microbiol Mol Biol Rev 77:73–111
Laxmi M, Bhardwaj A, Mehta S, Mehta A (2015) Development and characterization of nanoemulsion as carrier for the enhancement of bioavailability of artemether. Artif Cells Nanomed Biotechnol 43:334–344
Lee J, Zhang L (2015) The hierarchy quorum sensing network in Pseudomonas aeruginosa. Protein Cell 6:26–41
Lefebvre E, Lembre P, Picard J, El-Guermah L, Seyer D, Larreta Garde V (2018) Ephemeral biogels to control anti-biofilm agent delivery: from conception to the construction of an active dressing. Mater Sci Eng C 82:210–216
Li J, Zhong W, Zhang K, Wang D, Hu J, Chan-Park MB (2020) Biguanide-derived polymeric nanoparticles kill MRSA biofilm and suppress infection in vivo. ACS Appl Mater Interfaces 12:21231–21241
Liu Y, Jia Y, Yang K, Li R, Xiao X, Zhu K, Wang Z (2020) Metformin restores tetracyclines susceptibility against multidrug resistant bacteria. Adv Sci 7:1902227
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 25:402–408
Loo CY, Young PM, Cavaliere R, Whitchurch CB, Lee WH, Rohanizadeh R (2014) Silver nanoparticles enhance Pseudomonas aeruginosa PAO1 biofilm detachment. Drug Dev Ind Pharm 40:719–729
Loo CY, Rohanizadeh R, Young PM, Traini D, Cavaliere R, Whitchurch CB, Lee WH (2016) Combination of silver nanoparticles and curcumin nanoparticles for enhanced anti-biofilm activities. J Agric Food Chem 64:2513–2522
Mah TF, O’Toole GA (2001) Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol 9:34–39
Martínez-Castañon G-A, Nino-Martinez N, Martinez-Gutierrez F, Martinez-Mendoza J, Ruiz F (2008) Synthesis and antibacterial activity of silver nanoparticles with different sizes. J Nanopart Res 10:1343–1348
Miró-Canturri A, Ayerbe-Algaba R, Smani Y (2019) Drug repurposing for the treatment of bacterial and fungal infections. Front Microbiol 10:41
Moradali MF, Ghods S, Rehm BH (2017) Pseudomonas aeruginosa lifestyle: a paradigm for adaptation, survival, and persistence. Front Cell Infect Microbiol 7:39
Mosallam FM, Helmy EA, Bendary MM, El-Batal AI (2021a) Potency of a novel synthesized Ag-eugenol nanoemulsion for treating some bacterial and fungal pathogens. J Mater Res. https://doi.org/10.1557/s43578-021-00226-1
Mosallam FM, Helmy EA, Bendary MM, El-Batal AI (2021b) Potency of a novel synthesized Ag-eugenol nanoemulsion for treating some bacterial and fungal pathogens. J Mater Res 36:1524–1537
Mullard A (2012) Drug repurposing programmes get lift off. Nat Rev Drug Discov 11:505–506
Muller M, Merrett ND (2014) Pyocyanin production by Pseudomonas aeruginosa confers resistance to ionic silver. Antimicrob Agents Chemother 58:5492–5499
Murphy M, Ting K, Zhang X, Soo C, Zheng Z (2015) Current development of silver nanoparticle preparation, investigation, and application in the field of medicine. J Nanomater 2015:1–12
Pamp SJ, Tolker-Nielsen T (2007) Multiple roles of biosurfactants in structural biofilm development by Pseudomonas aeruginosa. J Bacteriol 189:2531–2539
Pang Z, Raudonis R, Glick BR, Lin TJ, Cheng Z (2019) Antibiotic resistance in Pseudomonas aeruginosa: mechanisms and alternative therapeutic strategies. Biotechnol Adv 37:177–192
Pathania R, Kaushik R, Khan MA (2018) Essential oil nanoemulsions and their antimicrobial and food applications. Curr Res Nutr Food Sci J 6:626–643
Pham DTN, Khan F, Phan TTV, Park SK, Manivasagan P, Oh J, Kim YM (2019) Biofilm inhibition, modulation of virulence and motility properties by FeOOH nanoparticle in Pseudomonas aeruginosa. Braz J Microbiol 50:791–805
Pourmbarak Mahnaie M, Mahmoudi H (2020) Effect of glutathione-stabilized silver nanoparticles on expression of las I and las R of the genes in Pseudomonas aeruginosa strains. Eur J Med Res 25:17
Prakash A, Baskaran R, Nithyanand P, Vadivel V (2020) Effect of nanoemulsification on the antibacterial and anti-biofilm activities of selected spice essential oils and their major constituents against Salmonella enterica Typhimurium. J Clust Sci 31:1123–1135
Prestinaci F, Pezzotti P, Pantosti A (2015) Antimicrobial resistance: a global multifaceted phenomenon. Pathog Glob Health 109:309–318
Price-Whelan A, Dietrich LE, Newman DK (2006) Rethinking ‘secondary’ metabolism: physiological roles for phenazine antibiotics. Nat Chem Biol 2:71–78
Qais FA, Shafiq A, Ahmad I, Husain FM, Khan RA, Hassan I (2020) Green synthesis of silver nanoparticles using Carum copticum: assessment of its quorum sensing and biofilm inhibitory potential against Gram negative bacterial pathogens. Microb Pathog 144:104172
Qais FA, Ahmad I, Altaf M, Manoharadas S, Al-Rayes BF, Abuhasil MSA, Almaroai YA (2021) Biofabricated silver nanoparticles exhibit broad-spectrum antibiofilm and antiquorum sensing activity against Gram-negative bacteria. RSC Adv 11:13700–13710
Rangel-Vega A, Bernstein LR, Mandujano Tinoco E-A, García-Contreras S-J, García-Contreras R (2015) Drug repurposing as an alternative for the treatment of recalcitrant bacterial infections. Front Microbiol 6:282
Rasko DA, Sperandio V (2010) Anti-virulence strategies to combat bacteria-mediated disease. Nat Rev Drug Discov 9:117–128
Remita S, Mostafavi M, Delcourt M (1996) Bimetallic Ag–Pt and Au–Pt aggregates synthesized by radiolysis. Radiat Phys Chem 47:275–279
Roberts AE, Maddocks SE, Cooper RA (2015) Manuka honey reduces the motility of Pseudomonas aeruginosa by suppression of flagella-associated genes. J Antimicrob Chemother 70:716–725
Santajit S, Indrawattana N (2016) Mechanisms of antimicrobial resistance in ESKAPE pathogens. Biomed Res Int 2016:2475067
Shah SR, Tatara AM, D’Souza RN, Mikos AG, Kasper FK (2013) Evolving strategies for preventing biofilm on implantable materials. Mater Today 16:177–182
Shah S, Gaikwad S, Nagar S, Kulshrestha S, Vaidya V, Nawani N, Pawar S (2019) Biofilm inhibition and anti-quorum sensing activity of phytosynthesized silver nanoparticles against the nosocomial pathogen Pseudomonas aeruginosa. Biofouling 35:34–49
Singh BR, Singh BN, Singh A, Khan W, Naqvi AH, Singh HB (2015) Mycofabricated biosilver nanoparticles interrupt Pseudomonas aeruginosa quorum sensing systems. Sci Rep 5:13719
Slavin YN, Asnis J, Hafeli UO, Bach H (2017) Metal nanoparticles: understanding the mechanisms behind antibacterial activity. J Nanobiotechnol 15:65
Thangamani S, Mohammad H, Younis W (2015) Drug repurposing for the treatment of staphylococcal infections. Curr Pharm Des 21:2089–2100
Van Delden C, Iglewski BH (1998) Cell-to-cell signaling and Pseudomonas aeruginosa infections. Emerg Infect Dis 4:551–560
Veesenmeyer JL, Hauser AR, Lisboa T, Rello J (2009) Pseudomonas aeruginosa virulence and therapy: evolving translational strategies. Crit Care Med 37:1777–1786
Ventola CL (2015) The antibiotic resistance crisis: part 1: causes and threats. Pharm Ther 40:277
Vestby LK, Nesse LL (2015) Wound care antiseptics-performance differences against Staphylococcus aureus in biofilm. Acta Vet Scand 57:1–5
Wang L, Hu C, Shao L (2017) The antimicrobial activity of nanoparticles: present situation and prospects for the future. Int J Nanomed 12:1227–1249
Whiteley M, Bangera MG, Bumgarner RE, Parsek MR, Teitzel GM, Lory S, Greenberg EP (2001) Gene expression in Pseudomonas aeruginosa biofilms. Nature 413:860–864
Wikler MA (2006) Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically: approved standard. CLSI (NCCLS) 26:M7-A7
Xiao Y, Liu F, Li S, Jiang N, Yu C, Zhu X, Qin Y, Hui J, Meng L, Song C (2020) Metformin promotes innate immunity through a conserved PMK-1/p38 MAPK pathway. Virulence 11:39–48
Yang Y, Alvarez PJ (2015) Sublethal concentrations of silver nanoparticles stimulate biofilm development. Environ Sci Technol Lett 2:221–226
Yi J, Zhang Y, Lin W, Niu B, Chen Q (2019) Effect of polyhexamethylene biguanide functionalized silver nanoparticles on the growth of Staphylococcus aureus. FEMS Microbiol Lett 366:4
Zaidi S, Misba L, Khan AU (2017) Nano-therapeutics: a revolution in infection control in post antibiotic era. Nanomed Nanotechnol Biol Med 13:2281–2301
Zhao Y, Wang C, Chow AH, Ren K, Gong T, Zhang Z, Zheng Y (2010) Self-nanoemulsifying drug delivery system (SNEDDS) for oral delivery of Zedoary essential oil: formulation and bioavailability studies. Int J Pharm 383:170–177
Ferdous Z, Nemmar A (2020) Health impact of silver nanoparticles: a review of the biodistribution and toxicity following various routes of exposure. Int J Mol Sci 21:2375
Acknowledgements
The authors would like, Department of Microbiology and Immunology-Faculty of Pharmacy-Zagazig University-Zagazig-Egypt and Drug Microbiology Lab, Drug Radiation Research Department, National Center for Radiation Research and Technology (NCRRT), Egypt.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). No funding provided.
Author information
Authors and Affiliations
Contributions
Conceptualization, HAA, GHS FMM, and SEG; methodology, SEG and FMM; software, HAA, SEG; validation, HAA, FMM.; SEG; formal analysis, HAA, FMM.; SEG; investigation, HAA, SEG; data curation, SEG and FMM; writing—original draft preparation, HAA, SEG and FMM; writing review and editing, HAA, GHS; supervision, HAA, GHS and FMM.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare there is no conflict of interest.
Ethical approval and consent to participate
The ethical standards for animal welfare approved by The Institutional Animal Care and Use Committee, Zagazig University (ZU-IACUC), Egypt (Approval number: ZU IACUC/3/F/114/2020). All procedures in this study were performed in accordance with relevant guidelines.
Constant of participate and publish
All authors agree to participate and publish.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gomaa, S.E., Shaker, G.H., Mosallam, F.M. et al. Knocking down Pseudomonas aeruginosa virulence by oral hypoglycemic metformin nano emulsion. World J Microbiol Biotechnol 38, 119 (2022). https://doi.org/10.1007/s11274-022-03302-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-022-03302-8