Abstract
The waste-to-energy (WTE) technologies are now recovering energy and materials from over 300 million tonnes of municipal solid wastes worldwide. Extensive studies have investigated substituting natural construction materials with WTE residues to relieve the environmental cost of natural resource depletion. This study examined the beneficial uses of WTE residues in civil engineering applications and the corresponding environmental standards in Europe, the U.S., and China. This review presents the opportunities and challenges for current technical approaches and the environmental standards to be met to stabilize WTE residues. The principal characteristics of WTE residues (bottom ash and fly ash) and the possible solutions for their beneficial use in developed and developing countries are summarized. The leaching procedures and environmental standards for pH, heavy metals, and polychlorinated dibenzo-p-dioxins/furans (PCDD/Fs) are compared. The current practice and engineering properties of materials using WTE residues, including mixtures with stone aggregate or sand, cement-based or hot-mix asphalt concrete (pavement), fill material in the embankments, substitute of Portland cement or clinker production, and ceramic-based materials (bricks and lightweight aggregate) are comprehensively reviewed.
Graphical abstract

Similar content being viewed by others
Introduction
Municipal solid waste (MSW) is a heterogeneous source with significant energy and material potential. Worldwide, the generation of MSW is consistently increasing yearly, from 2.01 billion tonnes in 2016 to 2.59 billion tonnes by 2030 and 3.40 billion tonnes annually by 2050 [1]. The MSW management depends on various factors, including MSW composition, gross domestic product (GDP), population density, land source, education, policy/regulations, infrastructures, etc. [2,3,4,5,6,7]. After all possible recycling and composting (e.g., fermentation, anaerobic digestion, etc.), two options for a significant fraction are called the post-recycled MSW: landfilling or waste-to-energy (WTE). The term waste-to-energy (or WTE) is now commonly used for all types of controlled combustion with energy and metals/materials recovery. The disposal of MSW has become a high cost; thus, the order of priority of various waste management methods indicated that sanitary landfilling is lower than WTE [8].
The combustion process converts chemical energy in postrecycled MSW to produce superheated steam to drive a turbine for electricity generation [9,10,11,12,13]. Grate combustion is the most common and simplest technology for MSW applied worldwide, operating at 850–1000 °C with complete oxidation, which can reduce the MSW volume by 85%–90% and the mass by 80% [8, 14, 15]. WTE has many advantages over landfilling, including land saving, electricity generation, and reducing associated greenhouse gas (GHG) [16].
The combustion process of MSW produces two residues: bottom ash and fly ash, in the daily generation ratio of 6:1 to 10:1 [17]. The bottom ash is the combustion residue discharged from the end of the grate furnace, about 20% of the total MSW, classified as nonhazardous waste. In comparison, fly ash is the air pollution control (APC) residue, commonly about 1%–5% of the total MSW, which is classified as hazardous waste because it contains heavy metals and polychlorinated dibenzo-p-dioxins/furans (PCDD/Fs). The proper beneficial uses of WTE residues as resourceful materials require environmental specifications, regulations, and local legislation related to specific leaching procedures and standards regionally that were used as a guideline to evaluate the treatment effectiveness [18]. However, the reuse of WTE fly ash is a challenging topic worldwide due to its hazardous characteristics, which may require proper pretreatment [19,20,21].
As a renewable energy source, there were over 512 WTE plants in Europe in 2016, providing a total capacity of 93 million tonnes [22]. In 2020, European Union 27 countries (EU-27), 61 million tonnes of MSW (27% of total MSW) were treated by WTE, estimated to generate 14 million tonnes of residues annually, as shown in Fig. 1 (fly ash is estimated to be about 3% of the total MSW for EU and the U.S.) [23]. Following the circular economy principles, the European Commission encourages the utilization of the mineral fraction of WTE bottom ash (after proper ferrous and nonferrous metals recycling) as secondary construction materials in the civil engineering sector [24, 25]. Even though a significant variance is present in the reuse rate, from 0 to 100% in different countries, the overall reutilization rate in the EU in construction works is approximately 54%, as indicated by the previous findings that utilization does not depend on how well-regulated bottom ash utilization is, but as a result of political commitment to bottom ash recycling and economic interest [26]. Besides, European countries have restricted laws and legislation that prevent the mix of bottom ash and fly ash, and the management involves appropriate treatment prior to a final disposal of fly ash, including backfilling to underground mines or quarry, and chemical−physical stabilization with binding agents (commonly cement) followed by landfilling at the current stage [26, 27].
Currently, 75 WTE facilities are operated in the 25 states of the U.S., mainly in the Northeast, combusting about 29 million tonnes (11% of total MSW) in 2018 and generating about 7 million tonnes of residues annually [28, 29]. A large amount of land, less dense population, and low economic cost of MSW sanitary landfill caused the WTE facilities in the U.S. to be less common than in the EU countries (Fig. 1). U.S. WTE industries process combined ash (the mix of fly ash and bottom ash in the daily generation ratios) as a single stream and dispose of it in the landfills after it passes the Toxic Characteristic Leaching Procedure (TCLP) [17, 30]. About 10% of WTE combined ash is reused in road construction and landfill cover [31, 32]. In recent years, researchers and scientists in the U.S. have been driving public attention to the GHG emissions from landfills to convert the waste management framework to WTE technologies and enhance the energy and metals/materials recovery, which requires the support and development from the legislation and interests in markets [33,34,35,36,37,38,39].
China has risen to 228 million tonnes of MSW generated nationwide in 2018, with 102 million tonnes of WTE capacity; the MSW generation is concentrated in eastern coastal provinces due to the dense pollution, developed economy, and urbanization [40]. The annual generation of total residues in China is estimated to be about 26 million tonnes (fly ash is estimated to be 5% of the total MSW, considering that the differentials of combustion processes in China generated more residues from the APC system). Cement stabilization/solidification is the most common way of fly ash disposal in landfills, which can be implemented easily at a relatively low cost [41]. Besides, the existing cement kiln treats approximately 6% of the total fly ash generated in China [42]. However, there is no clear data source for the bottom ash reutilization rate [31].
This review innovatively compared the beneficial uses of WTE residues in civil engineering applications from technical perspectives and their corresponding environmental standards in European countries, the U.S., and China, presenting the opportunities and challenges to verify the effectiveness of WTE residues stabilization, the gaps of promoting WTE residues to reutilization, and the demands from construction. The overall characteristics of WTE residues (bottom ash and fly ash), and their practice in civil engineering applications, were also summarized, which indicates the possible solutions and framework to apply the beneficial uses of WTE residues in current and future scenarios to meet the demands of both developed and developing countries.
Common standards and speciation
Leaching procedure and standards
Heavy metals are the predominant concern for the environmental impacts when the WTE residues are disposed of in landfills or reused as construction materials. Meanwhile, some elements or components (e.g., chlorine, dissolved organic carbon (DOC), etc.) also have environmental concerns; if the waste is discharged to the landfills, it may generate pollutants in the soil or groundwater through the leaching. A comparison of the most commonly used leaching procedures used in European countries (the UK is listed as a representative), the U.S., and China are shown in Fig. 2, where the detailed procedure and parameters are summarized from the standards listed in Sects. “EU leaching procedure and standards”, “The U.S. leaching procedure and standards”, and “China leaching procedure and standards” [41, 43,44,45,46,47,48,49]. Owing to the pH being a primary influence on the leachability of heavy metals, the investigations and measurement of pH in the different regions are also included.
EU leaching procedure and standards
The European Committee for Standardization (CEN) developed standards and guidance on the leaching tests and chemical analysis to evaluate the End-of-life waste materials: CEN/TC 292 “Characterization of waste” and CEN/TC 351 “Construction products-Assessment of the release of dangerous substances” [45, 50,51,52]. CEN/TC 292 consists of basic characterization (Liquid-to-solid ratio (L/S), leachant composition, pH redox, complexing capacity, and physical parameters), compliance testing, and onsite verification. CEN/TS 16637-2 of CEN/TC 351 applied tank test: dynamic monolithic leaching test at liquid-to-surface area ratio (L/A) of 8 cm3/cm2 by demineralized water as a function of time [53]. The following specific tests are proposed for the leaching properties as summarized in Table 1 for (1) pH dependence tests (CEN/TS 14429, CEN/TS 14997) [54, 55]; (2) Percolation tests (CEN/TS 14405, CEN/TS 16637-3 of CEN/TC 351) [56, 57]; and (3) Batch tests (EN 12457–1, 2, 3, 4) [50, 58,59,60,61].
The EU members are required by EU legislation to follow the CEN standards; thus, the individual countries derived their own regulations and criteria based on their specific situations [62]. One of the widely mentioned version in academic publications is the BS EN 12457–2 (Characterization of waste-Leaching-Compliance test for leaching of granular waste materials and sludges-Part 2): One-stage batch test at a liquid-to-solid ratio of 10 mL/g for materials with particle size below 4 mm (with or without particle size reduction) [44]. The leaching results by deionized water via BS EN 12457–2 are used to compare a solid to the UK waste acceptance criteria (WAC, Table 2), which classify landfill sites as suitable for hazardous, nonhazardous, or inert waste [43, 63, 64].
The U.S. leaching procedure and standards
TCLP and Resource Conservation and Recovery Act (RCRA) standards
The EPA Method 1311-TCLP is currently applied in the U.S. industry, as described in Table 3 [49]. It determines whether the waste is below the levels mandated by the RCRA and, thus, can be disposed of in nonhazardous landfills [68]. The leaching results of the TCLP test are compared to eight RCRA metal regulatory standards (Table 4) to determine whether the wastes can be disposed of in nonhazardous landfills (MSW or sanitary landfills) or hazardous landfills in the U.S. [49, 69, 70].
Leaching Environmental Assessment Framework (LEAF)
The LEAF is useful in estimating the environmental impacts of utilizing secondary materials, primarily as construction materials or disposal scenarios [46]. The LEAF evaluation system consists of four leaching methods summarized in Table 1. Method 1313-Liquid–Solid Partitioning as a Function of Extract pH Using a Parallel Batch Extraction Procedure; Method 1314-Liquid–Solid Partitioning as a Function of Liquid–Solid Ratio for Constituents in Solid Materials Using an Up-Flow Percolation Column Procedure; Method 1315-Mass Transfer Rates of Constituents in Monolithic or Compacted Granular Materials Using a Semi-Dynamic Tank Leaching Procedure; Method 1316-Liquid–Solid Partitioning as a Function of Liquid–Solid Ratio Using a Parallel Batch Extraction Procedure [48, 65,66,67].
Among these four methods, Method 1313 of pH dependence (Table 1) is the most important test to investigate the leachability of constituents [48]. The parallel batch extractions of solid material receive equilibrium conditions over a wide range of eluate pH values (0.5–13). Specimens are crushed and milled to the particle sizes (Table 5) for different minimum dry mass, contact time, and extracted with HNO3 solutions of various concentrations at a liquid/solid ratio of 10 mL solution per gram of dry sample. The samples are mixed in a turn-over-turn at 28 rpm and room temperature, 20 °C. The equilibrium (eluate) pH is recorded after the rotating reaction. The extracted solutions are filtered through 0.45 \(\mu m\) filtration membranes for leachability analysis.
The UK WAC limits meet the BS EN 12457–2 leaching test. A study by International Solid Waste Association (ISWA) stated that the leaching criteria for utilization of WTE residues must be at least the same as or lower than the leaching criteria (WAC) for disposal to landfill [32]. Currently, there are no criteria or landfill limits for the U.S. EPA LEAF tests, while the procedure and parameters of Method 1313 using water leaching are the same as BS EN 12457–2. Thus, it is suggested by authors that using deionized water leaching, following the Method 1313 procedure in their previous research, and the results were compared to the WAC limits as a reference for the future enaction of LEAF criteria [17, 30, 76, 77]. This comparison can contribute to estimating the environmental influence of reusing WTE residues as secondary construction materials in the U.S.
China leaching procedure and standards
This section summarized the commonly used leaching procedure and standards by previous articles from China. The corrosivity of WTE residues is measured by the Solid Waste-Glass Electrode Test-Method of Corrosivity (GB/T 15555.12–1995) by using 100 g-dry samples with 1 L distilled water (liquid-to-solid ratio (L/S) at 10 mL/g) mixing in a shaker for 8 h at room temperature, and settling down for 16 h before the pH value measurement. The residues were considered corrosive when pH value <2 or \(\ge\)12.5 [72, 78]. Meanwhile, the Code for Fly Ash Test-Method (DL/T5532-2017) is used for the pH value measurement of WTE residues, following the 10 g-dry samples immersed in the 200 mL distilled water (L/S at 20 mL/g), mixed for 10 min on the shaker, settled down for 30 min before the pH value measurement [72, 79].
The procedure measures the total concentration of heavy metals in WTE residues: Soil Quality-Analysis of Soil Heavy Metals-Atomic Absorption Spectrometry with Aqua Regia Digestion (NY/T 1613–2008) [80]. Previous researchers adjusted the procedure, using 1 g of WTE residue samples (sieved less than 0.149 mm) added with 10 mL of aqua regia (HNO3:HCl=1:3 in volume, L/S=100 mL/g), setting in the boiling water bath for 2-h of heating, shaking 1–2 times, then cooling the digested solutions to room temperature and using the supernatant for Inductively coupled plasma optical emission spectroscopy (ICP-OES) measurement [72].
The leaching toxicity of WTE residues was measured by the Solid Waste-Extraction Procedure for Leaching Toxicity-Acetic Acid Buffer Solution Method (HJ/T300-2007) [41, 71,72,73,74]. The procedure of HJ/T300-2007 is compared to that of the U.S. EPA TCLP test, as shown in Table 3. The heavy metals leaching toxicity measurement in Chinese standards is similar to that in U.S. EPA TCLP standards. Landfill Site of Municipal Solid Waste (GB 16889–2008) is used as the standard to evaluate the leachability of heavy metals of WTE residues after proper treatment for safe disposal in nonhazardous landfills in China (Table 4) [75].
Dioxin-related procedure and speciation
Dioxin refers to a group of toxic chemical compounds sharing certain chemical structures, and biological characteristics, including PCDDs, polychlorinated dibenzofurans (PCDFs), and certain polychlorinated biphenyls (PCBs), those with chlorines at positions 2,3,7 and 8 are toxic [81]. The public perception of WTE fly ash is sometimes negative because it contains dioxins, another challenge of reusing it in civil engineering applications [82,83,84,85]. The U.S. EPA established a comprehensive dioxin database, methods, and tools to monitor and measure dioxin for controlling emissions and preventing exposure [86].
EPA Method 1613B measures tetra-through octa-chlorinated dioxins and furans (PCDD/Fs) by high-resolution gas chromatography/high-resolution mass spectrometry (HRGC/HRMS) using isotope dilution to quantify very low concentrations of individual PCDD/Fs congeners [87]. This method has been widely applied as pretreatment steps (extraction, concentration, and column separation) for dioxins concentration measurement of WTE fly ash in academic research papers in recent years [88,89,90]. Besides, EPA Method 8280B (detection limit 1–5 ppm in ash samples) and Method 8290A (detection limit at parts-per trillion (ppt) to parts-per quadrillion (ppq)), while less sensitive than Method 1613B, were also used in the previous papers [91,92,93].
For landfill disposal, the concentration of dioxins in WTE fly ash must meet the environmental standard of 3 ng/g in China according to the China national standard GB 16889–2008, which is significantly lower than the U.S. hazardous limit of dioxin disposal at 10 ng/g and EU Statutory Order on Persistent Organic Pollutants at 15 ng/g [41, 74, 75, 77, 94]. However, due to WTE fly ash being categorized as hazardous waste, there is still a long journey to be beneficially reused as a resourceful material in civil engineering applications. A previous study also mentioned that dioxins and furans do not easily leach, while releasing these contaminants is of major concern because of their toxicity [95]. In August 2020, China launched the first technical specification (HJ 1134–2020) for reusing treated WTE fly ash as a resource for further application, which required the total remaining dioxins (not exceeding 50 ng-TEQ/kg-dry via the treatment processes, including low-temperature thermal decomposition, high-temperature sintering, or high-temperature melting, etc.), heavy metals leachability (standards GB 8978 by using HJ 557), total Cl (<2%, preferred to <1%), the standards for reuse as a cement manufacturing feed material (GB 30485 and HJ 662), and not allowed to be used in sintering brick [96,97,98,99,100].
Characteristics of WTE residues
For bottom ash, the ash discharge system after the combustion chamber significantly influences its characteristics (Table 6) and potential beneficial uses. The wet-discharge system by Ulrich Martin drops the combusted ash particles from the end of the moving grate into a water tank for quenching and forming agglomerated bottom ash, which is the most common ash discharge process [101, 102]. The water and air sealing wall in the discharger prevents the flue gas and other pollutants from being released outside the furnace. The ram continuously pushes the bottom ash to the drop-off edge. The freshly quenched bottom ash was characterized by amorphous and microcrystalline calcium-silicate-hydrate phases and the formation of quench products (e.g., calcite, Friedel’s salt, hydrocalumite, portlandite, etc.) were controlled by very fine particles (<0.425 mm) [103]. The processing parameters in the quenching water tank (e.g., water content, etc.) may also influence the characteristics of bottom ash. The bottom ash in the U.S. (Fig. 3a) are stone-like granulate particles, while the bottom ash in China WTE facilities is dustier and looser.
The wet-discharged bottom ash commonly contains moisture of about 10%–60%, which is classified as a nonhazardous material; except for moisture, the bottom ash typically contains 50%–70% mineral fraction, 15%–30% glass and ceramics, 5%–13% ferrous metals, 2%–5% nonferrous metals, and 1%–5% unburned organics [17, 31, 68, 104,105,106]. Modern WTE facilities commonly apply ferrous (magnetic separation) and nonferrous (eddy current) metal recycling units to extract metal scraps from bottom ash; thus, the remaining stone-like fractions after metal recycling can be used in civil engineering applications depending on the ash characteristics [107]. The particle size distribution of bottom ash is significant when the material is used as a substitute for secondary building material or other utilization purposes [108]. A previous review study reported that 60%–90% of ash is found in 0.02–10 mm, whereas 0–30% bottom ash may contain >10 mm larger particles, which are ferrous and nonferrous metals, slag, and construction-type waste materials, which can be further recycled in metal separation processes [31]. Tian 2022 also found that 80% of the bottom ash after metal recycling is 1–20 mm, as shown in Fig. 3 [17].
To increase the mechanical metal recycling rate, the dry discharge system was developed in WTE facilities in Japan and Switzerland, which showed a 45% increase in ferrous metals and a 50% increase in nonferrous metals over the wet-discharge method, also resulting in a significant fine fraction generated (approximately 45% of total bottom ash) due to the absence of water during quenching [109].
Table 7 presents the elemental concentrations of WTE bottom ash and fly ash [17, 110]. Fly ash is dominant in Si (202 g/kg, average value, same as followed) and Ca (150 g/kg), and has a high concentration of Ca (222 g/kg) and Cl (145 g/kg) due to the hydrated lime slurry injection in the APC system and its reaction with HCl in the flue gas. Pb (3513 mg/kg), Zn (9 g/kg), and Cd (178 mg/kg) are mainly considered as the concern of leaching of toxicity, which may release into the soil or groundwater and cause environmental risks. Tian et al. systematically investigated the detailed characteristics of fly ash [110].
The ternary composition reviewed from the literature is normalized and presented in Fig. 4, when compared with the composition of ordinary Portland cement (OPC), the common cementitious supplementary materials from industrial residues (blast-furnace slag, coal fly ash Class C/F, coal bottom ash), waste glass, silica fume, and some clays commonly applied in cement material (bentonite, kaolin, metakaolin) [166,167,168,169,170,171,172,173]. The experience of utilizing other industrial residues or aluminosilicate materials also references the beneficial uses of WTE residues.
CaO–Al2O3–SiO2 ternary diagram for the composition of industrial residues and other aluminosilicate materials data reviewed by the author (Tian 2022) [17]
Beneficial uses of WTE residues in civil engineering applications
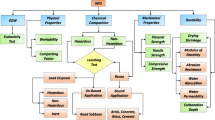
Numerous studies (Fig. 5) of the uses of WTE residues are categorized based on demands of civil engineering applications. The applications of WTE bottom ash are more widely accepted and discussed in this review study than fly ash because it is a nonhazardous material, and its generation is 6–10 times the latter.
Alternative aggregates in concrete or cement-based concrete pavement
U.S. American Society for Testing and Materials (International standards organization, ASTM) C33/C33M Standard Specification for Concrete Aggregates defines the requirements for grading and quality of the coarse and fine aggregates for use in concrete with different nominal sizes (sieves with Square Openings) [174]. Typically, the coarse aggregate sizes are larger than 4.75 mm and generally range between 9.5 mm and 37.5 mm; the fine aggregate sizes are less than 4.75 mm, and the limit for material finer than the 75 μm (No. 200 sieve) shall be 5.0% maximum [174,175,176]. Natural coarse aggregate is extracted from mines, consisting of gravel (deposits), crushed gravel, and crushed stone for particle size, angularity, and texture demands. In addition to stone, sand, as another essential industrial construction material, is the most consumed natural resource in the world after water and has become a limited resource in recent years due to exhausted extraction. The sand (fine aggregates) is classified as fine sand (0.075–0.425 mm), medium sand (0.425–2 mm), and coarse sand (2–4.75 mm), consisting of pit sand (2–4.75 mm), river sand (0.06–2 mm), sea sand (0.06–2 mm, containing salt, so it is avoided for concrete structure), manufactured sand (0.6–4.75 mm) [177,178,179,180]. Industrial residues in similar particle size ranges and strengths are expected to be used as supplementary sand materials to relieve the shortage of sand.
The granulated WTE bottom ash is used extensively to replace coarse aggregates due to the increasing environmental cost of natural mining stone. In contrast, the sorting of sizes in WTE bottom ash varies depending on the applications of the particle size ranges discussed above. Most of the previous studies explored the performance of WTE bottom ash in particle sizes >4.75 mm [111, 181, 182]. Some modern WTE facilities use a 9.5 mm screen to separate the sizes of WTE bottom ash mainly for enhancing the recovery of nonferrous metals; therefore, bottom ash <9.5 mm to replace coarse aggregate was investigated in a previous study [183].
In Denmark, UK, the Netherlands, and Norway, the utilization rate of the mineral fraction of bottom ash is more than 60% in concrete structures or cement-based concrete pavement [31, 32, 184, 185]. The compressive strength varies from 17 MPa (2500 psi) to 28 MPa (4000 psi) and is higher in residential and commercial structural concrete. Research using mixed bottom ash and fly ash (ratio 90/10) completely replacing both natural coarse and fine aggregates showed a compressive strength above 15 MPa, which is suitable for use as nonstructural concrete [24]. A prior study by the author examines the performance of WTE combined ash (the mix of bottom ash and fly ash in the daily generation ratio) after washing and crushing in the sizes of 9.5–25 mm and 2–9.5 mm as coarse aggregate 100% substitute natural crushed stone received exceeding 28 MPa of compressive strength in produced structural concrete [30]. To produce granulate aggregate materials with low dust and chloride and higher mechanical strength, typical pretreatments of bottom ash involve washing and natural weathering before further processing [24, 183, 186,187,188], which results in significant improvements in the quality of the mineral fractions for cement-based concrete production [189].
As for the replacement of fine aggregate, the fine size previous studies widely investigated the size range of bottom ash <4.75 mm or 5 mm [181, 182, 190,191,192,193,194], and some studies investigated the performance of bottom ash <2.36 mm or 2 mm [112, 195, 196]. The fine size bottom ash is a lightweight porous material containing metallic aluminum, which caused the reaction with alkaline compounds in cementitious phases and generated hydrogen gas, resulting in bubbling and swelling in cement products [17, 30]. The highest cumulative amount of hydrogen gas collected in the particle size ranges (<0.6 mm, 0.6–1 mm, 1–2 mm, 2–4.75 mm) within 20 days was 8.4–39.4 L/kg-dry bottom ash [197]. To relieve the swelling, academic research has explored alkaline treatment for bottom ash (immersion in sodium hydroxide solutions for 15 days), resulting in up to 50% replacement and exhibiting compressive strength of 25 MPa [111, 182]. A previous study by the author found that the compressive strength of 28 MPa was obtained in cement mortar using <2 mm combined ash as a fine aggregate up to 50% volume replacement in the mixture design with water compensation to the absorption rate (13%) of ash, reaching 50% of the compressive strength by only using natural sand [17]. Similar mixture adjustments were also concluded in the recent T.-P Huynh et al. [194] study when the fine bottom ash was adjusted to the saturated surface dry condition before use and the novel method (densified mixture design algorithm [198]) was used as the mixture design, using fine bottom ash as a 30%–50% replacement for natural sand in mortar mixtures caused 35% loss of compressive strength, and 100% replacement caused 48% loss in the compressive strength.
Cement stabilization/solidification can effectively encapsulate the heavy metals from WTE residues and prevent their leaching into the environment. The ash-derived construction products comply with civil engineering and stringent environmental standards related to the leachability of metals and are used commercially [45, 123, 199, 200].
Alternative aggregates in hot-mix asphalt/bituminous concrete pavement
Unlike Portland cement concrete, which is made of cement, stone aggregate, sand, and water and is used as the surface course of rigid pavement, flexible pavement is used asphalt or bituminous to bind aggregates in a hot mix for the surface course. The bitumen encapsulation mechanisms are applied in hot-mix asphalt concrete to solidify and utilize WTE bottom ash as the stone aggregate and be used as pavement for road construction [201,202,203]. Numerous studies have investigated the performance of bitumen-bound bottom ash asphalt mixture, indicating that the ash−concrete mixture needed higher asphalt content than the mixture of a commercial asphalt concrete pavement [204,205,206]. Another research found that the increase in bottom ash substitution in the asphalt pavement mixture lowered the tensile strength and concluded the optimal formula of 10%–40% bottom ash as a substitute for natural aggregate and 3.5%–9.5% bitumen as the binder [201]. Some studies indicated that the percentage of bottom ash substitution was limited to 20% to control the tensile strength ratio within 75% [185, 201].
The TCLP leaching test results showed that heavy metal concentrations were below the detectable levels [201, 206, 207]. The low leachability of in bottom ash-containing asphalt mixture resulted in the hydrophobic nature of the binding system and the surface cover, which prevented the contact of bottom ash with water and significantly decreased infiltration and element mobility [185, 208].
Base, subbase, and subgrade soil for pavement construction
Besides its uses as alternative aggregates, WTE bottom ash is widely used as an unbound or hydraulically bound structural material for the base, subbase, and subgrade soil, which has been widely investigated in numerous field experiments [209,210,211,212,213]. In unbound form, the shear strength, elastic modulus, and bearing capacity of bottom ash showed similar performance to natural sand, and the California bearing ratio (CBR) values of bottom ash are 20%–30% higher than the recommended subbase values [214]. The size of unbound bottom ash generally fulfills the grading requirement as the Type 1 unbound mixture, but grading adjustments can be required. It has been found that unbound bottom ash provided 70% of the strength of crushed rock as a desirable subbase material in field tests [213]. Additionally, the water content as the critical factor of aging reactions was less than 0.1–0.5 L/kg after ten years; thus, the aging phenomenon was not observed in deformation properties under loaded conditions [211].
Hydraulically bound bottom ash utilizes cement or other binders to improve the mechanical performance of the pavement base, which requires a compact to 95% of the maximum dry density to meet the SHW Series 800 standard [214]. Adding cement or lime can improve the compressive strength and elastic modulus of bound bottom ash to receive the confining pressure at 70 kPa [212, 214, 215]. The leachability of heavy metals of unbounded and hydraulically bounded bottom ash in road construction was widely studied in a previous study, which indicated that although the asphalt on the top layer prevents infiltration of rainwater, the large surface area of unbonded/bounded bottom ash in the base/subbase/subgrade soil resulted in the susceptible exposure of contaminant to the environment [213, 216, 217].
Zimar et al. [218] explored WTE fly ash for treating expansive/high-plastic clays and utilized the WTE fly ash stabilized clay as subgrade or subbase soil to improve the performance of highway pavement when wetted and shrunk during drying. Due to the increasing cost of raw construction materials, multiple studies have investigated the utilization of stabilized industrial residues as subgrade soils for road constructions [219,220,221,222]. With the development of effective stabilization technology for WTE fly ash, it is promising to develop stabilized WTE fly ash in road constructions rather than disposing of it in landfills. For instance, Colangelo et al. developed the preliminary washed WTE fly ash mixed with 20% of cement for road base constructions [223].
Filling material in embankment construction
Another essential practical reuse option for bottom ash is embankment construction [224]. Embankments are compacted soil or rock-based earth material barriers with stability to avoid terrain level changes, typically near the roadway or railway to provide structural support. Based on the types of construction materials, embankments are classified into reinforced embankments, earthfill embankments, and rockfill embankments [225]. When the mechanical properties of bottom ash are similar to those of a controlled low-strength material with a compressive strength of 8.3 MPa or less, bottom ash is a suitable alternative to conventional compacted fill [31, 226]. Compacted bottom ash at the optimum water content has a lower density value. It yields a higher friction angle and cohesion value than most construction fills, resulting in higher shear strength. The permeability of WTE bottom ash is also comparable to that of sand, allowing the free drainage of water and preventing the buildup of pore water pressures [227]. Reusing WTE bottom ash in embankment construction also prevents direct exposure to humans and reduces leaching risk to water suppliers. In several European countries, including Germany, France, the Netherlands, and Denmark, the WTE bottom ash has been widely used in practice as an embankment fill with reuse rates of 83% in Germany, 75% in France, and 90% in the Netherlands [224].
Supplementary cementitious material
Portland cement is the most common type of cement, a hydraulic binder that can set and harden via the hydration reaction with water. The supplementary cementitious materials are used either as substitute cement (blended cement) or added separately to the mixture, contributing to the properties of hardened cementitious phases through hydraulic or pozzolanic activity or filler effects (inert materials) [166]. The most commonly used commercial supplementary cementitious materials contain silica fume, coal fly ash (Class C/F), blast-furnace slag (a byproduct from pig iron production), fine limestone, metakaolin, and natural pozzolans [166].
Because the large sizes of WTE bottom ash can be successfully used as a stone aggregate substitute in concrete production, the fine bottom ash with high porosity has been widely investigated as a supplementary cementitious material. Li et al. [228] found that the cementitious reactivity is lower than that of Portland cement, and its addition to cement may lead to retardation of cement hydration. The replacement was controlled below 30%, resulting in the blended cement of strength class 32.5 according to GB 175–2007 (Chinese National Standard), and the leachability of heavy metal is far lower than GB 5085.3–2007 [228,229,230]. Also, the function of potential filler material (minor additional constituent, 5% in weight) by using milled fines (0.125–3 mm) was discussed by Loginova et al. [231]. The pozzolanic reactivity via the Si and Al components in ash (mainly amorphous phases) reacts with hydration products, Ca(OH)2, to form calcium silicate hydrate gel [228, 232, 233]. It is acknowledged that the presence of metallic aluminum causes deleterious expansion due to hydrogen evolution. Therefore, Carsana et al. [124] proposed the wet-grinding of bottom ash to a fine slurry to react the metallic aluminum particles with water, and then added it to the mix, which received even higher compressive strength in self-compacting concrete due to the pozzolanic reaction. Other pretreatments were mentioned in the previous research before the fine bottom ash was used as supplementary cementitious materials to enhance the ash properties, including thermally treated (550–800 °C) [234, 235] and chemically activated by hydroxides [120]. Garcia-Lodeiro et al. [135] investigated the performance of hybrid cement with bottom ash (83%) and fly ash (17%) in the replacement of 40% of cement clinker, which exhibited an upward trend of 32.5 MPa for 28-day compressive strength. Tian [17] also concluded that the washed fine combined ash powder that received 50% compressive strength of the reference group by 25% replacement cement.
Using WTE fly ash as supplementary cementitious material has been widely investigated due to its advantages in chemical composition and particle sizes. Different from the single purpose of stabilization/solidification for landfill disposal or foundation reinforcement soil, via the mix of fly ash, cement, water, and sometimes adding the accelerating admixtures or activators [236,237,238,239], structural aggregate materials were used in the civil products to receive the required mechanical strength. It is found that about 10% replacement of Portland cement by WTE fly ash can receive even higher mortar strength, and 25% replacement is the suggested maximum dosage, which can also pass the leaching standards in different countries [77, 141, 240]. Ground granulate blast-furnace slag (GGBFs) is popular to be used with Portland cement and mixed with cement in a ternary hydration system for better heavy metal stabilization effects and is eco-friendly [241, 242]. A previous study by the author concluded that the reactive compounds in WTE fly ash chemically reacted with water and enhanced the hydration degrees of the cementitious system [77]. Aubert et al. developed three successive steps for WTE fly ash pretreatment: water washing, phosphating with phosphoric acid to stabilize heavy metals, and to calcinate at a temperature higher than 600 °C to eliminate organic compounds [243]. Tian et al. [77] proved that the phosphoric acid stabilized fly ash could still react with the cementitious hydration products and received the optimal cement replacement at 25%. To control the leachability of heavy metals, melting (1300–1500 °C) was applied to fly ash before it was blended into cement by Wang et al. [244]. Ren et al. [245] investigated the pretreatment of WTE fly ash with CO2 via slurry carbonation and dry carbonation with subsequent water washing, resulted in the CO2-pretreated WTE fly ash can replace cement by up to 30% without sacrificing the long-term strength and mechanical properties, which are also far below the regulatory limits.
Alkali-activated material
Alkali-activated materials (also known as geopolymers), including natural aluminosilicate clay or inorganic industrial residues (containing aluminate and silicate components) as precursors, added to strong alkaline solutions (NaOH, KOH, Na2SiO3, Na2CO3, or their mixtures) as activators, form highly amorphous binding phases which can form hardened structures through geopolymerization [246]. Every kilogram of Portland cement manufactured emitted 0.66–0.82 kg of CO2, resulting in the cement industry contributing to 5%–7% of global anthropogenic CO2 emissions [247]. The alkali-activated binders are identified to have the potential for sustainable utilization in the construction materials industry, offering a like-for-like replacement of Portland cement across its full applications [248].
Zhang et al. [249] started the attempts to use ground WTE bottom ash as a precursor with the increasing interest in alkali-activated materials, followed by Lancellotti et al. [250] and Chen et al. [251] as two representative studies to understand the reaction mechanisms of WTE bottom ash; then, it has flourished in the last five years, widely investigated WTE bottom ash and fly ash as alkali-activated materials [252, 253]. 8 mol/L alkaline NaOH was suggested in the study by Chen et al. by only using bottom ash as the precursor, forming Si–O or Si–O ring structure in final products with 1–3 MPa compressive strength in the hardened pastes, while most of the heavy metals except Cr were effectively immobilized [251]. Zhu et al. [254] used mixed sodium hydroxide (8 mol/L) and sodium silicate solution at a mass ratio of 1:2 as the alkali-activator to react with bottom ash. To enhance the mechanical strength, the WTE bottom ash was mixed with other geopolymer precursors, such as metakaolin, or coal fly ash, observed in the combination of C–(A)–S–H, (C,N)–A–S–H, and N–A–S–H gels depending on the addition ratios, and received optimal replacement at about 40%–50% [255, 256]. Maldonado-Alameda et al. [257] investigated the effects of WTE bottom ash particle size on alkali-activated binder performance. Zhu et al. [258] used the separated glass materials from WTE bottom ash and received the heist compressive strength of 70 MPa in the hardened paste products. Suescum-Morales et al. [259] investigated the accelerated CO2 curing effects on WTE bottom ash-coal fly ash mix and resulted in the formation of aragonite.
An essential reason to incorporate WTE fly ash with geopolymer precursors (coal fly ash, metakaolin, GGBFs, etc.) is to effectively immobilize the heavy metals in the fly ash and reduce the leachability in civil products [149, 260,261,262,263,264,265]. Tan et al. [266] developed a co-disposal of construction and demolition waste and WTE fly ash, and Ye et al. [267] developed a co-disposal of Bayer red mud and WTE fly ash via alkali activation. The compressive strength of WTE fly ash-alkali-activated products mostly received the compressive strength of 1–10 MPa (a few studies exceeded 30 MPa) varied by different fly ash addition, the types of other precursors involved, and the composition of alkali activators [268]. The stabilized products by alkali activation can pass the limits recommended by regional standards (e.g., Chinese CSEPA GB 5085.1–2007) [269, 270].
Geopolymer artificial aggregates and geopolymer bricks as green manufacturing processes, compared to the traditional sintered lightweight aggregate or fired bricks at temperatures over 1000–1200 °C, have been explored in several previous studies [271,272,273,274]. Tian et al. 2022 [76] utilized the WTE challenging tailings, including fine combined ash (30% in volume) and filter cake ash (15% in volume) collected from wastewater of combined ash water washing, to mix with metakaolin and react through alkali activation, resulting in the leachability of products that can pass the very restricted UK WAC nonhazardous limits.
Feed material for Portland cement production
Portland cement is produced by heating limestone and clay silicate materials at higher temperatures of about 1400–1500 °C in a rotating kiln, mixing with gypsum after it is cooled, and finally ground to a highly uniform fine power [275]. Numerous studies have investigated the usage of WTE residues as a replacement for raw materials in cement production because of the advantages of their chemical composition [15, 20, 276, 277]. Clavier et al. [278] assessed the feasibility of incorporating WTE bottom ash into raw cement kiln feed materials at 2.8%; leaching tests of characterization data of a cement product were comparable to the results from OPC, which ventured WTE bottom ash-amended cement production in North America, that assisted the navigation of a new recycling market for WTE bottom ash. Clavier et al. [20] concluded from the existing studies that the untreated WTE ash (bottom ash or fly ash) addition in raw materials is limited to below 10%, while may increase viable addition to 30%–50% to control harmful substances with some exceptions.
Besides, the high temperature of the cement kiln is efficient in decomposing the harmful organic compounds in the WTE residues, especially the PCDD/Fs in the WTE fly ash, which motivates the recycling of WTE fly ash to be beneficially used in civil engineering applications. The Cl concentration in Portland cement is regulated as <0.02% or <0.035% in certain countries to minimize steel corrosion in the reinforced concrete or industrial equipment (cement kiln, flue gas ducts, and fans) [156]. Thus, it may downgrade the cement quality without the pretreatments of WTE fly ash for reducing total Cl and heavy metals [279,280,281]. The allowable limits of Cl in the WTE fly ash and bottom ashes were 1.75% and 3.5%, respectively, compared to its average concentrations of 15% and 0.8% in Table 2 [126]. Washing is the most common pretreatment method to reduce water-soluble chlorides and leachable heavy metals [137, 184, 282]. The uses as the raw material replacement for cement clinker production ranged from 5%–10% to 25% WTE bottom ash [20, 283, 284]. The washed WTE fly ash can be used up to 35% for cement manufacturing [285, 286].
Ceramic-based materials
Ceramic materials contain products, including tiles, bricks, refractories, etc., manufactured at high temperatures (900–1200 °C), used in numerous civil engineering applications due to their good insulation, high hardness, and chemical resistance [287]. Ceramics are commonly used in construction, and are made from a mixture of minerals, typically silica sand, clay, or other materials, mixed with water (~ 30%) [288]. Incorporating WTE residues with clay in ceramic-based materials manufacturing reduces the usage of clay and the environmental cost of mineral extraction.
Using WTE bottom ash in the bricks production has been widely investigated by the previous researchers, indicating that the mechanical strength of bricks is affected by the sintering temperature, sintering method, bottom ash percentage, and bottom ash chemical composition [289]. Increasing the sintering temperature up to 950 °C increases the density of sintered bricks, and higher temperatures (\(\ge\)1000 \(^\circ{\rm C}\)) can cause an increase in the concentration of coarse pore, which is known as bloating phenomenon. The increase in bottom ash percentage also leads to a higher percentage of porosity, which causes a decrease in the mechanical properties [290]. Both flexural strength and the elastic modulus are reduced to about half when bloating occurs [291]. However, the presence of pores can provide special thermal and acoustic insulation characteristics, and the compressive strength values obtained passed European standards even for 100% bottom ash brick, with a significant reduction in the leaching of heavy metals by leaching test (UNE-EN 772–5) [292].
It is noted that the reactive heavy metals and transition metals in bottom ash can promote the sintering process and save energy during the firing process [293]. As for the transformation of bottom ash, calcination changes the crystalline phase, thereby giving higher green density and low firing shrinkage, and giving the high density, low water absorption, and high Vickers microhardness to sintered ceramics [62, 294]. The pH-dependent leaching results of ceramics produced by calcinated untreated bottom ash were compared, and the leachabilities of heavy metals were reduced through encapsulation [294]. Leaching results showed a low risk for metal contamination as heavy metals were well fixed into the coexistence system of amorphous and crystalline phases [295].
Zhang et al. [296] found that the optimal mixture for WTE fly ash in ceramic bricks was fly ash:red ceramic clay:felspar:gang sand=20:60:10:10 by mass, and the optimal sintering temperature was 950 °C, while leachability of As, Hg, Pb, Zn, and Cd exceeded the China standard limit (GB5085.3–2007) by Horizontal Vibration Extraction Procedure (HVEP) and Available Leaching Toxicity (ALT) leaching procedures [230]. Zhang et al. 2007 [297] found that WTE fly ash (20% in the mix) can be mixed with cream-colored clay, fired clay, and limestone to produce ceramic tiles, which received a compressive strength of 18.6 MPa after sintering at 960 °C, reaching the requirements of China GB4100-83 and effectively immobilizing the heavy metals. Researchers have been aiming to improve the quality of the WTE fly ash ceramic bricks in recent years [298]. For example, the fired bricks developed by Sun et al. [299] incorporated 10% washed fly ash, and their heavy metals leachability could satisfy regulation limitations and can be used in harsh scenarios. Another improvement in the quality and environmental safety of ash-ceramic bricks by Chen et al. [300] was adding electric arc furnace slag (EAFS) as the pore plugger to overcome the disadvantages brought by involving WTE fly ash. However, Chinese technical specification for WTE fly ash HJ 1134-2020 specified WTE fly ash, and its treated products cannot be used for sintering bricks production at the current legislation stage due to there being no well-developed APC in most of the brick factories to capture the toxic emission from the sintering process of the WTE fly ash.
Starting from Bethanis and Cheeseman [301] and Cheeseman et al. [302], WTE bottom ash fine fractions (<8 mm) were used to produce sintered lightweight aggregate in the temperature range of 900–1140 °C. The sintered lightweight aggregate pellets were produced in a pelletizer with a controlled water amount of 24%, mixing the WTE bottom ash with other clay or industrial residue (previous studies used rice husk, pulverized fuel ash, red mud, etc.), dried overnight at 105 °C, fired at various temperatures, then received the density of 1.55–2.6 g/cm3 and crushing strength of 4–7 MPa [301,302,303]. Sun et al. [304] increased the crushing strength of sintered lightweight aggregate to 27 MPa with 1046.73 kg/m3 of bulk density and 1783.44 kg/m3 of apparent density by the mix ratio of WTE bottom ash:red mud=1:1 at 1070 °C. Shao et al. [305] improved the strength of fly ash-lightweight aggregate by preheating (at 400 °C), received optimal raw material ratio of WTE fly ash, civil sludge, contaminated soil, and flint clay at 30%:40%:15%:15%, and its leaching of heavy metals was far less than the Chinese standard GB5085.3–2007 [230]. Han et al. 2022 [306] developed the super-lightweight aggregate (bulk density < 500 kg/m3) by mixing 70% WTE fly ash and 30% bentonite with 0.3% SiC addition as a bloating agent, and the sintered products could pass HJ 1134–2020 [96].
Conclusions
This paper summarized WTE residue characteristics, leaching procedure and environmental standards, and the current practice, challenges, and opportunities of WTE residues beneficially used in civil engineering applications. It is estimated that about 46.3 million tonnes of WTE residues are generated in the EU, the U.S., and China; currently, 7.3 million tonnes (16%) are used beneficially in construction.
WTE generates two residues: bottom ash (nonhazardous waste, about 20% of MSW) and fly ash (hazardous waste with leachable heavy metals, high chloride content, and minor PCDD/Fs, about 1%–5% MSW). The particle size distribution primarily influences the applications of bottom ash beneficial uses. The stabilization effects of heavy metals in fly ash are the dominant concern during the utilization, with the second concern controlling the potential emission of PCDD/Fs during product manufacturing or practice scenarios.
European standardization committees developed standards and guidance on the leaching tests and chemical analysis to evaluate End-of-life waste materials. Individual European countries adopted the procedure and established limits based on regional conditions. The derived civil engineering products must be examined by leaching tests to prevent potential environmental risks. The U.S. widely applies the TCLP procedure to fit with RCRA limits for landfill disposal. At the same time, EPA LEAF consists of four leaching methods to estimate the environmental impacts of utilizing secondary materials, primarily as construction materials or disposal scenarios. It is highlighted that China launched the first technical specification (HJ 1134–2020) for reusing the treated WTE fly ash as a resource for further applications.
For the beneficial uses of WTE residues, coarse-size fractions of bottom ash and combined ash after washing can be used as coarse aggregate to substitute crushed stone in structural concrete, cement-based, or asphalt hot-mix concrete pavement. Fine fractions can partially replace sand as fine aggregates; however, swelling due to the metallic aluminum in fine ash mainly challenges its utilization in cementitious phases. Attributed to the particle size and strength advantages, bottom ash can also be applied as an unbound or hydraulically bound structural material in the base, subbase, or subgrade soil in the pavement. Utilizing chemically stabilized fly ash as pavement subgrade soil is a future trend for avoiding landfill disposal. Embankment construction is also an essential practical reuse of bottom ash as compacted soil/rock for structural support.
Fly ash presents higher reactivity and mechanical strength when used as supplementary cementitious material than bottom ash. Bottom ash exhibits reactivity as an alkali-activated material, in contrast to the alkali-activation studies about fly ash is more related to the effects of heavy metals stabilization. Portland cement production assists in navigating a new recycling market for bottom ash and fly ash, which also contributes to destroying the PCDD/Fs. Washing is required to reduce the Cl content, and substituting raw feed materials is strictly limited to guarantee cement quality. Ceramic brick is another option for bottom ash reuse, while it may be limited in China due to technical speciation. Sintered lightweight aggregate can be produced by both bottom ash and fly ash. Innovative geopolymer aggregate or cold-binding cement aggregate was also investigated.
Future perspectives
-
WTE opportunities in developing countries: there are several completed or under development of WTE projects in Africa (e.g., Morocco, South Africa, Ethiopia, Ghana, etc.), South Asia (e.g., Indonesia, Malaysia, India, Thailand, Philippines, Vietnam, etc.), middle east (e.g., Turkey, Iran, etc.), and South America (e.g., Brazil, Chile, Mexico, etc.), which also combined international support and collaboration [307,308,309,310,311,312,313,314,315].
-
Current studies focus on MSW management regionally to meet the rapid urbanization and electricity demands. Establishing the economically viable WTE residues management system and beneficial uses strategies in the meantime of WTE facilities infrastructure benefits the sustainable operation of WTE plants at the next stage.
-
The acceleration of meeting the environmental standards with the construction standards for WTE residues will increase the utilization rate and stimulate the interests of markets that require comprehensive technical speciation and legislation.
Data availability
Some or all data, models, or code that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- APC:
-
Air pollution control (system)
- BA:
-
Bottom ash
- BS:
-
British Standards
- CEN/TC:
-
European Committee for Standardization/Technical Committee
- CEN/TS:
-
European Committee for Standardization/Technical Specification
- EC:
-
European Commission
- EN:
-
European Standards
- EPA:
-
Environmental Protection Agency (US)
- EU:
-
European Union
- GB:
-
The National Standards of the People's Republic of China (Mandatory)
- GB/T:
-
The National Standards of the People's Republic of China (Recommended)
- GGBFs:
-
Ground granulated blast-furnace slag
- GHG:
-
Greenhouse gas
- HJ/T:
-
Environmental Protection/Technical Specification (China)
- L/A:
-
Liquid-to-surface area ratio
- L/S:
-
Liquid-to-solid ratio
- LEAF:
-
Leaching Environmental Assessment Framework (US)
- MSW:
-
Municipal solid waste
- PCDD/Fs:
-
Polychlorinated dibenzo-p-dioxins/furans
- RCRA:
-
Resource Conservation and Recovery Act (US)
- TCLP:
-
Toxic Characteristic Leaching Procedure (US)
- WAC:
-
Waste acceptance criteria (UK)
- WTE:
-
Waste-to-energy
References
Kaza, S., Yao, L., Bhada-Tata, P., et al. 2018. World Bank Group: What A Waste 2.0-A global snapshot of solid waste management to 2050. Washington, DC: World Bank. Available at: http://hdl.handle.net/10986/30317. Accessed 20 Nov 2022.
Liu, J., Li, Q., Gu, W., et al. 2019. The impact of consumption patterns on the generation of municipal solid waste in China: Evidences from provincial data. International Journal of Environmental Research and Public Health 16(10): 1717. https://doi.org/10.3390/ijerph16101717.
Varjani, S., Shahbeig, H., Popat, K., et al. 2022. Sustainable management of municipal solid waste through waste-to-energy technologies. Bioresource Technology 355: 127247. https://doi.org/10.1016/j.biortech.2022.127247.
Hoang, A.T., Varbanov, P.S., Nižetić, S., et al. 2022. Perspective review on municipal solid waste-to-energy route: Characteristics, management strategy, and role in circular economy. Journal of Cleaner Production 359: 131897. https://doi.org/10.1016/j.jclepro.2022.131897.
Fiorentino, G., Ripa, M., Protano, G., et al. 2015. Life cycle assessment of mixed municipal solid waste: Multi-input versus multi-output perspective. Waste Management 46: 599–611. https://doi.org/10.1016/j.wasman.2015.07.048.
Pires, A., Martinho, G., and Chang, N.B. 2011. Solid waste management in European countries: A review of systems analysis techniques. Journal of Environmental Management 92(4): 1033–1050. https://doi.org/10.1016/j.jenvman.2010.11.024.
Malinauskaite, J., Jouhara, H., Czajczyńska, D., et al. 2017. Municipal solid waste management and waste-to-energy in the context of a circular economy and energy recycling in Europe. Energy 141: 2013–2044. https://doi.org/10.1016/j.energy.2017.11.128.
Themelis, N.J., Elena, M., Barriga, D., et al. 2013. Guidebook for the application of waste to energy technologies in Latin America and the Caribbean. Available at: https://secureservercdn.net/198.71.233.185/epm.300.myftpupload.com/wp-content/uploads/2022/02/WTE-Guidebook.pdf. Accessed 14 Dec 2022.
World Energy Council. 2016. World Energy Resources Waste to Energy 2016, pp. 1–76. Available at: https://www.worldenergy.org/wp-content/uploads/2017/03/WEResources_Waste_to_Energy_2016.pdf. Accessed 22 June 2022.
Lee, U., Chung, J.N., and Ingley, H.A. 2014. High-temperature steam gasification of municipal solid waste, rubber, plastic and wood. Energy and Fuels, American Chemical Society 28(7): 4573–4587. https://doi.org/10.1021/ef500713j.
Prurapark, R., Owjaraen, K., Saengphrom, B., et al. 2020. Effect of temperature on pyrolysis oil using high-density polyethylene and polyethylene terephthalate sources from mobile pyrolysis plant. Frontiers in Energy Research 8: 541535. https://doi.org/10.3389/fenrg.2020.541535.
Zeng, R., Wang, S., Cai, J., et al. 2018. A review of pyrolysis gasification of MSW, In Proceedings of the 7th International Conference on Energy, Environment and Sustainable Development (ICEESD 2018), Vol. 163. pp. 166–171. https://doi.org/10.2991/iceesd-18.2018.27.
Zhou, C. 2014. Gasification and pyrolysis characterization and heat transfer phenomena during thermal conversion of municipal solid waste. Ph.D. Thesis. Stockholm, Sweden: Industrial Engineering and Management, KTH Royal Institute of Technology. https://www.diva-portal.org/smash/get/diva2:757954/FULLTEXT01.pdf.
Nakamura, M.R., Castaldi, M.J., and Themelis, N.J. 2010. Stochastic and physical modeling of motion of municipal solid waste (MSW) particles on a waste-to-energy (WTE) moving grate. International Journal of Thermal Sciences 49(6): 984–992. https://doi.org/10.1016/j.ijthermalsci.2009.12.006.
Sun, X., Li, J., Zhao, X., et al. 2016. A review on the management of municipal solid waste fly ash in American, Procedia. Environmental Sciences 31: 535–540. https://doi.org/10.1016/j.proenv.2016.02.079.
Arafat, H.A., Jijakli, K., and Ahsan, A. 2015. Environmental performance and energy recovery potential of five processes for municipal solid waste treatment. Journal of Cleaner Production 105: 233–240. https://doi.org/10.1016/j.jclepro.2013.11.071.
Tian, Y. 2022. Characterization, stabilization, and utilization of waste-to-energy residues in civil engineering applications. Ph.D. Thesis. Columbia University. https://doi.org/10.7916/6wgq-vx06.
Liu, A., Ren, F., Lin, W.Y., et al. 2015. A review of municipal solid waste environmental standards with a focus on incinerator residues. International Journal of Sustainable Built Environment 4(2): 165–188. https://doi.org/10.1016/j.ijsbe.2015.11.002.
Tang, Q., Zhang, Y., Gao, Y., et al. 2017. Use of cement-chelated, solidified, municipal solid waste incinerator (MSWI) fly ash for pavement material: Mechanical and environmental evaluations. Canadian Geotechnical Journal 54(11): 1553–1566. https://doi.org/10.1139/cgj-2017-0007.
Clavier, K.A., Paris, J.M., Ferraro, C.C., et al. 2020. Opportunities and challenges associated with using municipal waste incineration ash as a raw ingredient in cement production—A review. Resources, Conservation and Recycling 160: 104888. https://doi.org/10.1016/j.resconrec.2020.104888.
Zhang, T., Yang, Y., Zhou, K., et al. 2022. Hydrothermal oxidation degradation of dioxins in fly ash with water-washing and added Ce–Mn catalyst. Journal of Environmental Management 317: 115430. https://doi.org/10.1016/j.jenvman.2022.115430.
Scarlat, N., Fahl, F., and Dallemand, J.F. 2019. Status and opportunities for energy recovery from municipal solid waste in Europe. Waste and Biomass Valorization 10(9): 2425–2444. https://doi.org/10.1007/s12649-018-0297-7.
Eurostat. 2022. Municipal waste landfilled, incinerated, recycled and composted, EU, 1995–2020. Available at: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=File:Municipal_waste_landfilled,_incinerated,_recycled_and_composted,_EU,_1995-2020.png#file. Accessed 12 Nov 2022.
Ginés, O., Chimenos, J.M., Vizcarro, A., et al. 2009. Combined use of MSWI bottom ash and fly ash as aggregate in concrete formulation: environmental and mechanical considerations. Journal of Hazardous Materials 169(1–3): 643–650. https://doi.org/10.1016/j.jhazmat.2009.03.141.
European Commission. 2019. Report from the Commission of the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions-On the Implementation of the Circular Economy Action Plan. Available at: https://doi.org/10.1259/arr.1905.0091. Accessed 12 Dec 2022.
Blasenbauer, D., Huber, F., Lederer, J., et al. 2020. Legal situation and current practice of waste incineration bottom ash utilisation in Europe. Waste Management 102: 868–883. https://doi.org/10.1016/j.wasman.2019.11.031.
Maresca, A., Bisinella, V., and Astrup, T.F. 2022. Life cycle assessment of air-pollution-control residues from waste incineration in Europe: Importance of composition, technology and long-term leaching. Waste Management 144: 336–348. https://doi.org/10.1016/j.wasman.2022.03.032.
United States Environmental Protection Agency (US EPA). 2022. Energy Recovery from the Combustion of Municipal Solid Waste (MSW). Available at: https://www.epa.gov/smm/energy-recovery-combustion-municipal-solid-waste-msw#:~:text=RelatedTopics%3A-,Energy. Accessed 12 Nov 2022.
United States Environmental Protection Agency (US EPA). 2020. Advancing Sustainable Materials Management: 2018 Fact Sheet. Available at: https://www.epa.gov/sites/default/files/2021-01/documents/2018_ff_fact_sheet_dec_2020_fnl_508.pdf. Accessed 11 Nov 2022.
Tian, Y., Thanos Bourtsalas, A.C., Kawashima, S., et al. 2020. Performance of structural concrete using Waste-to-Energy (WTE) combined ash. Waste Management 118: 180–189. https://doi.org/10.1016/j.wasman.2020.08.016.
Dou, X., Ren, F., Nguyen, M.Q., et al. 2017. Review of MSWI bottom ash utilization from perspectives of collective characterization, treatment and existing application. Renewable and Sustainable Energy Reviews 79: 24–38. https://doi.org/10.1016/j.rser.2017.05.044.
Ørnebjerg, H., Franck, J., Lamers, F., et al. 2006. Working group on thermal treatment of waste. Management of bottom ash from WTE plants: An overview of management options and treatment methods.
Anshassi, M., Smallwood, T., and Townsend, T.G. 2022. Life cycle GHG emissions of MSW landfilling versus incineration: Expected outcomes based on US landfill gas collection regulations. Waste Management 142: 44–54. https://doi.org/10.1016/j.wasman.2022.01.040.
Zhao, H., Themelis, N.J., (Thanos) Bourtsalas, A.C., et al. 2019. Methane Emissions from Landfills, Columbia University. Available at: http://energyrecoverycouncil.org/wp-content/uploads/2019/10/ERC-2018-directory.pdf. Accessed 20 June 2023.
Kaplan, P.O., Decarolis, J.F., and Barlaz, M.A. 2019. WTE: Life cycle assessment comparison to landfilling, In Themelis, N., Bourtsalas, A. Recovery of Materials and Energy from Urban Wastes. Encyclopedia of Sustainability Science and Technology Series. pp. 499–521. Available at: https://doi.org/10.1007/978-1-4939-2493-6_409-3. Accessed 20 June 2023.
Themelis, N.J., and A.C. Thanos Bourtsalas. 2021. Methane generation and capture of US Landfills. Journal of Environmental Science and Engineering A 10(6): 199–206. https://doi.org/10.17265/2162-5298/2021.06.001.
Scharff, H. 2014. Landfill reduction experience in The Netherlands. Waste Management 34(11): 2218–2224. https://doi.org/10.1016/j.wasman.2014.05.019.
Assamoi, B., and Lawryshyn, Y. 2012. The environmental comparison of landfilling vs. incineration of MSW accounting for waste diversion. Waste Management 32(5): 1019–1030. https://doi.org/10.1016/j.wasman.2011.10.023.
Wang, Y., Levis, J.W., and Barlaz, M.A. 2021. Life-Cycle assessment of a regulatory compliant US municipal solid waste landfill. Environmental Science and Technology 55(20): 13583–13592. https://doi.org/10.1021/acs.est.1c02526.
Ding, Y., Zhao, J., Liu, J.W., et al. 2021. A review of China’s municipal solid waste (MSW) and comparison with international regions: Management and technologies in treatment and resource utilization. Journal of Cleaner Production. 293: 126144. https://doi.org/10.1016/j.jclepro.2021.126144.
Ma, W., Chen, D., Pan, M., et al. 2019. Performance of chemical chelating agent stabilization and cement solidification on heavy metals in MSWI fly ash: A comparative study. Journal of Environmental Management 247: 169–177. https://doi.org/10.1016/j.jenvman.2019.06.089.
Liu, F., Liu, H., Yang, N., et al. 2021. Comparative study of municipal solid waste incinerator fly ash reutilization in China: Environmental and economic performances. Resources, Conservation and Recycling 169: 105541. https://doi.org/10.1016/j.resconrec.2021.105541.
Council of the European Union. 2003. 2003/33/EC. Council Decision establishing criteria and procedures for the acceptance of waste at landfills pursuant to Article 16 of and Annex II to Directive 1999/31/EC. Official Journal of the European Communities 27–49.
British Standard (BSI), BS EN 12457-2:2002. 2002. Characterisation of waste—Leaching—Compliance test for leaching of granular waste materials and sludges—Part 2: One stage batch test at a liquid to solid ratio of 10 L/kg for materials with particle size below 4 mm (without or with size reduction). European Committee for Standardization. https://shop.bsigroup.com/ProductDetail?pid=000000000030017799. Accessed 11 Nov 2022.
European Committee for Standardization, CEN/TC 292. 2012. Characterization of waste. Available at: https://ec.europa.eu/environment/pdf/waste/mining/Finalreport_SA_CEN_395_2007_08.pdf. Accessed 11 Nov 2022.
United States Environmental Protection Agency (US EPA). 2017. Leaching Environmental Assessment Framework (LEAF) Methods and Guidance. Available at: https://www.epa.gov/hw-sw846/leaching-environmental-assessment-framework-leaf-methods-and-guidance. Accessed 11 Nov 2022.
Kosson, D.S., Garrabrants, A.C., Thorneloe, S.A., et al. 2019. Leaching Environmental Assessment Framework (LEAF) How-To Guide. Available at: https://www.epa.gov/sites/default/files/2019-05/documents/final_leaching_environmental_assessment_framework_leaf_how-to_guide.pdf. Accessed 20 June 2023.
United States Environmental Protection Agency (US EPA). 2017. Method 1313: Liquid-Solid Partitioning as a Function of Extract pH Using a parallel batch extraction procedure. Available at: https://www.epa.gov/sites/production/files/2017-10/documents/method_1313_-_final_8-3-17.pdf. Accessed 20 June 2023.
United States Environmental Protection Agency (US EPA). 2004. SW-846 Test Method 1311: Toxicity Characteristic Leaching Procedure. Available at: https://www.epa.gov/sites/default/files/2015-12/documents/1311.pdf. Accessed 20 June 2023.
Saveyn, H., Eder, P., Garbarino, E., et al. 2014. Study on methodological aspects regarding limit values for pollutants in aggregates in the context of the possible development of end-of-waste criteria under the EU waste framework directive. Publications Office. https://doi.org/10.2791/1125.
European Committee of Standardization, 2008. CEN/TC 351—Construction Products—Assessment of release of dangerous substances.
Van der Sloot, H.A., Dijkstra, J.J., Hjelmar, O., et al. 2008. Evaluation of a horizontal approach to assess the possible release of dangerous substances from construction products in support of the requirements of the construction products directive. Available at: http://resolver.tudelft.nl/uuid:e2c9b8f4-367a-43b9-82ff-a6ee2b3230ee. Accessed 20 June 2023.
European Committee of Standardization 2014. CEN/TS 16637–2 Construction products. Assessment of release of dangerous substances Horizontal dynamic surface leaching test.
European Committee for Standardization. 2015. CEN/TS 14429:2015. Characterization of waste - Leaching behaviour tests - Influence of pH on leaching with initial acid/base addition.
European Committee for Standardization. 2015. CEN/TS 14997:2015. Characterization of waste. Leaching behaviour test. Influence of pH on leaching with continuous pH control.
European Committee of Standardization. 2004. CEN/TS 14405:2004. Characterization of waste. Leaching behaviour tests. Up-flow percolation test (under specified conditions).
European Committee of Standardization. 2004. CEN/TS 16637-3: Construction products: Assessment of release of dangerous substances Part 3. Horizontal up-flow percolation test.
European Committee of Standardization. 2002. CEN EN 12457–1:2002. Characterisation of waste - Leaching - Compliance test for leaching of granular waste materials and sludges—Part 1: One stage batch test at a liquid to solid ratio of 2 L/kg for materials with high solid content and with particle size below 4 mm (without or with size reduction).
European Committee of Standardization. 2002. CEN EN 12457-2:2002. Characterisation of waste - Leaching - Compliance test for leaching of granular waste materials and sludges - Part 2: One stage batch test at a liquid to solid ratio of 10 L/kg for materials with particle size below 4 mm (without or with size reduction).
European Committee of Standardization. 2002. CEN EN 12457–3:2002. Characterisation of waste - Leaching - Compliance test for leaching of granular waste materials and sludges - Part 3: Two stage batch test at a liquid to solid ratio of 2 L/kg and 8 L/kg for materials with high solid content and with particle size below 4 mm (without or with size reduction).
European Committee of Standardization. 2002. CEN EN 12457–4:2002. Characterisation of waste - Leaching - Compliance test for leaching of granular waste materials and sludges - Part 4: One stage batch test at a liquid to solid ratio of 10 L/kg for materials with particle size below 10 mm (without or with size reduction).
Bourtsalas A. 2015. Processing the Problematic Fine Fraction of Incinerator Bottom. Ph.D Thesis. Imperial College London. Available at: https://core.ac.uk/download/pdf/77007771.pdf. Accessed 20 June 2023.
Amutha Rani, D., Boccaccini, A.R., Deegan, D., et al. 2008. Air pollution control residues from waste incineration: Current UK situation and assessment of alternative technologies. Waste Management 28(11): 2279–2292. https://doi.org/10.1016/j.wasman.2007.10.007.
Zandi, M., Russell, N.V., Edyvean, R.G.J., et al. 2007. Interpretation of standard leaching test BS EN 12457–2: Is your sample hazardous or inert? Journal of Environmental Monitoring 9(12): 1426–1429. https://doi.org/10.1039/b712957b.
United States Environmental Protection Agency. 2017. Method 1314: Liquid-Solid Partitioning as a Function of Liquid-Solid Ratio For Constituents in Solid Materials Using an Up-Flow Percolation Column Procedure.
United States Environmental Protection Agency (US EPA). 2017. Method 1315: Mass Transfer Rates of Constituents in Monolithic or Compacted Granular Materials Using a Semi-drynamic Tank Leaching Procedure
United States Environmental Protection Agency (US EPA). 2017. Method 1316: Liquid-Solid Partitioning as a Function of Liquid-to-Solid Ratio in Solid Materials Using a Parallel Batch Procedure.
Yang, R., Liao, W.P., and Wu, P.H. 2012. Basic characteristics of leachate produced by various washing processes for MSWI ashes in Taiwan. Journal of Environmental Management 104: 67–76. https://doi.org/10.1016/j.jenvman.2012.03.008.
Hazardous Waste Experts. 2015. Heavy metal waste regulation: Which substances make up the RCRA 8 metals? Available at: https://www.hazardouswasteexperts.com/heavy-metal-waste-regulation-which-substances-make-up-the-rcra-8-metals/. Accessed 20 June 2023.
United States Environmental Protection Agency (US EPA). 2022. Resource Conservation and Recovery Act (RCRA) Overview. Available at: https://www.epa.gov/rcra/resource-conservation-and-recovery-act-rcra-overview. Accessed June 29 2022.
Chinese Academy of Environmental Sciences. 2007. HJ/T 300–2007 Solid waste. Extraction procedure for leaching toxicity. Acetic acid buffer solution method.
Fan, C., Wang, B., Ai, H., et al. 2022. A comparative study on characteristics and leaching toxicity of fluidized bed and grate furnace MSWI fly ash. Journal of Environmental Management 305: 114345. https://doi.org/10.1016/j.jenvman.2021.114345.
Liu, K., Huang, T., Huang, X., et al. 2016. The application of homemade Neosinocalamus affinis AC in electrokinetic removal technology on heavy metal removal from the MSWI fly ash. Science and Reports 6: 39312. https://doi.org/10.1038/srep39312.
Pan, S., Yao, Q., Cai, W., et al. 2022. Characterization of dioxins and heavy metals in chelated fly ash. Energies 15: 4868. https://doi.org/10.3390/en15134868.
Chinese Academy of Environmental Sciences. 2008. GB 16889–2008. Standard for Pollution Control on the Landfill Site of Municipal Solid Waste.
Tian, Y., Bourtsalas, A.C.T., Kawashima, S., et al. 2022. Performance of Waste-to-Energy fine combined ash/filter cake ash-metakaolin based artificial aggregate. Construction and Building Materials 327: 127011. https://doi.org/10.1016/j.conbuildmat.2022.127011.
Tian, Y., Themelis, N.J., Zhao, D., et al. 2022. Stabilization of Waste-to-Energy (WTE) fly ash for disposal in landfills or use as cement substitute. Waste Management 150: 227–243. https://doi.org/10.1016/j.wasman.2022.06.043.
China National Environmental Protection Agency. 1995. GB/T 15555.12–1995. Solid waste. Glass electrode test. Method of corrosivity.
Electricity & Power Industry Standard (Recommended). 2017. China Energy Industry Power Design Standardization Technical Committee, DL/T5532–2017 Code for fly ash test method.
China Ministry of Agriculture. 2008. Agriculture Industry Standard (Recommended). NY/T 1613–2008 Soil quality. Analysis of soil heavy metals-atomic absorption spectrometry with aqua rehia digestion.
United States Environmental Protection Agency (US EPA). 2022. Learn about Dioxin. Available at: https://www.epa.gov/dioxin/learn-about-dioxin#tab-2. Accessed 20 Nov 2022.
Cheng, H., and Hu, Y. 2010. Municipal solid waste (MSW) as a renewable source of energy: Current and future practices in China. Bioresource Technology 101(11): 3816–3824. https://doi.org/10.1016/j.biortech.2010.01.040.
Gan, M., Wong, G., Fan, X., et al. 2021. Enhancing the degradation of dioxins during the process of iron ore sintering co-disposing municipal solid waste incineration fly ash. Journal of Cleaner Production 291: 125286. https://doi.org/10.1016/j.jclepro.2020.125286.
Xiao, H., Cheng, Q., Liu, M., et al. 2020. Industrial disposal processes for treatment of polychlorinated dibenzo-p-dioxins and dibenzofurans in municipal solid waste incineration fly ash. Chemosphere 243: 125351. https://doi.org/10.1016/j.chemosphere.2019.125351.
Yan, D., Peng, Z., Yu, L., et al. 2018. Helge Karstensen, Characterization of heavy metals and PCDD/Fs from water-washing pretreatment and a cement kiln co-processing municipal solid waste incinerator fly ash. Waste Management 76: 106–116. https://doi.org/10.1016/j.wasman.2018.03.006.
United States Environmental Protection Agency (US EPA). 2022. Dioxin Databases, Methods and Tools. Available at: https://www.epa.gov/dioxin/dioxin-databases-methods-and-tools. Accessed 20 Nov 2022.
United States Environmental Protection Agency (US EPA). 1994. Method 1613 Tetra-through Octa-Chlorinated Dioxins and Furans by Isotope Dilution HRGC/HRMS Disclaimer. Available at: https://www.epa.gov/sites/default/files/2015-08/documents/method_1613b_1994.pdf. Accessed 20 Nov 2022.
Wei, J., Li, H., and Liu, J. 2021. Fate of dioxins in a municipal solid waste incinerator with state-of-the-art air pollution control devices in China. Environmental Pollution 289: 117798. https://doi.org/10.1016/j.envpol.2021.117798.
Fu, J., Cai, P., Zhan, M., et al. 2022. Formation and control of dioxins during thermal desorption remediation of chlorine and non-chlorine organic contaminated soil. Journal of Hazardous Materials. 436: 129124. https://doi.org/10.1016/j.jhazmat.2022.129124.
He, H., Lu, S., Peng, Y., et al. 2022. Emission characteristics of dioxins during iron ore Co-sintering with municipal solid waste incinerator fly ash in a sintering pot. Chemosphere 287: 131884. https://doi.org/10.1016/j.chemosphere.2021.131884.
United States Environmental Protection Agency (US EPA). 2007. Method 8280B Polychlorinated dibenzo-p-dioxins (PCDDs) and polychlorinated dibenzofurans (PCDFs) by high-resolution gas chromatography low-resolution mass spectrometry (HRGC LRMS). Available at: https://www.epa.gov/sites/default/files/2015-12/documents/8280b_0.pdf. Accessed 20 Nov 2022.
United States Environmental Protection Agency (US EPA). 2007. EPA Method 8290A, Appendix A (SW-846): Procedure for the Collection, Handling, Analysis, and Reporting of Wipe Tests Performed Within the Laboratory. Available at: https://www.epa.gov/sites/default/files/2016-01/documents/sw846method8290a.pdf. Accessed 20 Nov 2022.
Chang, Y.M., Fan, W.P., Dai, W.C., et al. 2011. Characteristics of PCDD/F content in fly ash discharged from municipal solid waste incinerators. Journal of Hazardous Materials 192(2): 521–529. https://doi.org/10.1016/j.jhazmat.2011.05.055.
United States Environmental Protection Agency. 2011. Fact Sheet on the Management of Dioxin Contaminated Soils. Available at: https://semspub.epa.gov/work/11/174546.pdf. Accessed 19 Nov 2022.
Astrup, T. 2008. Management of APC residues from W-t-E Plants—An overview of management options and treatment methods. p. 116. Available at: https://orbit.dtu.dk/en/publications/management-of-apc-residues-from-w-t-e-plants-an-overview-of-manag. Accessed 20 June 2023.
National Environmental Protection Standard of the People’s Republic of China. 2020. HJ 1134–2020: Technical specification for pollution control of fly-ash from municipal solid waste incineration. Available at: https://www.chinesestandard.net/PDF/English.aspx/HJ1134-2020. Accessed 24 Nov 2022.
National Standard of the People’s Republic of China. 1996. GB 8978–1996. Integrated Wastewater Discharge Standard.
Ministry of Environmental Protection of China. 2010. HJ 557–2010. Solid waste-Extraction procedure for leaching toxicity-Horizontal vibration method.
National Standard of the People’s Republic of China. 2013. GB 30485–2013. Standard for pollution control on co-processing of solid wastes in cement kiln.
National Environmental Protection Standard of the People’s Republic of China. 2013. HJ 662–2013. Environmental protection technical specification for co-processing of Solid wastes in cement kiln.
Themelis, N.J., and Thanos Bourtsalas, A.C. 2019. Recovery of Materials and Energy from Urban Waste. In Encyclopedia of Sustainability Science and Technology Series. New York, NY, USA: Springer. https://doi.org/10.1007/978-1-4939-7850-2_394
Nakamura, M., Castaldi, M.J., and Themelis, N.J. 2006. Numerical analysis of size reduction of municipal solid waste particles on the traveling grate of a waste-to-energy combustion chamber. In Proceedings of the 14th Annual North American Waste-to-Energy Conference. Tampa, FL, USA: ASME. May 1–3, 2006. pp. 125–130. https://doi.org/10.1115/NAWTEC14-3193.
Inkaew, K., Saffarzadeh, A., and Shimaoka, T. 2016. Modeling the formation of the quench product in municipal solid waste incineration (MSWI) bottom ash. Waste Management 52: 159–168. https://doi.org/10.1016/j.wasman.2016.03.019.
Chimenos, J., Segarra, M., Fernández, M., et al. 1999. Characterization of the bottom ash in municipal solid waste incinerator. Journal of Hazardous Materials 64(3): 211–222. https://doi.org/10.1016/S0304-3894(98)00246-5.
Berkhout, S.P.M., Oudenhoven, B.P.M., and Rem, P.C. 2011. Optimizing non-ferrous metal value from MSWI bottom ashes. Journal of Environmental Protection 2(5): 564–570. https://doi.org/10.4236/jep.2011.25065.
Šyc, M., Krausová, A., Kameníková, P., et al. 2017. Material analysis of Bottom ash from waste-to-energy plants. Waste Management 73: 360–366. https://doi.org/10.1016/j.wasman.2017.10.045.
Holm, O., and Simon, F.G. 2017. Innovative treatment trains of bottom ash (BA) from municipal solid waste incineration (MSWI) in Germany. Waste Management 59: 229–236. https://doi.org/10.1016/j.wasman.2016.09.004.
Yu, J., Sun, L., Xiang, J., et al. 2013. Physical and chemical characterization of ashes from a municipal solid waste incinerator in China. Waste Management & Research 31(7): 663–673. https://doi.org/10.1177/0734242X13485793.
Bourtsalas, A. 2012. Review of WTE ash utilization processes under development in northwest Europe. Available at: https://gwcouncil.org/wp-content/uploads/2022/07/WTEBA.pdf. Accessed 21 Feb 2023.
Tian, Y., Themelis, N.J., Thanos, A.C., et al. 2023. Systematic study of the formation and chemical/mineral composition of waste-to-energy (WTE) fly ash. Materials Chemistry and Physics 293: 126849. https://doi.org/10.1016/j.matchemphys.2022.126849.
Pera, J., Coutaz, L., Ambroise, J., et al. 1997. Use of incinerator bottom ash in concrete. Cement and Concrete Research 27(1): 1–5. https://doi.org/10.1016/S0008-8846(96)00193-7.
Saikia, N., Cornelis, G., Mertens, G., et al. 2008. Assessment of Pb-slag, MSWI bottom ash and boiler and fly ash for using as a fine aggregate in cement mortar. Journal of Hazardous Materials 154(1–3): 766–777. https://doi.org/10.1016/j.jhazmat.2007.10.093.
Bruder-Hubscher, V., Lagarde, F., Leroy, M.J., et al. 2001. Utilisation of bottom ash in road construction: Evaluation of the environmental impact. Waste Management & Research 19(6): 545–56. http://www.ncbi.nlm.nih.gov/pubmed/12201685.
Xia, Y., He, P., Shao, L., et al. 2017. Metal distribution characteristic of MSWI bottom ash in view of metal recovery. Journal of Environmental Sciences (China) 52: 178–189. https://doi.org/10.1016/j.jes.2016.04.016.
Yao, J., Li, W., Xia, F., et al. 2011. Investigation of Cu leaching from municipal solid waste incinerator bottom ash with a comprehensive approach. Frontiers in Energy 5(3): 340–348. https://doi.org/10.1007/s11708-010-0131-9.
Saccani, A., Sandrolini, F., Andreola, F., et al. 2005. Influence of the pozzolanic fraction obtained from vitrified bottom-ashes from MSWI on the properties of cementitious composites. Materials and Structures 38: 367–371. https://doi.org/10.1617/14173.
Liu, Y., Li, Y., Li, X., et al. 2008. Leaching behavior of heavy metals and PAHs from MSWI bottom ash in a long-term static immersing experiment. Waste Management 28(7): 1126–1136. https://doi.org/10.1016/j.wasman.2007.05.014.
Shim, Y.S., Rhee, S.W., and Lee, W.K. 2005. Comparison of leaching characteristics of heavy metals from bottom and fly ashes in Korea and Japan. Waste Management 25(5): 473–480. https://doi.org/10.1016/j.wasman.2005.03.002.
Shih, P.H., Chang, J.E., and Chiang, L.C. 2003. Replacement of raw mix in cement production by municipal solid waste incineration ash. Cement and Concrete Research 33(11): 1831–1836. https://doi.org/10.1016/S0008-8846(03)00206-0.
Polettini, A., Pomi, R., and Fortuna, E. 2009. Chemical activation in view of MSWI bottom ash recycling in cement-based systems. Journal of Hazardous Materials 162(2–3): 1292–1299. https://doi.org/10.1016/j.jhazmat.2008.06.018.
Jiao, F., Zhang, L., Dong, Z., et al. 2016. Study on the species of heavy metals in MSW incineration fly ash and their leaching behavior. Fuel Processing Technology 152: 108–115. https://doi.org/10.1016/j.fuproc.2016.06.013.
Yao, J., Li, W.B., Kong, Q.N., et al. 2010. Content, mobility and transfer behavior of heavy metals in MSWI bottom ash in Zhejiang province, China. Fuel 89(3): 616–622. https://doi.org/10.1016/j.fuel.2009.06.016.
Sorlini, S., Collivignarelli, M.C., and Abbà, A. 2017. Leaching behaviour of municipal solid waste incineration bottom ash: from granular material to monolithic concrete. Waste Management & Research 35(9): 978–990. https://doi.org/10.1177/0734242X17721340.
Carsana, M., Gastaldi, M., Lollini, F., et al. 2016. Improving durability of reinforced concrete structures by recycling wet-ground MSWI bottom ash. Materials and Corrosion 67(6): 573–582. https://doi.org/10.1002/maco.201608881.
Kowalski, P.R., Kasina, M., and Michalik, M. 2017. Metallic elements occurrences in the municipal waste incineration bottom ash. Energy Procedia 125: 56–62. https://doi.org/10.1016/j.egypro.2017.08.060.
Pan, J.R., Huang, C., Kuo, J.J., et al. 2008. Recycling MSWI bottom and fly ash as raw materials for Portland cement. Waste Management 28(7): 1113–1118. https://doi.org/10.1016/j.wasman.2007.04.009.
Phoungthong, K., Xia, Y., Zhang, H., et al. 2016. Leaching toxicity characteristics of municipal solid waste incineration bottom ash. Frontiers of Environmental Science and Engineering 10(2): 399–411. https://doi.org/10.1007/s11783-015-0819-5.
Jeong, S.M., Osako, M., and Kim, Y.J. 2005. Utilizing a database to interpret leaching characteristics of lead from bottom ashes of municipal solid waste incinerators. Waste Management 25: 694–701. https://doi.org/10.1016/j.wasman.2004.12.015.
Wu, B., Wang, D., Chai, X., et al. 2016. Characterization of chlorine and heavy metals for the potential recycling of bottom ash from municipal solid waste incinerators as cement additives, Front. Environmental Science and Engineering 10: 1–9. https://doi.org/10.1007/s11783-016-0847-9.
Bertolini, L., Carsana, M., Cassago, D., et al. 2004. MSWI ashes as mineral additions in concrete. Cement and Concrete Research 34: 1899–1906. https://doi.org/10.1016/j.cemconres.2004.02.001.
Yang, S., Saffarzadeh, A., Shimaoka, T., et al. 2014. Existence of Cl in municipal solid waste incineration bottom ash and dechlorination effect of thermal treatment. Journal of Hazardous Materials 267: 214–220. https://doi.org/10.1016/j.jhazmat.2013.12.045.
Zhang, T., and Zhao, Z. 2014. Optimal use of MSWI bottom ash in concrete. International Journal of Concrete Structures and Materials 8: 173–182. https://doi.org/10.1007/s40069-014-0073-4.
Li, Y., Hao, L., and Chen, X. 2016. Analysis of MSWI bottom ash reused as alternative material for cement production, procedia. Environmental Sciences 31: 549–553. https://doi.org/10.1016/j.proenv.2016.02.084.
Lin, K.L., and Chang, C.T. 2006. Leaching characteristics of slag from the melting treatment of municipal solid waste incinerator ash. Journal of Hazardous Materials 135: 296–302. https://doi.org/10.1016/j.jhazmat.2005.11.064.
Garcia-Lodeiro, I., Carcelen-Taboada, V., Fernández-Jiménez, A., et al. 2016. Manufacture of hybrid cements with fly ash and bottom ash from a municipal solid waste incinerator. Construction and Building Materials 105: 218–226. https://doi.org/10.1016/j.conbuildmat.2015.12.079.
Saikia, N., Kato, S., and Kojima, T. 2007. Production of cement clinkers from municipal solid waste incineration (MSWI) fly ash. Waste Management 27(9): 1178–1189. https://doi.org/10.1016/j.wasman.2006.06.004.
Chen, X., Bi, Y., Zhang, H., et al. 2016. Chlorides removal and control through water-washing process on MSWI fly ash. Procedia Environmental Sciences 31: 560–566. https://doi.org/10.1016/j.proenv.2016.02.086.
Aubert, J.E., Husson, B., and Vaquier, A. 2004. Metallic aluminum in MSWI fly ash: Quantification and influence on the properties of cement-based products. Waste Management 24(6): 589–596. https://doi.org/10.1016/j.wasman.2004.01.005.
Colangelo, F., Messina, F., and Cioffi, R. 2015. Recycling of MSWI fly ash by means of cementitious double step cold bonding pelletization: Technological assessment for the production of lightweight artificial aggregates. Journal of Hazardous Materials 299: 181–191. https://doi.org/10.1016/j.jhazmat.2015.06.018.
Cyr, M., Idir, R., and Escadeillas, G. 2012. Use of metakaolin to stabilize sewage sludge ash and municipal solid waste incineration fly ash in cement-based materials. Journal of Hazardous Materials 243: 193–203. https://doi.org/10.1016/j.jhazmat.2012.10.019.
Goh, C.-C., Show, K.-Y., and Cheong, H.-K. 2003. Municipal solid waste fly ash as a blended cement material. Journal of Materials in Civil Engineering 15: 513–523. https://doi.org/10.1061/(asce)0899-1561(2003)15:6(513).
Guo, X., Shi, H., Hu, W., et al. 2014. Durability and microstructure of CSA cement-based materials from MSWI fly ash. Cement and Concrete Composites 46: 26–31. https://doi.org/10.1016/j.cemconcomp.2013.10.015.
Guo, X., Shi, H., Wu, K., et al. 2016. Performance and risk assessment of alinite cement-based materials from municipal solid waste incineration fly ash (MSWIFA). Materials and Structures 49: 2383–2391. https://doi.org/10.1617/s11527-015-0655-x.
Hartmann, S., Koval’, L., Škrobánková, H., et al. 2015. Possibilities of municipal solid waste incinerator fly ash utilisation. Waste Management & Research 33(8): 740–747. https://doi.org/10.1177/0734242X15587545.
Jin, M., Zheng, Z., Sun, Y., et al. 2016. Resistance of metakaolin-MSWI fly ash based geopolymer to acid and alkaline environments. Journal of Non-Crystalline Solids 450: 116–122. https://doi.org/10.1016/j.jnoncrysol.2016.07.036.
Kalmykova, Y., and Karlfeldt Fedje, K. 2013. Phosphorus recovery from municipal solid waste incineration fly ash. Waste Management 33(6): 1403–1410. https://doi.org/10.1016/j.wasman.2013.01.040.
Karlfeldt Fedje, K., Ekberg, C., Skarnemark, G., et al. 2010. Removal of hazardous metals from MSW fly ash-An evaluation of ash leaching methods. Journal of Hazardous Materials 173(1–3): 310–317. https://doi.org/10.1016/j.jhazmat.2009.08.094.
Keppert, M., Pavlík, Z., Tydlitát, V., et al. 2012. Properties of municipal solid waste incineration ashes with respect to their separation temperature. Waste Management & Research 30(10): 1041–1048. https://doi.org/10.1177/0734242X12448513.
Lancellotti, I., Kamseu, E., Michelazzi, M., et al. 2010. Chemical stability of geopolymers containing municipal solid waste incinerator fly ash. Waste Management 30(4): 673–679. https://doi.org/10.1016/j.wasman.2009.09.032.
Lassesson, H., Fedje, K.K., and Steenari, B.M. 2014. Leaching for recovery of copper from municipal solid waste incineration fly ash: Influence of ash properties and metal speciation. Waste Management & Research 32(8): 755–762. https://doi.org/10.1177/0734242X14542147.
Li, J.S., Xue, Q., Wang, P., et al. 2016. Leaching characteristics of chlorine from municipal solid waste incineration fly ash by up-flow percolation column tests. Environmental Earth Sciences. 75(1): 1–7. https://doi.org/10.1007/s12665-015-4772-1.
Li, X., Bertos, M.F., Hills, C.D., et al. 2007. Accelerated carbonation of municipal solid waste incineration fly ashes. Waste Management 27(9): 1200–1206. https://doi.org/10.1016/j.wasman.2006.06.011.
Mu, Y., Saffarzadeh, A., and Shimaoka, T. 2017. Influence of ignition process on mineral phase transformation in municipal solid waste incineration (MSWI) fly ash: Implications for estimating loss-on-ignition (LOI). Waste Management 59: 222–228. https://doi.org/10.1016/j.wasman.2016.09.028.
Shi, H.S., and Kan, L.L. 2009. Leaching behavior of heavy metals from municipal solid wastes incineration (MSWI) fly ash used in concrete. Journal of Hazardous Materials 164(2–3): 750–754. https://doi.org/10.1016/j.jhazmat.2008.08.077.
Sørensen, M.A., Mogensen, E.P.B., Lundtorp, K., et al. 2001. High temperature co-treatment of bottom ash and stabilized fly ashes from waste incineration. Waste Management 21(6): 555–562. https://doi.org/10.1016/S0956-053X(00)00113-6.
Wei, Y.L., and Cheng, C.L. 2016. Determination of total Cl in incinerator fly ashes utilized as cement raw materials. Construction and Building Materials 124: 544–549. https://doi.org/10.1016/j.conbuildmat.2016.07.132.
Wu, K., Shi, H., De Schutter, G., et al. 2012. Experimental study on alinite ecocement clinker preparation from municipal solid waste incineration fly ash. Materials and Structures/Materiaux et Constructions 45(8): 1145–1153. https://doi.org/10.1617/s11527-012-9822-5.
Wu, S., Xu, Y., Sun, J., et al. 2015. Inhibiting evaporation of heavy metal by controlling its chemical speciation in MSWI fly ash. Fuel 158: 764–769. https://doi.org/10.1016/j.fuel.2015.06.003.
Zhang, Y., Cetin, B., Likos, W.J., et al. 2016. Impacts of pH on leaching potential of elements from MSW incineration fly ash. Fuel 184: 815–825. https://doi.org/10.1016/j.fuel.2016.07.089.
Astrup, T., Dijkstra, J.J., Comans, R.N.J., et al. 2006. Geochemical modeling of leaching from MSWI air-pollution-control residues. Environmental Science and Technology 40(11): 3551–3557. https://doi.org/10.1021/es052250r.
De Boom, A., and Degrez, M. 2012. Belgian MSWI fly ashes and APC residues: A characterisation study. Waste Management 32(6): 1163–1170. https://doi.org/10.1016/j.wasman.2011.12.017.
Bayuseno, A.P., and Schmahl, W.W. 2011. Characterization of MSWI fly ash through mineralogy and water extraction. Resources, Conservation and Recycling 55(5): 524–534. https://doi.org/10.1016/j.resconrec.2011.01.002.
Wang, X., Li, A., and Zhang, Z. 2016. The effects of water washing on cement-based stabilization of MWSI fly ash, procedia. Environmental Sciences 31: 440–446. https://doi.org/10.1016/j.proenv.2016.02.095.
Mangialardi, T. 2004. Effects of a washing pre-treatment of municipal solid waste incineration fly ash on the hydration behaviour and properties of ash-Portland cement mixtures. Advances in Cement Research 16(2): 45–54. https://doi.org/10.1680/adcr.16.2.45.36254.
Wang, L., Jin, Y., Nie, Y., et al. 2010. Recycling of municipal solid waste incineration fly ash for ordinary Portland cement production: A real-scale test. Resources, Conservation and Recycling 54(12): 1428–1435. https://doi.org/10.1016/j.resconrec.2010.06.006.
Lothenbach, B., Scrivener, K., and Hooton, R.D. 2011. Supplementary cementitious materials. Cement and Concrete Research 41(12): 1244–1256. https://doi.org/10.1016/j.cemconres.2010.12.001.
Kula, I., Olgun, A., Erdogan, Y., et al. 2001. Effects of colemanite waste, cool bottom ash, and fly ash on the properties of cement. Cement and Concrete Research 31(3): 491–494. https://doi.org/10.1016/S0008-8846(00)00486-5.
Siddique, R., and Bennacer, R. 2012. Use of iron and steel industry by-product (GGBS) in cement paste and mortar. Resources, Conservation and Recycling 69: 29–34. https://doi.org/10.1016/j.resconrec.2012.09.002.
Jiang, Y., Ling, T.C., Mo, K.H., et al. 2019. A critical review of waste glass powder—multiple roles of utilization in cement-based materials and construction products. Journal of Environmental Management 242: 440–449. https://doi.org/10.1016/j.jenvman.2019.04.098.
Nochaiya, T., Wongkeo, W., and Chaipanich, A. 2010. Utilization of fly ash with silica fume and properties of Portland cement-fly ash-silica fume concrete. Fuel 89(3): 768–774. https://doi.org/10.1016/j.fuel.2009.10.003.
Shi, Z., Geiker, M.R., De Weerdt, K., et al. 2017. Role of calcium on chloride binding in hydrated Portland cement–metakaolin–limestone blends. Cement and Concrete Research 95: 205–216. https://doi.org/10.1016/j.cemconres.2017.02.003.
Abdullahi, M., Odigure, J.O., Kovo, A.S., et al. 2016. Characterization and predictive reaction model for cement-sand-kaolin composite for CO2 sequestration. Journal of CO2 Utilization. 16: 169–181. https://doi.org/10.1016/j.jcou.2016.06.008.
Flessati, L., Della Vecchia, G., and Musso, G. 2021. Mechanical Behavior and Constitutive Modeling of Cement-Bentonite Mixtures for Cutoff Walls. Journal of Materials in Civil Engineering. 33(3): 04020483. https://doi.org/10.1061/(asce)mt.1943-5533.0003584.
American Society for Testing and Materials (ASTM) International. 2018. C33/C33M-18. Standard Specification for Concrete Aggregates. Available at: https://doi.org/10.1520/C0033. Accessed 20 Nov 2022.
BSI Standards Publication. 2013. BS EN 12620:2013. Aggregates for concrete, BSI Customer Relations. Available at: https://knowledge.bsigroup.com/products/aggregates-for-concrete-1/standard. Accessed 1 Dec 2022.
Kosmatka, S.H., Kerkhoff, B., and Panarese, W.C. 2008. Aggregates for Concretes. In Design and Control of Concrete Mixtures, Chapter 5. Portland Cement Association. Available at: http://www.ce.memphis.edu/1101/notes/concrete/PCA_manual/Chap05.pdf. Accessed 2 Dec 2022.
Supriani, F., Islam, M., and Afrizal, Y. 2019. Sand type characteristics analysis and mapping in Bengkulu. IOP Conference Series: Earth and Environmental Science 314: 012058. https://doi.org/10.1088/1755-1315/314/1/012058.
National Park Service. 2018. Coastal Sediments—Material Size. National Park Service. Available at: https://www.nps.gov/articles/coastal-sediments-material-size.htm. Accessed 17 Sep 2018.
Yang, J., Yu, W., Fang, H., et al. 2018. Detection of size of manufactured sand particles based on digital image processing. PLoS One 13(12): e0206135. https://doi.org/10.1371/journal.pone.0206135.
CementConcrete.org. 2022. Types of Sand: Uses, Properties, Grain size & Classification. Available at: https://cementconcrete.org/construction-materials/types-of-sand-grain-size/2667/. Accessed 2 Dec 2022
Al-Rawas, A.A., Hago, A.W., Taha, R., et al. 2005. Use of incinerator ash as a replacement for cement and sand in cement mortars. Building and Environment 40(9): 1261–1266. https://doi.org/10.1016/j.buildenv.2004.10.009.
Quenee, B., Li, G., Siwak, J.M., et al. 2000. The use of MSWI (Municipal solid waste incineration) bottom ash as aggregates in hydraulic concrete. Waste Management Series 1: 422–437. https://doi.org/10.1016/S0713-2743(00)80054-9.
Roessler, J., Paris, J., Ferraro, C.C., et al. 2016. Use of waste to energy bottom ash as an aggregate in portland cement concrete: impacts of size fractionation and carbonation. Waste and Biomass Valorization. 7(6): 1521–1530. https://doi.org/10.1007/s12649-016-9545-x.
Lam, C.H.K., Ip, A.W.M., Barford, J.P., et al. 2010. Use of incineration MSW ash: A review. Sustainability 2(7): 1943–1968. https://doi.org/10.3390/su2071943.
Silva, R.V., de Brito, J., Lynn, C.J., et al. 2019. Environmental impacts of the use of bottom ashes from municipal solid waste incineration: a review. Resources, Conservation and Recycling 140: 23–35. https://doi.org/10.1016/j.resconrec.2018.09.011.
Chimenos, J.M., Fernandez, A.I., Miralles, L., et al. 2005. Change of mechanical properties during short-term natural weathering of MSWI bottom ash. Environmental Science and Technology 39(19): 7725–7730. https://doi.org/10.1021/es050420u.
Del Valle-Zermeño, R., Chimenos, J.M., Giró-Paloma, J., et al. 2014. Use of weathered and fresh bottom ash mix layers as a subbase in road constructions: Environmental behavior enhancement by means of a retaining barrier. Chemosphere 117(1): 402–409. https://doi.org/10.1016/j.chemosphere.2014.07.095.
Steketee, J.J., Duzijn, R.F., and Born, J.G.P. 1997. Quality improvement of MSWI bottom ash by enhanced aging, washing and combination processes. Studies in Environmental Science 71: 13–23. https://doi.org/10.1016/S0166-1116(97)80184-7.
Keulen, A., van Zomeren, A., Harpe, P., et al. 2016. High performance of treated and washed MSWI bottom ash granulates as natural aggregate replacement within earth-moist concrete. Waste Management 49: 83–95. https://doi.org/10.1016/j.wasman.2016.01.010.
Pavlík, Z., Keppert, M., Pavlíková, M., et al. 2012. MSWI bottom ash as eco-aggregate in cement mortar design. WIT Transactions on Ecology and the Environment 165: 127–138. https://doi.org/10.2495/ARC120121.
Syahrul, M., Sani, M., Muftah, F., et al. 2010. The properties of special concrete using washed bottom ash (WBA) as partial sand replacement. International Journal of Sustainable Construction Engineering & Technology 1(2): 65–76.
Cho, B.H., Nam, B.H., An, J., et al. 2020. Municipal solid waste incineration (MSWI) ashes as construction materials-a review. Materials 13(14): 1–30. https://doi.org/10.3390/ma13143143.
Cheng, A., Hsu, H.M., Chao, S.J., et al. 2011. Properties of cement-based materials containing melting incinerator bottom ash. Advances in Materials Research 250–253: 834–838. Available at: https://doi.org/10.4028/www.scientific.net/AMR.250-253.834. Accessed 20 June 2023.
Huynh, T.P., and Ngo, S.H. 2022. Waste incineration bottom ash as a fine aggregate in mortar: An assessment of engineering properties, durability, and microstructure. Journal of Building Engineering 52: 104446. https://doi.org/10.1016/j.jobe.2022.104446.
Lynn, C.J., Dhir, R.K., and Ghataora, G.S. 2016. Municipal incinerated bottom ash characteristics and potential for use as aggregate in concrete. Construction and Building Materials 127: 504–517. https://doi.org/10.1016/j.conbuildmat.2016.09.132.
Tang, P., Yu, Q.L., Yu, R., et al. 2013. The application of MSWI bottom ash fines in high performance concrete. In Sustainable Construction Materials. pp. 435–438. Poster session presented at International Conference on the Chemistry of Construction Materials, Berlin, Germany. Available at: http://repository.tue.nl/761333. Accessed 20 June 2023.
Nithiya, A., Saffarzadeh, A., and Shimaoka, T. 2018. Hydrogen gas generation from metal aluminum-water interaction in municipal solid waste incineration (MSWI) bottom ash. Waste Management 73: 342–350. https://doi.org/10.1016/j.wasman.2017.06.030.
Hwang, C.L., and Hung, M.F. 2005. Durability design and performance of self-consolidating lightweight concrete. Construction and Building Materials 19(8): 619–626. https://doi.org/10.1016/j.conbuildmat.2005.01.003.
Cai, Z., Bager, D.H., and Christensen, T.H. 2004. Leaching from solid waste incineration ashes used in cement-treated base layers for pavements. Waste Management 24(6): 603–612. https://doi.org/10.1016/j.wasman.2004.01.010.
Van der Sloot, H.A. 2002. Characterization of the leaching behaviour of concrete mortars and of cement-stabilized wastes with different waste loading for long term environmental assessment. Waste Management 22(2): 181–186. https://doi.org/10.1016/S0956-053X(01)00067-8.
Chen, J.S., Chu, P.Y., Chang, J.E., et al. 2008. Engineering and environmental characterization of municipal solid waste bottom ash as an aggregate substitute utilized for asphalt concrete. Journal of Materials in Civil Engineering 20(6): 432–439. https://doi.org/10.1061/(asce)0899-1561(2008)20:6(432).
Kandhal, P. 2009. Waste materials in hot mix asphalt—An overview. Use of Waste Materials in Hot-Mix Asphalt 92: 3–3–14. https://doi.org/10.1520/stp19841s.
Xue, Y., Hou, H., Zhu, S., et al. 2009. Utilization of municipal solid waste incineration ash in stone mastic asphalt mixture: Pavement performance and environmental impact. Construction and Building Materials 23(2): 989–996. https://doi.org/10.1016/j.conbuildmat.2008.05.009.
Zeng, M., and Ksaibati, K. 2003. Evaluation of moisture susceptibility of asphalt mixtures containing bottom ash. Transportation Research Record 1832(1): 25–33. https://doi.org/10.3141/1832-04.
Ogunro, V.O., Inyang, H.I., Hooper, F., et al. 2004. Gradation control of bottom ash aggregate in superpave bituminous mixes. Journal of Materials in Civil Engineering 16(6): 604–613. https://doi.org/10.1061/(ASCE)0899-1561(2004)16:6(604).
Zhang, X., Gress, D., Karpinski, S., et al. 1999. Utilization of municipal solid waste combustion bottom ash as a paving material. Transportation Research Record 1652(1): 257–263. https://doi.org/10.3141/1652-32.
Huang, C.M., Te Chiu, C., Li, K.C., et al. 2006. Physical and environmental properties of asphalt mixtures containing incinerator bottom ash. Journal of Hazardous Materials 137(3): 1742–1749. https://doi.org/10.1016/j.jhazmat.2006.05.016.
Gress, D., Zhang, X., Tarr, S., et al. 1997. Physical and Environmental Properties of Asphalt-Amended Bottom Ash. Transportation Research Record 345: 10–18. Available at: https://onlinepubs.trb.org/Onlinepubs/trr/1992/1345/1345-002.pdf. Accessed 20 June 2023.
Rogbeck, J., and Hardén, J. 1996. Ash gravel-a material for recycling. Waste Management 16: 109–112. https://doi.org/10.1016/S0956-053X(96)00032-3.
Olsson, S., Kärrman, E., and Gustafsson, J.P. 2006. Environmental systems analysis of the use of bottom ash from incineration of municipal waste for road construction. Resources, Conservation and Recycling 48(1): 26–40. https://doi.org/10.1016/j.resconrec.2005.11.004.
Arm, M., Suer, P., Arvidsson, H., et al. 2011. Technical and environmental long-term properties of industrial residues—summary of field and laboratory investigations. Waste Management 31(1): 101–107. https://doi.org/10.1016/j.wasman.2010.09.004.
Ahmed, A.T. 2011. Approximated calculation model for plastic deformations of granular waste materials. Construction and Building Materials 25(8): 3610–3616. https://doi.org/10.1016/j.conbuildmat.2011.03.056.
Åberg, A., Kumpiene, J., and Ecke, H. 2006. Evaluation and prediction of emissions from a road built with bottom ash from municipal solid waste incineration (MSWI). Science of the Total Environment 355(1–3): 1–12. https://doi.org/10.1016/j.scitotenv.2005.03.007.
Lynn, C. J., Ghataora, G. S., and Dhir, OBE R. K. 2017. Municipal incinerated bottom ash (MIBA) characteristics and potential for use in road pavements. International Journal of Pavement Research and Technology 10(2): 185–201. https://doi.org/10.1016/j.ijprt.2016.12.003.
Paine, K. A., Dhir, R. K., and Doran, V. P. A. 2002. Incinerator bottom ash: Engineering and environmental properties as a cement bound paving material. International Journal of Pavement Engineering 3(1): 43–52. https://doi.org/10.1080/10298430290023458.
De Windt, L., Dabo, D., Lidelöw, S., et al. 2011. MSWI bottom ash used as basement at two pilot-scale roads: Comparison of leachate chemistry and reactive transport modeling. Waste Management 31(2): 267–280. https://doi.org/10.1016/j.wasman.2010.06.002.
Guyonnet, D., Bodénan, F., Brons-Laot, G., et al. 2008. Multiple-scale dynamic leaching of a municipal solid waste incineration ash. Waste Management 28(10): 1963–1976. https://doi.org/10.1016/j.wasman.2007.07.007.
Zimar, Z., Robert, D., Sidiq, A., et al. 2022. Waste-to-energy ash for treating highly expansive clays in road pavements. Journal of Cleaner Production 374: 133854. https://doi.org/10.1016/j.jclepro.2022.133854.
Li, H., Cui, C., Cai, J., et al. 2022. Utilization of steel slag in road semi-rigid base: A review. Coatings 12(7): 994. https://doi.org/10.3390/coatings12070994.
Arvind, S., and Shashikant, S. 2022. A study on lime stabilized slag-fly ash in construction of pavement for long term stability. AIP Conference Proceeding 2413: 030016. https://doi.org/10.1063/5.0091328.
Gu, X., Yu, B., Dong, Q., et al. 2018. Application of secondary steel slag in subgrade: Performance evaluation and enhancement. Journal of Cleaner Production 181: 102–108. https://doi.org/10.1016/j.jclepro.2018.01.172.
Renjith, R., Robert, D., Setunge, S., et al. 2021. Optimization of fly ash based soil stabilization using secondary admixtures for sustainable road construction. Journal of Cleaner Production 294: 126264. https://doi.org/10.1016/j.jclepro.2021.126264.
Colangelo, F., Cioffi, R., Montagnaro, F., et al. 2012. Soluble salt removal from MSWI fly ash and its stabilization for safer disposal and recovery as road basement material. Waste Management 32(6): 1179–1185. https://doi.org/10.1016/j.wasman.2011.12.013.
Oehmig, W.N., Roessler, J.G., Blaisi, N.I., et al. 2015. Contemporary practices and findings essential to the development of effective MSWI ash reuse policy in the United States. Environmental Science & Policy 51: 304–312. https://doi.org/10.1016/j.envsci.2015.04.024.
Wu, W., Berhe, T.G., and Ashour, T. Embankments and dams. In Modern Earth Buildings: Materials, Engineering, Constructions and Applications, Hall, M. R., Lindsay, R., and Krayenhoff, M., Eds., pp. 538–558. https://doi.org/10.1533/9780857096166.4.538.
Yan, D.Y.S., Tang, I.Y., and Lo, I.M.C. 2014. Development of controlled low-strength material derived from beneficial reuse of bottom ash and sediment for green construction. Construction and Building Materials 64: 201–207. https://doi.org/10.1016/j.conbuildmat.2014.04.087.
Muhunthan, B., Taha, R., and Said, J. 2004. Geotechnical engineering properties of incinerator ash mixes. Journal of the Air & Waste Management Association 54(8): 985–991. https://doi.org/10.1080/10473289.2004.10470959.
Li, X.G., Lv, Y., Ma, B.G., et al. 2012. Utilization of municipal solid waste incineration bottom ash in blended cement. Journal of Cleaner Production 32: 96–100. https://doi.org/10.1016/j.jclepro.2012.03.038.
National Standard of the People’s Republic of China. 2007. GB 175–2007. Common Portland Cements.
National Standard of the People’s Republic of China. 2007. GB 5085.3–2007. Identification standards for hazardous wastes - Identification for extraction toxicity.
Loginova, E., Schollbach, K., Proskurnin, M., et al. 2021. Municipal solid waste incineration bottom ash fines: Transformation into a minor additional constituent for cements. Resources Conservation and Recycling 166: 105354. https://doi.org/10.1016/j.resconrec.2020.105354.
Polettini, A., Pomi, R., and Carcani, G. 2005. The effect of Na and Ca salts on MSWI bottom ash activation for reuse as a pozzolanic admixture. Resources, Conservation and Recycling 43(4): 403–418. https://doi.org/10.1016/j.resconrec.2004.07.004.
Lin, K.L., and Lin, D.F. 2006. Hydration characteristics of municipal solid waste incinerator bottom ash slag as a pozzolanic material for use in cement. Cement and Concrete Composites 28(9): 817–823. https://doi.org/10.1016/j.cemconcomp.2006.03.003.
Qiao, X.C., Tyrer, M., Poon, C.S., et al. 2008. Novel cementitious materials produced from incinerator bottom ash. Resources, Conservation and Recycling 52(3): 496–510. https://doi.org/10.1016/j.resconrec.2007.06.003.
Tang, P., Florea, M.V.A., Spiesz, P., et al. 2016. Application of thermally activated municipal solid waste incineration (MSWI) bottom ash fines as binder substitute. Cement and Concrete Composites 70: 194–205. https://doi.org/10.1016/j.cemconcomp.2016.03.015.
Liang, S., Chen, J., Guo, M., et al. 2020. Utilization of pretreated municipal solid waste incineration fly ash for cement-stabilized soil. Waste Management 105: 425–432. https://doi.org/10.1016/j.wasman.2020.02.017.
Chen, L., Wang, L., Cho, D.W., et al. 2019. Sustainable stabilization/solidification of municipal solid waste incinerator fly ash by incorporation of green materials. Journal of Cleaner Production 222: 335–343. https://doi.org/10.1016/j.jclepro.2019.03.057.
Cerbo, A. A. V., Ballesteros, F., Chen, T. C., et al. 2017. Solidification/stabilization of fly ash from city refuse incinerator facility and heavy metal sludge with cement additives. Environmental Science and Pollution Research 24(2): 1748–1756. https://doi.org/10.1007/s11356-016-7943-z.
Billen, P., Verbinnen, B., de Smet, M., et al. 2015. Comparison of solidification/stabilization of fly ash and air pollution control residues from municipal solid waste incinerators with and without cement addition. Journal of Material Cycles and Waste Management 17(2): 229–236. https://doi.org/10.1007/s10163-014-0292-4.
Yan, K., Gao, F., Sun, H., et al. 2019. Effects of municipal solid waste incineration fly ash on the characterization of cement-stabilized macadam. Construction and Building Materials 207: 181–189. https://doi.org/10.1016/j.conbuildmat.2019.02.048.
Wan, S., Zhou, X., Zhou, M., et al. 2018. Hydration characteristics and modeling of ternary system of municipal solid wastes incineration fly ash-blast furnace slag-cement. Construction and Building Materials 180: 154–166. https://doi.org/10.1016/j.conbuildmat.2018.05.277.
Aubert, J.E., Husson, B., and Sarramone, N. 2007. Utilization of municipal solid waste incineration (MSWI) fly ash in blended cement. Part 2. Mechanical strength of mortars and environmental impact. Journal of Hazardous Materials 146(1–2): 12–19. https://doi.org/10.1016/j.jhazmat.2006.11.044.
Aubert, J.E., Husson, B., and Sarramone, N. 2006. Utilization of municipal solid waste incineration (MSWI) fly ash in blended cement. Part 1: Processing and characterization of MSWI fly ash. Journal of Hazardous Materials 136(3): 624–631. https://doi.org/10.1016/j.jhazmat.2005.12.041.
Wang, K.S., Lin, K.L., and Huang, Z.Q. 2001. Hydraulic activity of municipal solid waste incinerator fly-ash-slag-blended eco-cement. Cement and Concrete Research 31(1): 97–103. https://doi.org/10.1016/S0008-8846(00)00423-3.
Ren, P., Ling, T.C., and Mo, K.H. 2022. CO2 pretreatment of municipal solid waste incineration fly ash and its feasible use as supplementary cementitious material. Journal of Hazardous Materials 424: 127457. https://doi.org/10.1016/j.jhazmat.2021.127457.
Garcia-Lodeiro, I., Palomo, A., and Fernández-Jiménez, A. 2014. An overview of the chemistry of alkali-activated cement-based binders. In Handbook of Alkali-Activated Cements, Mortars and Concretes. Sawston, UK: Woodhead Publishing Limited. https://doi.org/10.1533/9781782422884.1.19.
Turner, L.K., and Collins, F.G. 2013. Carbon dioxide equivalent (CO2-e) emissions: A comparison between geopolymer and OPC cement concrete. Construction and Building Materials 43: 125–130. https://doi.org/10.1016/j.conbuildmat.2013.01.023.
Provis, J.L. 2018. Alkali-activated materials. Cement and Concrete Research 114: 40–48. https://doi.org/10.1016/j.cemconres.2017.02.009.
Zhang, Z., Li, G.Q., Yang, Y.X., et al. 2014. Thermodynamics of the vitrified bottom ash slag from municipal solid waste incinerators-phase relations of CaO-SiO2-Na2O oxide system. Advanced Materials Research 881–883: 574–578. https://doi.org/10.4028/www.scientific.net/AMR.881-883.574.
Lancellotti, I., Cannio, M., Bollino, F., et al. 2015. Geopolymers: An option for the valorization of incinerator bottom ash derived “end of waste”. Ceramics International 41(2): 2116–2123. https://doi.org/10.1016/j.ceramint.2014.10.008.
Chen, Z., Liu, Y., Zhu, W., et al. 2016. Incinerator bottom ash (IBA) aerated geopolymer. Construction and Building Materials 112: 1025–1031. https://doi.org/10.1016/j.conbuildmat.2016.02.164.
Zhu, W., Chen, X., Struble, L.J., et al. 2019. Quantitative characterization of aluminosilicate gels in alkali-activated incineration bottom ash through sequential chemical extractions and deconvoluted nuclear magnetic resonance spectra. Cement and Concrete Composites 99: 175–180. https://doi.org/10.1016/j.cemconcomp.2019.03.014.
Carvalho, R., Silva, R.V., de Brito, J., et al. 2021. Alkali activation of bottom ash from municipal solid waste incineration: Optimization of NaOH- and Na2SiO3-based activators. Journal of Cleaner Production 291: 125930. https://doi.org/10.1016/j.jclepro.2021.125930.
Zhu, W., Chen, X., Struble, L.J., et al. 2018. Characterization of calcium-containing phases in alkali-activated municipal solid waste incineration bottom ash binder through chemical extraction and deconvoluted Fourier transform infrared spectra. Journal of Cleaner Production 192: 782–789. https://doi.org/10.1016/j.jclepro.2018.05.049.
Wongsa, A., Boonserm, K., Waisurasingha, C., et al. 2017. Use of municipal solid waste incinerator (MSWI) bottom ash in high calcium fly ash geopolymer matrix. Journal of Cleaner Production 148: 49–59. https://doi.org/10.1016/j.jclepro.2017.01.147.
Maldonado-Alameda, A., Giro-Paloma, J., Andreola, F., et al. 2022. Weathered bottom ash from municipal solid waste incineration: Alkaline activation for sustainable binders. Construction and Building Materials. 327: 126983. https://doi.org/10.1016/j.conbuildmat.2022.126983.
Maldonado-Alameda, A., Giro-Paloma, J., Svobodova-Sedlackova, A., et al. 2020. Municipal solid waste incineration bottom ash as alkali-activated cement precursor depending on particle size. Journal of Cleaner Production 242: 118443. https://doi.org/10.1016/j.jclepro.2019.118443.
Zhu, W., Chen, X., Zhao, A., et al. 2019. Synthesis of high strength binders from alkali activation of glass materials from municipal solid waste incineration bottom ash. Journal of Cleaner Production 212: 261–269. https://doi.org/10.1016/j.jclepro.2018.11.295.
Suescum-Morales, D., Silva, R.V., Bravo, M., et al. 2022. Effect of incorporating municipal solid waste incinerated bottom ash in alkali-activated fly ash concrete subjected to accelerated CO2 curing. Journal of Cleaner Production 370: 133533. https://doi.org/10.1016/j.jclepro.2022.133533.
Galiano, Y. L., Pereira, C. F., and Vale, J. 2011. Stabilization/solidification of a municipal solid waste incineration residue using fly ash-based geopolymers. Journal of Hazardous Materials 185(1): 373–381. https://doi.org/10.1016/j.jhazmat.2010.08.127.
Yang, W., Cao, X., Zhang, Q., et al. 2022. Coupled microwave hydrothermal dechlorination and geopolymer preparation for the solidification/stabilization of heavy metals and chlorine in municipal solid waste incineration fly ash. Science of the Total Environment 853: 158563. https://doi.org/10.1016/j.scitotenv.2022.158563.
Xu, D., Huang, Y., Jin, X., et al. 2022. Synergistic treatment of heavy metals in municipal solid waste incineration fly ash with geopolymer and chemical stabilizers. Process Safety and Environment Protection 160: 763–774. https://doi.org/10.1016/j.psep.2022.02.052.
Labianca, C., Ferrara, C., Zhang, Y., et al. 2022. Alkali-activated binders—a sustainable alternative to OPC for stabilization and solidification of fly ash from municipal solid waste incineration. Journal of Cleaner Production 380: 134963. https://doi.org/10.1016/j.jclepro.2022.134963.
Grabias-Blicharz, E., and Franus, W. 2023. A critical review on mechanochemical processing of fly ash and fly ash-derived materials. Science of the Total Environment 860: 160529. https://doi.org/10.1016/j.scitotenv.2022.160529.
Grabias-Blicharz, E., Panek, R., Franus, M., et al. 2022. Mechanochemically assisted coal fly ash conversion into zeolite. Materials 15(20): 7174. https://doi.org/10.3390/ma15207174.
Tan, J., de Vlieger, J., Desomer, P., et al. 2022. Co-disposal of construction and demolition waste (CDW) and municipal solid waste incineration fly ash (MSWI FA) through geopolymer technology. Journal of Cleaner Production 362: 132502. https://doi.org/10.1016/j.jclepro.2022.132502.
Ye, N., Chen, Y., Yang, J., et al. 2016. Co-disposal of MSWI fly ash and Bayer red mud using an one-part geopolymeric system. Journal of Hazardous Materials 318: 70–78. https://doi.org/10.1016/j.jhazmat.2016.06.042.
Liu, J., Wang, Z., Xie, G., et al. 2022. Resource utilization of municipal solid waste incineration fly ash—cement and alkali-activated cementitious materials: A review. Science of the Total Environment 852: 158254. https://doi.org/10.1016/j.scitotenv.2022.158254.
Li, X., Yu, L., Zhou, H., et al. 2021. An environment-friendly pretreatment process of municipal solid waste incineration fly ash to enhance the immobilization efficiency by alkali-activated slag cement. Journal of Cleaner Production 290: 125728. https://doi.org/10.1016/j.jclepro.2020.125728.
National Standard of the People’s Republic of China. 2007. GB 5085.1–2007. Identification standards for hazardous wastes - Identification for corrosivity.
Peyne, J., Gautron, J., Doudeau, J., et al. 2018. Development of low temperature lightweight geopolymer aggregate, from industrial Waste, in comparison with high temperature processed aggregates. Journal of Cleaner Production 189: 47–58. https://doi.org/10.1016/j.jclepro.2018.04.038.
Fang, Y., Ahmad, M.R., Lao, J.C., et al. 2023. Development of artificial geopolymer aggregates with thermal energy storage capacity. Cement and Concrete Composites 135: 104834. https://doi.org/10.1016/j.cemconcomp.2022.104834.
Bai, Y., Guo, W., Wang, Z., et al. 2022. Geopolymer bricks prepared by MSWI fly ash and other solid wastes: Moulding pressure and curing method optimisation. Chemosphere 307: 135987. https://doi.org/10.1016/j.chemosphere.2022.135987.
Iftikhar, S., Rashid, K., Ul Haq, E., et al. 2020. Synthesis and characterization of sustainable geopolymer green clay bricks: An alternative to burnt clay brick. Cement and Concrete Composites 259: 119659. https://doi.org/10.1016/j.conbuildmat.2020.119659.
Saleh, H.M., and Eskander, S.B. 2020. Innovative cement-based materials for environmental protection and restoration. In New Materials in Civil Engineering, pp. 613–641. Woburn, MA, USA: Butterworth-Heinemann. https://doi.org/10.1016/B978-0-12-818961-0.00018-1.
Clavier, K.A., Ferraro, C.C., and Townsend, T.G. 2021. Pilot-scale cement production using treated waste incineration bottom ash: Physical and environmental performance. Resources, Conservation and Recycling 175: 105862. https://doi.org/10.1016/j.resconrec.2021.105862.
Clavier, K.A., Paris, J.M., Ferraro, C.C., et al. 2021. Washed waste incineration bottom ash as a raw ingredient in cement production: Implications for lab-scale clinker behavior. Resources, Conservation and Recycling 169: 105513. https://doi.org/10.1016/j.resconrec.2021.105513.
Clavier, K.A., Watts, B., Liu, Y., et al. 2019. Risk and performance assessment of cement made using municipal solid waste incinerator bottom ash as a cement kiln feed. Resources, Conservation and Recycling 146: 270–279. https://doi.org/10.1016/j.resconrec.2019.03.047.
Wang, X., Zhang, L., Zhu, K., et al. 2021. Distribution and chemical species transition behavior of chlorides in municipal solid waste incineration fly ash during the pressure-assisted sintering treatment. Chemical Engineering Journal 415: 128873. https://doi.org/10.1016/j.cej.2021.128873.
Ebert, B.A.R., and Kirkelund, G.M. 2022. Effects of chlorides and sulphates on heavy metal leaching from mortar with raw and electrodialytically treated MSWI fly ash. Waste and Biomass Valorization 13(5): 2673–2688. https://doi.org/10.1007/s12649-022-01686-0.
Yu, Z., Chen, Y., Liu, P., et al. 2015. Accelerated simulation of chloride ingress into concrete under drying-wetting alternation condition chloride environment. Construction and Building Materials 93: 205–213. https://doi.org/10.1016/j.conbuildmat.2015.05.090.
Wang, K.S., Chiang, K.Y., Lin, K.L., et al. 2001. Effects of a water-extraction process on heavy metal behavior in municipal solid waste incinerator fly ash. Hydrometallurgy 62(2): 73–81. https://doi.org/10.1016/S0304-386X(01)00186-4.
Krammart, P., and Tangtermsirikul, S. 2004. Properties of cement made by partially replacing cement raw materials with municipal solid waste ashes and calcium carbide waste. Construction and Building Materials 18(8): 579–583. https://doi.org/10.1016/j.conbuildmat.2004.04.014.
Margallo, M., Aldaco, R., and Irabien, Á. 2014. Environmental management of bottom ash from municipal solid waste incineration based on a life cycle assessment approach. Clean Technologies and Environmental Policy 16(7): 1319–1328. https://doi.org/10.1007/s10098-014-0761-4.
Bogush, A.A., Stegemann, J.A., Zhou, Q., et al. 2020. Co-processing of raw and washed air pollution control residues from energy-from-waste facilities in the cement kiln. Journal of Cleaner Production 254: 119924. https://doi.org/10.1016/j.jclepro.2019.119924.
Mao, Y., Wu, H., Wang, W., et al. 2020. Pretreatment of municipal solid waste incineration fly ash and preparation of solid waste source sulphoaluminate cementitious material. Journal of Hazardous Materials 385: 121580. https://doi.org/10.1016/j.jhazmat.2019.121580.
Silva, R.V., de Brito, J., Lynn, C.J., et al. 2017. Use of municipal solid waste incineration bottom ashes in alkali-activated materials, ceramics and granular applications: A review. Waste Management 68: 207–220. https://doi.org/10.1016/j.wasman.2017.06.043.
Designing Buildings, Use of ceramics in construction. 2022. https://www.designingbuildings.co.uk/wiki/Use_of_ceramics_in_construction. Accessed 9 Nov 2022.
Lu, Y., Tian, A., Zhang, J., et al. 2020. Physical and chemical properties, pretreatment, and recycling of municipal solid waste incineration fly ash and bottom ash for highway engineering: A literature review. Advances in Civil Engineering 2020: 8886134. https://doi.org/10.1155/2020/8886134.
Ramli, M.I.I., Salleh, M.A.A.M., Abdullah, M.M.A.B., et al. 2022. The influence of sintering temperature on the pore structure of an alkali-activated kaolin-based geopolymer ceramic. Materials 15(7): 2667. https://doi.org/10.3390/ma15072667.
Appendino, P., Ferraris, M., Matekovits, I., et al. 2004. Production of glass-ceramic bodies from the bottom ashes of municipal solid waste incinerators. Journal of the European Ceramic Society 24(5): 803–810. https://doi.org/10.1016/S0955-2219(03)00264-4.
Terrones-Saeta, J.M., Suárez-Macías, J., Iglesias-Godino, F.J., et al. 2020. Study of the incorporation of biomass bottom ashes in ceramic materials for the manufacture of bricks and evaluation of their leachates. Materials 13(9): 2099. https://doi.org/10.3390/ma13092099.
Rambaldi, E., Esposito, L., Andreola, F., et al. 2010. The recycling of MSWI bottom ash in silicate based ceramic. Ceramics International 36(8): 2469–2476. https://doi.org/10.1016/j.ceramint.2010.08.005.
Bourtsalas, A., Vandeperre, L.J., Grimes, S.M., et al. 2014. Production of pyroxene ceramics from the fine fraction of incinerator bottom ash. Waste Management 45: 217–225. https://doi.org/10.1016/j.wasman.2015.02.016.
Zhang, Z., Wang, J., Liu, L., et al. 2020. Preparation of additive-free glass-ceramics from MSW incineration bottom ash and coal fly ash. Construction and Building Materials 254: 119345. https://doi.org/10.1016/j.conbuildmat.2020.119345.
Zhang, H., Zhao, Y., and Qi, J. 2011. Utilization of municipal solid waste incineration (MSWI) fly ash in ceramic brick: product characterization and environmental toxicity. Waste Management 31(2): 331–341. https://doi.org/10.1016/j.wasman.2010.10.017.
Zhang, H., Zhao, Y., and Qi, J. 2007. Study on use of MSWI fly ash in ceramic tile. Journal of Hazardous Materials 141(1): 106–114. https://doi.org/10.1016/j.jhazmat.2006.06.100.
National Standard of the People’s Republic of China. 2015. GB/T 4100–2015. Ceramic tiles.
Sun, J., Zhou, H., Jiang, H., et al. 2021. Recycling municipal solid waste incineration fly ash in fired bricks: An evaluation of physical-mechanical and environmental properties. Construction and Building Materials 294: 123476. https://doi.org/10.1016/j.conbuildmat.2021.123476.
Chen, H., Liu, Y., Cui, H., et al. 2022. Effects of electric arc furnace slag on promoting quality and environmental safety of fired bricks incorporating municipal solid waste incineration fly ash. Construction and Building Materials 345: 128327. https://doi.org/10.1016/j.conbuildmat.2022.128327.
Bethanis, S., and Cheeseman, C.R. 2004. Production of lightweight aggregate from incinerator bottom ash and pulverised fuel ash. Environmental Waste Management II 78: 55–64. Available at: https://www.witpress.com/Secure/elibrary/papers/WM04/WM04006FU.pdf. Accessed 20 Nov 2022.
Cheeseman, C.R., Makinde, A., and Bethanis, S. 2005. Properties of lightweight aggregate produced by rapid sintering of incinerator bottom ash. Resources, Conservation and Recycling 43(2): 147–162. https://doi.org/10.1016/j.resconrec.2004.05.004.
Giro-Paloma, J., Mañosa, J., Maldonado-Alameda, A., et al. 2019. Rapid sintering of weathered municipal solid waste incinerator bottom ash and rice husk for lightweight aggregate manufacturing and product properties. Journal of Cleaner Production 232: 713–721. https://doi.org/10.1016/j.jclepro.2019.06.010.
Sun, Y., Li, J., Chen, Z., et al. 2021. Production of lightweight aggregate ceramsite from red mud and municipal solid waste incineration bottom ash: Mechanism and optimization. Construction and Building Materials 287:122993. https://doi.org/10.1016/j.conbuildmat.2021.122993.
Shao, Y., Shao, Y., Zhang, W., et al. 2022. Preparation of municipal solid waste incineration fly ash-based ceramsite and its mechanisms of heavy metal immobilization. Waste Management 143: 54–60. https://doi.org/10.1016/j.wasman.2022.02.021.
Han, S., Song, Y., Ju, T., et al. 2022. Recycling municipal solid waste incineration fly ash in super-lightweight aggregates by sintering with clay and using SiC as bloating agent. Chemosphere 307: 135895. https://doi.org/10.1016/j.chemosphere.2022.135895.
Nabavi-Pelesaraei, A., Bayat, R., Hosseinzadeh-Bandbafha, H., et al. 2017. Modeling of energy consumption and environmental life cycle assessment for incineration and landfill systems of municipal solid waste management—A case study in Tehran Metropolis of Iran. Journal of Cleaner Production 148: 427–440. https://doi.org/10.1016/j.jclepro.2017.01.172.
Tan, S.T., Ho, W.S., Hashim, H., et al. 2015. Energy, economic and environmental (3E) analysis of waste-to-energy (WTE) strategies for municipal solid waste (MSW) management in Malaysia. Energy Conversion and Management 102: 111–120. https://doi.org/10.1016/j.enconman.2015.02.010.
Fetanat, A., Mofid, H., Mehrannia, M., et al. 2019. Informing energy justice based decision-making framework for waste-to-energy technologies selection in sustainable waste management: A case of Iran. Journal of Cleaner Production 228: 1377–1390. https://doi.org/10.1016/j.jclepro.2019.04.215.
Afrane, S., Ampah, J. D., Jin, C., et al. 2021. Techno-economic feasibility of waste-to-energy technologies for investment in Ghana: A multicriteria assessment based on fuzzy TOPSIS approach. Journal of Cleaner Production 318: 128515. https://doi.org/10.1016/j.jclepro.2021.128515.
Stafford, W.H.L. 2019. WtE Best Practices and Perspectives in Africa. In: Municipal Solid Waste Energy Conversion in Developing Countries: Technologies, Best Practices, Challenges and Policy, pp. 185–217. Amsterdam, the Netherlands: Elsevier. https://doi.org/10.1016/B978-0-12-813419-1.00006-1.
Adenuga, O.T., Mpofu, K., and Modise, K.R. 2020. An approach for enhancing optimal resource recovery from different classes of waste in South Africa: Selection of appropriate waste to energy technology. Sustainable Futures 2: 100033. https://doi.org/10.1016/j.sftr.2020.100033.
Alao, M. A., Popoola, O. M., and Ayodele, T. R. 2021. Selection of waste-to-energy technology for distributed generation using IDOCRIW-Weighted TOPSIS method: A case study of the city of Johannesburg, south Africa. Renewable Energy 178: 162–183. https://doi.org/10.1016/j.renene.2021.06.031.
Alao, M. A., Popoola, O. M., and Ayodele, T. R. 2022. Waste-to-energy nexus: An overview of technologies and implementation for sustainable development. Cleaner Energy Systems 3: 100034. https://doi.org/10.1016/j.cles.2022.100034.
UN Environment Programme. 2017. Ethiopia’s waste-to-energy plant is a first in Africa. Available at: https://www.unep.org/news-and-stories/story/ethiopias-waste-energy-plant-first-africa. Accessed 20 June 2023.
Acknowledgements
This study was conducted with financial support from Earth Engineering Center (EEC), Columbia University, and Global WtERT Council, Inc. The authors also acknowledged the academic instructions from Professors Nickolas J. Themelis, A.C.(Thanos) Bourtsalas, and Shiho Kawashima of Columbia University.
Funding
Open Access funding provided by the MIT Libraries.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. Yixi Tian is the Guest Editor of the special issue Thermal processing of post-recycling urban wastes (WTE).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tian, Y., Dai, S. & Wang, J. Environmental standards and beneficial uses of waste-to-energy (WTE) residues in civil engineering applications. Waste Dispos. Sustain. Energy 5, 323–350 (2023). https://doi.org/10.1007/s42768-023-00140-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42768-023-00140-8