Abstract
Infected wounds pose a significant global health challenge due to the persistence of bacterial biofilms and limited tissue self-repair. Nitric oxide (NO) functions as a potent antimicrobial agent, demonstrating a dual capacity for both antimicrobial action and tissue rejuvenation across varying concentrations. However, achieving controlled NO release at distinct stages of infected wound progression, simultaneously targeting biofilm removal and wound recovery, remains a formidable challenge. In this work, we introduce a smart electrospun fibrous membrane, featuring an interior laden with NO-loaded HKUST-1 particles and a porous external surface. Notably, the results reveal the photothermal property of HKUST-1 when exposed to near-infrared (NIR) light, enabling precise management of NO release contingent upon light conditions. During the initial phase of infection treatment, a significant NO release is triggered by near-infrared photothermal stimulation, synergistically complementing photothermal therapy to effectively eliminate bacterial biofilms. Subsequently, in the wound-healing phase, NO is released from the degrading fibrous membrane in a controlled and gradual manner, synergizing with trace amounts of copper ions released during MOF degradation. This collaborative mechanism accelerates the formation of blood vessels within the wound, thereby facilitating the healing process. This study suggests a promising and innovative approach for the effective treatment of infected wounds.
Graphical Abstract
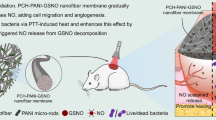
A smart electrospinning fibrous membrane that can intelligently release NO at different stages of infected wound treatment was designed, which could eliminate biofilm and promote the healing of infected wounds.







Similar content being viewed by others
Data Availability
All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Information. Additional data related to this paper may be requested from the authors.
References
MarieáMakabenta J. Nanotherapeutics using all-natural materials. Effective treatment of wound biofilm infections using crosslinked nanoemulsions. Mater Horiz. 2021;8:1776.
Zhao F, Su Y, Wang J, Romanova S, DiMaio DJ, Xie J, Zhao S. A highly efficacious electrical biofilm treatment system for combating chronic wound bacterial infections. Adv Mater. 2023;35:2208069.
Yu X, Zhao J, Fan D. A dissolving microneedle patch for antibiotic/enzymolysis/photothermal triple therapy against bacteria and their biofilms. Chem Eng J. 2022;437: 135475.
Xu Q, Chen S, Jiang L, Xia C, Zeng L, Cai X, Jin Z, Qin S, Ding W, He Q. Sonocatalytic hydrogen/hole-combined therapy for anti-biofilm and infected diabetic wound healing. Natl Sci Rev. 2023;10: nwad063.
Wang Y, Lv Q, Chen Y, Xu L, Feng M, Xiong Z, Li J, Ren J, Liu J, Liu B. Bilayer hydrogel dressing with lysozyme-enhanced photothermal therapy for biofilm eradication and accelerated chronic wound repair. Acta Pharmaceutica Sinica B. 2023;13:284.
Wang J, Chen X-Y, Zhao Y, Yang Y, Wang W, Wu C, Yang B, Zhang Z, Zhang L, Liu Y. pH-switchable antimicrobial nanofiber networks of hydrogel eradicate biofilm and rescue stalled healing in chronic wounds. ACS Nano. 2019;13:11686.
Hu H, Zhong D, Li W, Lin X, He J, Sun Y, Wu Y, Shi M, Chen X, Xu F. Microalgae-based bioactive hydrogel loaded with quorum sensing inhibitor promotes infected wound healing. Nano Today. 2022;42: 101368.
Darvishi S, Tavakoli S, Kharaziha M, Girault HH, Kaminski CF, Mela I. Advances in the sensing and treatment of wound biofilms. Angew Chem. 2022;134: e202112218.
Ouyang Q, Zeng Y, Yu Y, Tan L, Liu X, Zheng Y, Wu S. Ultrasound-responsive microneedles eradicate deep-layered wound biofilm based on TiO2 crystal phase engineering. Small. 2023;19:2205292.
Kasza K, Gurnani P, Hardie KR, Camara M, Alexander C. Challenges and solutions in polymer drug delivery for bacterial biofilm treatment: a tissue-by-tissue account. Adv Drug Deliv Rev. 2021;178: 113973.
LuTheryn G, Hind C, Campbell C, Crowther A, Wu Q, Keller SB, Glynne-Jones P, Sutton JM, Webb JS, Gray M. Bactericidal and anti-biofilm effects of uncharged and cationic ultrasound-responsive nitric oxide microbubbles on Pseudomonas aeruginosa biofilms. Front Cell Infect Microbiol. 2022;12: 956808.
Wang J, Rao L, Huang Z, Ma L, Yang T, Yu Z, Sun A, Ge Y. The nitric oxide synthase gene negatively regulates biofilm formation in Staphylococcus epidermidis. Front Cell Infect Microbiol. 2022. https://doi.org/10.3389/fcimb.2022.1015859.
Huang D, Wang J, Jia F, Fang Y, Gao Q, Gao Y, Li H, Ren K, Ji J. Nitric oxide pretreatment enhances ofloxacin susceptibility of biofilm concomitant with exopolysaccharide depletion. Colloid Interface Sci Commun. 2021;41: 100371.
Yuan Z, Lin C, He Y, Tao B, Chen M, Zhang J, Liu P, Cai K. Near-infrared light-triggered nitric-oxide-enhanced photodynamic therapy and low-temperature photothermal therapy for biofilm elimination. ACS Nano. 2020;14:3546.
Zou F, Wang Y, Zheng Y, Xie Y, Zhang H, Chen J, Hussain MI, Meng H, Peng J. A novel bioactive polyurethane with controlled degradation and l-Arg release used as strong adhesive tissue patch for hemostasis and promoting wound healing. Bioactive Mater. 2022;17:471.
Cao Y, Yin J, Shi Y, Cheng J, Fang Y, Huang C, Yu W, Liu M, Yang Z, Zhou H. Starch and chitosan-based antibacterial dressing for infected wound treatment via self-activated NO release strategy. Int J Biol Macromol. 2022;220:1177.
Malone-Povolny MJ, Maloney SE, Schoenfisch MH. Nitric oxide therapy for diabetic wound healing. Adv Healthcare Mater. 2019;8:1801210.
Zahid AA, Ahmed R, ur Rehman SR, Augustine R, Tariq M, Hasan A. Nitric oxide releasing chitosan-poly (vinyl alcohol) hydrogel promotes angiogenesis in chick embryo model. Int J Biol Macromol. 2019;136:901.
Yang Y, Huang K, Wang M, Wang Q, Chang H, Liang Y, Wang Q, Zhao J, Tang T, Yang S. Ubiquitination flow repressors: enhancing wound healing of infectious diabetic ulcers through stabilization of polyubiquitinated hypoxia-inducible factor-1α by theranostic nitric oxide nanogenerators. Adv Mater. 2021;33:2103593.
Ahmed R, Augustine R, Chaudhry M, Akhtar UA, Zahid AA, Tariq M, Falahati M, Ahmad IS, Hasan A. Nitric oxide-releasing biomaterials for promoting wound healing in impaired diabetic wounds: state of the art and recent trends. Biomed Pharmacother. 2022;149: 112707.
Zhang A, Wu H, Chen X, Chen Z, Pan Y, Qu W, Hao H, Chen D, Xie S. Targeting and arginine-driven synergizing photodynamic therapy with nutritional immunotherapy nanosystems for combating MRSA biofilms. Sci Adv. 2023;9: eadg9116.
Yang D, Li Y, Tan J, Li W, Xu Z, Xu J, Xu W, Hou C, Zhou J, Li G. Biomimetic antithrombotic tissue-engineered vascular grafts for converting cholesterol and free radicals into nitric oxide. Adv Healthcare Mater. 2023;12:2300340.
Xiang S, Wang M, Li L, Shen J. Synergistic antibacterial effect of multifunctional TiO2− X-based nanoplatform loading arginine and polydopamine for promoting infected wounds healing. Colloids Surf, B. 2023;226: 113332.
Fonseca J, Gong T, Jiao L, Jiang H-L. Metal–organic frameworks (MOFs) beyond crystallinity: amorphous MOFs, MOF liquids and MOF glasses. J Mater Chem A. 2021;9:10562.
Daglar H, Keskin S. Combining machine learning and molecular simulations to unlock gas separation potentials of MOF membranes and MOF/polymer MMMs. ACS Appl Mater Interfaces. 2022;14:32134.
Pettinari C, Pettinari R, Di Nicola C, Tombesi A, Scuri S, Marchetti F. Antimicrobial MOFs. Coord Chem Rev. 2021;446: 214121.
Wang Y, Jing D, Yang J, Zhu S, Shi J, Qin X, Yin W, Wang J, Ding Y, Chen T. Glucose oxidase-amplified CO generation for synergistic anticancer therapy via manganese carbonyl-caged MOFs. Acta Biomater. 2022;154:467.
Zhou G, Wang YS, Jin Z, Zhao P, Zhang H, Wen Y, He Q. Porphyrin–palladium hydride MOF nanoparticles for tumor-targeting photoacoustic imaging-guided hydrogenothermal cancer therapy. Nanoscale Horizons. 2019;4:1185.
Daglar H, Keskin S. Recent advances, opportunities, and challenges in high-throughput computational screening of MOFs for gas separations. Coord Chem Rev. 2020;422: 213470.
Yao S, Wang Y, Chi J, Yu Y, Zhao Y, Luo Y, Wang Y. Porous MOF microneedle array patch with photothermal responsive nitric oxide delivery for wound healing. Adv Sci. 2022;9:2103449.
Zhang P, Li Y, Tang Y, Shen H, Li J, Yi Z, Ke Q, Xu H. Copper-based metal–organic framework as a controllable nitric oxide-releasing vehicle for enhanced diabetic wound healing. ACS Appl Mater Interfaces. 2020;12:18319.
Yu S, Li G, Liu R, Ma D, Xue W. Dendritic Fe3O4@ poly (dopamine)@ PAMAM nanocomposite as controllable NO-releasing material: a synergistic photothermal and NO antibacterial study. Adv Func Mater. 2018;28:1707440.
Wu M, Lu Z, Wu K, Nam C, Zhang L, Guo J. Recent advances in the development of nitric oxide-releasing biomaterials and their application potentials in chronic wound healing. J Mater Chem B. 2021;9:7063.
Wu Y, Garren MR, Bright LME, Maffe P, Brooks M, Brisbois EJ, Handa H. Enhanced antibacterial efficacy against antibiotic-resistant bacteria via nitric oxide-releasing ampicillin polymer substrates. J Colloid Interface Sci. 2024;653:1763.
Liu Y, Zhang D, Deng J, Liu Y, Li W, Nie X. Preparation and safety evaluation of Centella asiatica total glycosides nitric oxide gel and its therapeutic effect on diabetic cutaneous ulcers. Evid-Based Complement Alternat Med. 2022. https://doi.org/10.1155/2022/1419146.
Ren X, Li J, Li J, Jiang Y, Li L, Yao Q, Ke Q, Xu H. Aligned porous fibrous membrane with a biomimetic surface to accelerate cartilage regeneration. Chem Eng J. 2019;370:1027.
Khan F, Bamunuarachchi NI, Pham DTN, Tabassum N, Khan MSA, Kim Y-M. Mixed biofilms of pathogenic Candida-bacteria: regulation mechanisms and treatment strategies. Crit Rev Microbiol. 2021;47:699.
Tran P, Enos T, Luth K, Hamood A, Ray C, Mitchell K, Reid TW. Organo-selenium-containing polyester bandage inhibits bacterial biofilm growth on the bandage and in the wound. Biomedicines. 2020;8:62.
Su Y, Andrabi SM, Shahriar SS, Wong SL, Wang G, Xie J. Triggered release of antimicrobial peptide from microneedle patches for treatment of wound biofilms. J Control Release. 2023;356:131.
Du T, Xiao Z, Zhang G, Wei L, Cao J, Zhang Z, Li X, Song Z, Wang W, Liu J. An injectable multifunctional hydrogel for eradication of bacterial biofilms and wound healing. Acta Biomater. 2023;161:112.
Sun P, Ye L, Tan X, Peng J, Zhao L, Zhou Y. Silver nanoparticle-assisted photodynamic therapy for biofilm eradication. ACS Appl Nano Mater. 2022;5:8251.
Wang Q, Qiu W, Li M, Li N, Li X, Qin X, Wang X, Yu J, Li F, Huang L. Multifunctional hydrogel platform for biofilm scavenging and O2 generating with photothermal effect on diabetic chronic wound healing. J Colloid Interface Sci. 2022;617:542.
Dong D, Chen R, Jia J, Zhao C, Chen Z, Lu Q, Sun Y, Huang W, Wang C, Li Y. Tailoring and application of a multi-responsive cellulose nanofibre-based 3D nanonetwork wound dressing. Carbohyd Polym. 2023;305: 120542.
Choi M, Park S, Park K, Jeong H, Hong J. Nitric oxide delivery using biocompatible perfluorocarbon microemulsion for antibacterial effect. ACS Biomater Sci Eng. 2019;5:1378.
Yang T, Zelikin AN, Chandrawati R. Progress and promise of nitric oxide-releasing platforms. Adv Sci. 2018;5:1701043.
Navale GR, Singh S, Ghosh K. NO donors as the wonder molecules with therapeutic potential: recent trends and future perspectives. Coord Chem Rev. 2023;481: 215052.
Pant J, Pedaparthi S, Hopkins SP, Goudie MJ, Douglass ME, Handa H. Antibacterial and cellular response toward a gasotransmitter-based hybrid wound dressing. ACS Biomater Sci Eng. 2019;5:4002.
Ma X, Cheng Y, Jian H, Feng Y, Chang Y, Zheng R, Wu X, Wang L, Li X, Zhang H. Hollow, rough, and nitric oxide-releasing cerium oxide nanoparticles for promoting multiple stages of wound healing. Adv Healthcare Mater. 2019;8:1900256.
Hall JR, Rouillard KR, Suchyta DJ, Brown MD, Ahonen MJR, Schoenfisch MH. Mode of nitric oxide delivery affects antibacterial action. ACS Biomater Sci Eng. 2019;6:433.
Dou J, Yang R, Jin X, Li P, Han X, Wang L, Chi B, Shen J, Yuan J. Nitric oxide-releasing polyurethane/S-nitrosated keratin mats for accelerating wound healing. Regener Biomater. 2022;9: rbac006.
Yuan Y, Ding L, Chen Y, Chen G, Zhao T, Yu Y. Nano-silver functionalized polysaccharides as a platform for wound dressings: a review. Int J Biol Macromol. 2022;194:644.
Zhao WY, Fang QQ, Wang XF, Wang XW, Zhang T, Shi BH, Zheng B, Zhang DD, Hu YY, Ma L. Chitosan-calcium alginate dressing promotes wound healing: a preliminary study. Wound Repair Regener. 2020;28:326.
Morbidelli L, Donnini S, Ziche M. Role of nitric oxide in the modulation of angiogenesis. Curr Pharm Des. 2003;9:521.
Dong C, Feng W, Xu W, Yu L, Xiang H, Chen Y, Zhou J. The coppery age: copper (Cu)-involved nanotheranostics. Adv Sci. 2020;7:2001549.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 32271386), Zhejiang Engineering Research Center for Tissue Repair Materials (Grant No: WIUCASZZXF21001), Wenzhou Science and Technology Major Project (ZY2022028), Wenzhou Science and Technology Project (Y20220142), the seed grants from the Wenzhou Institute, University of Chinese Academy of Sciences (WIUCASQD2020013, WIUCASQD2021030), the Opening Project of State Key Laboratory of High Performance Ceramics and Superfine Microstructure (Grant Nos: SKL202112SIC, SKL202213SIC), and the founding from First Affiliated Hospital of Wenzhou Medical University. Furthermore, the authors would like to thank Scientific Research Center of Wenzhou Medical University for consultation and instrument availability that supported this work.
Author information
Authors and Affiliations
Contributions
LS, CD, and LL contributed equally to this work. LS, CD, and LL: investigation, experimental operation, data curation, and writing—original draft. JX and YF: experimental operation. QK: supervision and funding acquisition. HX, CY, and JC: supervision, funding acquisition, and writing—review and Editing.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Supporting Information
Supplementary information contains experimental results including characterization of materials, cell viability of HUVECs, SEM images of bacterial, CLSM images of biofilms, cell migration of HaCaT, number of S. aureus in vivo, and primers used in RT-PCR.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Su, L., Dong, C., Liu, L. et al. Low-Temperature Trigger Nitric Oxide Nanogenerators for Anti-biofilm and Wound Healing. Adv. Fiber Mater. 6, 512–528 (2024). https://doi.org/10.1007/s42765-023-00369-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42765-023-00369-2