Abstract
Purpose: The aim of this study was to investigate the role of silicon (Si) in counteracting a cadmium (Cd) stress to pea plants (Pisum sativum L.) and to identify the mechanism by which Cd is bound within pea roots. Methods: These goals were achieved through (i) a histochemical study of Cd localization in pea roots, (ii) spectrophotometric determination of pectin content and the activity of pectin methylesterase (PME), (iii) speciation of Cd extracted from pea roots conducted through size exclusion chromatography (SEC) and inductively coupled plasma mass spectrometry (ICP/MS). Results: Cd was found mainly in the root stele of the Cd-stressed plants. The pectin content and PME activity were lower in the Cd-stressed plants, but Si supplementation reversed these effects. Selectivity was noticed in Cd extraction efficiency with water being the least effective and enzymatic-assisted extraction proving to be the most effective. Speciation analysis revealed significant heterogeneity in molar mass, ranging from approximately 295 to 95 kDa. Galacturonic acid was identified the dominant species responsible for Cd binding. The choice of solvent for extraction markedly influenced the Cd binding profile, indicating shifts in the distribution of species’ molar mass and their relative concentrations in extracts. Conclusions: Si alleviates Cd toxicity in pea plants, and one of the mechanisms through which it operates involves increasing pectin levels and PME activity. Pectin plays an active role in Cd detoxification in the root cell walls, forming electrostatic bonds with Cd cations through its carboxyl groups.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
1 Introduction
Phytotoxicity caused by many different biotic and abiotic factors leads to the general deterioration of plant growth and development. Environmental contamination with heavy metals such as cadmium (Cd) is an example of an abiotic factor. Cd contamination is particularly concerning due to the high toxicity of this metal and is one of the most widely discussed types of soil pollution (El Rasafi et al. 2022; Gill and Tuteja 2011; White and Brown 2010). Due to its chemical similarity to zinc ions, Cd can replace them in metabolic processes, negatively affecting plant metabolic pathways (Clemens 2006). Plants naturally have systems for metal bioaccumulation in the roots and mechanisms to reduce the metal’s toxicity (Clemens 2006). Nevertheless, most plants exhibit visible symptoms of Cd toxicity when the total Cd concentration in the soil exceeds 8 mg/kg, or the bioavailable Cd concentration becomes > 0.001 mg/kg, or the Cd concentration in the plant tissue reaches 3–30 mg/kg (Dutta et al. 2021). It has been shown that a Cd content in the dry matter of the plant greater than 110.6 mg/kg depresses the growth of the plant by about 50% (Khalid et al. 2019). For example, the Cd content in the dry matter of Pisum sativum grown in perlite, exceeding 7.84 mg/kg delays the flowering time and inhibits seed production (Głowacka et al. 2022). In our previous study, the Cd content was determined in the roots and shoots of peas grown in hydroponics at 281 mg/kg and 63 mg/kg, respectively, after 7 days of treatment with 50 µM CdSO4. This indicates that peas especially accumulate Cd in their underground parts and possess defence mechanisms that ensure a relatively low translocation index (Orzoł et al. 2022).
Complexation of Cd with various molecular, naturally synthetized species is a well-known detoxification mechanism present in plants. Cd tends to form complex-type compounds with electronegatively charged donors with a free electron pair, such as sulphur (from S-S and S-H groups present in peptides, for example) (Pons et al. 2021), oxygen-donors (from the carboxylic groups) and nitrogen-donor species (Bernabeu de Maria et al. 2023) primarily due to electrostatic attraction. Cd could be incorporated in soluble and insoluble fractions in plant tissues. When it is present in soluble cytosolic fraction, it has been shown that Cd can bind to phytochelatins (PCs) (Vacchina et al. 1999), which are specific sulphur-rich oligopeptides synthesized by plants in response to stress (Cobbett 2000). It was found that other divalent ion, lead, is strongly bound to insoluble (in water) cell wall compounds in P. sativum (Piechalak et al. 2002). In similar research, it was showed that Cd associates with pectins, cellulose, and lignin species having molecular weights higher than 12 kDa (Barałkiewicz et al. 2009). Cd is also present in a complexed form with malate in plant leaves (Ueno et al. 2005).
The plant cell wall serves as a critical defence against toxic substances, particularly heavy metals such as Cd, by acting as both a physical barrier and a binding site (Guo et al. 2021). Composed of a complex network of polysaccharides including pectins, cellulose, and hemicellulose, the cell wall plays a pivotal role in adsorbing and retaining heavy metals. Studies indicate that up to 85% of Cd can be sequestered in the pectins and hemicellulose fractions of the cell wall, significantly restricting the translocation of Cd to the plant’s shoots (Yu et al. 2020). Pectin, a major constituent of the cell wall, is primarily made up of galacturonic acid residues that impart a high negative charge, enhancing its capacity to bind metal ions such as Cd. The activity of pectin methylesterase (PME) is crucial in this context as it modifies pectins by reducing its degree of methyl esterification, thereby increasing the availability of free carboxylic groups. This increase in negative charge density within the cell wall not only boosts Cd binding but also aids in immobilizing Cd, potentially reducing its movement within the plant (Jia et al. 2019; Yu et al. 2020). Moreover, exposure to Cd is known to elevate the accumulation of low-methyl pectins in the root cell walls, thereby enhancing its chelating properties and reinforcing the cell wall’s role in mitigating heavy metal stress.
Silicon (Si) supplementation has emerged as a potential strategy to mitigate the phytotoxic effects of heavy metals such as Cd, chromium (Cr), and arsenic (As), by decreasing their accumulation in plant tissues. Studies have demonstrated that adding 1 mM of silicate to nutrient media significantly reduces both the uptake and translocation of Cd in plants, suggesting a protective role of Si (Greger et al. 2016). The interaction between Si and Cd in plants involves both physical binding mechanisms and biochemical detoxification processes. Antagonism between Si and Cd in plants involves a combination of reduced Cd uptake through modulation of transporter genes expression (Bari et al. 2020; Ma et al. 2015) and the formation of complexes that limit the bioavailability of Cd (Liu et al. 2013; Ma et al. 2015). Ma et al. (2015) during in vitro experiments on rice cells, suggested that a hemicellulose-bound form of Si with net negative charges is responsible for inhibition of Cd uptake by a mechanism of [Si-hemicellulose matrix] Cd complexation and subsequent co-deposition. Bari et al. (2020) showed that Si-induced alleviation of Cd stress is also closely related to phytochelatin-driven vacuolar storage of Cd in rice roots. However, the distribution of apoplasmic and symplasmic Cd was not affected by Si in maize roots, but significantly decreased the symplasmic concentration and increased the apoplasmic concentration of Cd in maize shoots (Vaculík et al. 2012).
Si can also limit uptake and bioaccumulation of Cd in the roots of pea plants (Cruzado-Tafur et al. 2023), but the mechanisms behind the protective action during heavy metal exposure are not fully understood. Therefore, this study focusses on the identification of the compounds responsible for Cd binding to the roots of plants. We focus on the cell wall, which is rich in potential Cd binding species like pectins through complexing via oxygen coordination. The novelty approach is designed to combine both physiological studies and chemical speciation. This manuscript extends the current state of knowledge by using speciation analysis rather than a fractionation approach. Speciation could directly give insight about species which are responsible for complexing Cd. In order, to elucidate the mechanisms of Cd binding in pea roots, particularly under conditions of Cd stress and Si supplementation, the histochemical analysis of Cd localization, comparison of pectin content and PME activity in stressed versus non-stressed roots, and elemental speciation were performed to identify the species responsible for Cd binding. Through detailed speciation analysis, this research explored how Si supplementation influences the Cd binding dynamics within the components of the cell wall, aiming to provide a clearer understanding of the interaction between Cd and Si and its implications for enhancing plant tolerance to heavy metal stress. We investigated the potential for Cd to form bonds with compounds of diverse chemical structures (represented by different molar masses) through electrostatic bonds with functional groups containing O, N, and S donor atoms. Additionally, we examined the binding preferences of Cd to hydrophilic and hydrophobic compounds within plant roots.
2 Materials and Methods
2.1 Plant Material
The material for the analyses consisted of roots collected from pea plants (Pisum sativum L.) ‘Pegaz’ grown in a hydroponic Hoagland solution of pH = 6 (Hoagland and Arnon 1950) and treated with 50 µM CdSO4 (Cd) and/or 1 mM Na2SiO3 (Si or Cd + Si) for one week, according to previously described experiments (Cruzado-Tafur et al. 2023; Orzoł et al. 2023). Plant material (roots) was collected 3 weeks after treatment with Si and/or Cd.
2.2 Location of Cd In Situ
The histochemical location of Cd in pea roots was performed with dithizone (Sigma-Aldrich, St. Louis, MO, USA), which forms coloured complexes with metal ions (Seregin and Ivanov 1997). Roots were immersed in the staining solution (30 mg dithizone, 60 mL of acetone, 20 mL deionized water and a few drops of 45% (v/v) acetic acid) and then incubated at RT for 2 h in the dark (He et al. 2020; Zhang et al. 2022). After being washed with distilled water, the roots were microscopically photographed (Nikon, Eclipse 80i, Nikon Europe B.V., Amstelveen, The Netherlands).
2.3 Pectin Determination
The cell wall material was prepared according to Schmohl and Horst’s protocol (Li et al. 2017; Schmohl and Horst 2000). Frozen roots of pea plants (1 g) were homogenized in 2 mL of 96% ethanol and incubated for 5 min at 4 °C. The samples were centrifuged at 23 000 × g for 10 min. The supernatant was then discarded, and ethanol was added to the precipitate. Subsequently, the samples were centrifuged again. The whole process was repeated five times. After the last centrifugation, the supernatant was removed, and the pellet was dried at 60 °C. The hydrolysis was performed according to Liu et al. (2022). The dry cell wall material was weighed (5 mg), then 2 ml of chilled H2SO4 was added and mixed for 10 min. After this time, 0.5 mL of distilled water was added and stirred for 2 h. This process was repeated, after which the samples were transferred to 10 mL tubes and replenished with distilled water. Subsequently, uronic acids were determined according to Li et al. (Li et al. 2016). Cell wall solution (0.2 mL) was added to the tubes with 1.2 mL of chill tetraborate reagent. The samples were boiled for 5 min, then they were cooled in an ice bath, and 20 µL of m-hydroxydiphenyl reagent was added. The absorbance was measured with a spectrophotometer (Tecan Infinite 200 PRO) at λ = 520 nm against a blank with NaOH instead of m-hydroxydiphenyl. Galacturonic acid was used as a calibration standard.
2.4 Cell Wall Protein Extraction and Enzymatic Activity Assay
The cell wall protein was extracted according to Baldwin et al. (2014). Proteins were extracted using 1 M LiCl buffer for pectin methylesterase (PME) enzyme assays. The root powder of frozen plants (500 mg) was homogenized in 300 µL of 50 mM sodium acetate buffer (pH = 5) with 1 M LiCl. The homogenates were incubated for 30 min. at 4 °C in an ice bath. The samples were centrifuged at 20 000 × g for 10 min. at 4 °C. The samples were extracted twice, after which the supernatants were pooled and desalted. The Bradford method was applied to determine the total protein content (Bradford 1976). PME was measured according to a modified method of Baldwin et al. (2014). The cell wall protein samples (5 µL) were incubated with 95 µL of 50 mM sodium phosphate buffer (pH = 7.5) with 0.025 U alcohol oxidase and 100 µg of 90% methylesterified citrus pectin. Subsequently, the samples were incubated for 15 min at 30 °C. After incubation, 250 µL of 20 mM pentane-2,4-dione, 0.05 M glacial acetic acid and 2 M ammonium acetate were added. The absorbance was measured with a spectrophotometer (Tecan Infinite 200 PRO) at λ = 412 nm after a 15-minute incubation. PME activity was determined with reference to a standard curve of methanol and expressed in nmol methanol × min− 1 × mg protein− 1 (1 U, units).
2.5 Extraction of Cd Species from Samples
The extraction was carried out with the use of four different solvents: (i) 18.2 MΩ double deionised water (Merck Millipore, Merck, Darmstadt, Germany), (ii) ammonium acetate 10 mM pH 6.2 solution (Avantor, Gliwice, Poland), (iii) pectinase (from Aspergillus aculeatus) (Sigma-Aldrich, Merck, Germany) and (iv) cellulase (from Trichoderma sp.) (Sigma-Aldrich, Merck, Germany); both enzymes were dispersed in water. The detailed procedure is described in the Supplementary material.
2.6 Extraction Efficiency
To determine extraction efficiency (ratio of extracted Cd to total Cd content in sample), the Cd quantification in both sample types was performed by ICP/MS technique and protocol described in Orzoł et al. (2022). The detailed procedure is described in the Supplementary material.
2.7 Separation and Characterization of Cd Species by SEC -ICP/MS
Speciation analysis was carried out using SEC chromatography and ICP-MS spectroscopy for Cd detection. The detailed procedure is described in the Supplementary material.
2.8 Species Identification and Characterization by MALDI-TOF/MS
Identification and characterization of compounds was performed by MALDI-TOF/MS spectrometry. The detailed procedure is described in the Supplementary material.
2.9 Statistical Analysis
A two-way analysis of variance (ANOVA) was performed to determine differences between groups, followed by the Tukey’s test with the significance level set at p ≤ 0.01. The statistical analysis was carried out using STATISTICA (ver. 13.1 Dell Inc. Tulsa, OK, USA). Validation was performed, as described by Cruzado-Tafur et al. (2023).
3 Results
3.1 The Localization of Cd, Pectin Content and the Activity of Pectin Methylesterase in the Pea Roots
Figure 1 shows the Cd localization in the roots of pea grown in control Hoagland solution and supplemented with 1 mM Si and/or treated 50 µM Cd. The brown colour indicating Cd-dithizone precipitation was observed mainly in the parts of the roots near the lateral root (Fig. 1– dotted arrows) of Cd-stressed plants (Fig. 1C). In the roots of plants stressed with Cd and Si supplemented, Cd-dithizone was only slightly visible (Fig. 1D). Furthermore, Cd was found in the root stele, where the vascular bundle with the xylem vessels (Fig. 1– arrows) is located.
Histochemical localization of Cd in the roots of P. sativum. The root of the control plant (A), plant supplemented with Si (B), Cd stressed plant (C), and Cd stressed plant supplemented with Si (D). The images show the region of the root close to the lateral root (dotted arrows) in the zoom images (indicated by white rectangles); visible are the xylem vessels (arrows). Detailed description in the text. Scale bar: 100 μm
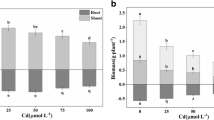
The pectin content in the roots (Fig. 2A) of the plants treated with Cd was significantly lower than in the other variants (by Control– 13%, Si– 13% and Cd + Si– 9%). The highest content of pectins was found in the control roots and in the roots supplemented with Si. Also, the content of pectins in roots supplemented with Si and treated with Cd was significantly higher than in roots subjected to Cd stress. As a result of the Cd treatment of the plants, the level of PME activity increased compared to its activity in the roots of the control pea plants (Fig. 2B). The highest enzyme activity was determined in plants both supplemented with Si and treated with Cd (0.02 U). In addition, treatment of plants with Cd was shown to significantly increase the activity of this enzyme (0.015 U). In comparison, PME activity in plants supplemented with Si increased by 34% relative to PME activity in the roots of control plants.
3.2 Cd Extraction Efficiency and Cd Speciation Analysis Revealing Cd Binding Species in the Pea Roots
In order to selectively leach Cd compounds from the sample, four types of solvents with different physicochemical descriptors (pH, ionic strength, dielectric constant, presence of specific chemical group in enzyme capable to specific degradation of substrate, etc.) and the ability to extract compounds differing in: hydrophilic properties, molar mass, solubility in water, etc., were used. In Table 1, the values of Cd extraction efficiency using these extractants are present in order to assess what part of Cd is extracted by each solvent.
The least effective solvent to extract Cd species from a sample is water. Other used solvents in studies represent similar extraction effectiveness of Cd. Noteworthy is the fact that most of the Cd content in the samples is the insoluble part by using those extraction procedures.
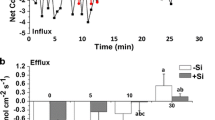
Individual solutions of extracts were subjected to speciation analysis for Cd. In order to determine the forms of Cd in the samples, the SEC-ICP-MS system was used. The representative chromatograms are shown in Fig. 3 for four types of extraction methodologies and for two types of sample: Cd-stressed as well as Cd-stressed and Si supplemented.
Extraction with water (Fig. 3A for Cd stressed only, and E for Cd stressed and Si supplemented type of sample) results in several fractions of species from about 275 kDa to 105 kDa. A sample containing Si changes the speciation of Cd, so that the fraction of compounds with the highest masses becomes dominant. The use of ammonium acetate leads to a homogeneous distribution of Cd in one and dominant fraction represent by species with apparent molar mass between 100 and 85 kDa; however, a low abundant fraction between 8 and 9.5 min in retention time was detected for a Cd stressed sample (Fig. 3B). Cd in extracts with the use of pectinase for both samples (Fig. 3C and G) was detected in a wide distribution of species between 210 kDa and 91 kDa. The presence of Si in the sample changes the intensity of the distribution in favour of the fraction with the biggest particles. Extraction with cellulase (Fig. 3D and H) leads to a wide distribution range of Cd species from 295 kDa to 95 kDa and Cd is more abundant in the fraction with low molar mass.
In order to identify the species which were bind to Cd, the MALDI-TOF/MS technique was utilized. This spectrometric technique is selected in non-targeted type of analysis. Figure 4 shows mass spectra collected for the fractions with the highest Cd content in SEC-ICP/MS. The analysis was performed in fragmentation mode and the names of identified compounds have been assigned to distinct peaks. First of all, the recorded spectra do not show the characteristic isotopic distribution of Cd bound to organic compounds based on C, H, and O. Compounds that were identified were: (i) glucuronic acid (as well as their unsaturated form)– is the main compounds presents in majority of fractions (ii) amino acids such as: glutamic and aspartic acids, methionine, cysteine and serine. In extract with ammonium acetate a 4-carboxyglutamic acid was detected as well as a 2-aminobutyric acid. Si-based compounds were not detected.
4 Discussion
Cd presence was notably observed in the stele of pea roots, with its concentration being modulated by Si supplementation. Consistent with previous findings (Cruzado-Tafur et al. 2023), our results indicate that Si supplementation effectively reduces the Cd concentration in the roots of Cd-stressed plants and limits its translocation to the shoots. This supports the effectiveness of Si in enhancing plant resilience against Cd stress. Several studies have shown that Si supply has beneficial effects on Cd-stressed pea plants. This include reducing of the inhibitory effect of Cd on the plant growth, root and stem development and seed production (El-Okkiah et al. 2022; Orzoł et al. 2023; Rahman et al. 2017). It is suggested that the mechanism responsible for this effect is the observed reduced accumulation of Cd in the roots and shoots of plants supplemented with Si. Our earlier study (Cruzado-Tafur et al. 2023) showed that pea plants accumulate more cadmium in the roots than in the shoots (translocation factor < 1). Furthermore, compared to plants that were subjected to Cd stress, the roots and shoots of plants supplemented with silicon had a lower cadmium bioconcentration factor (BCF). Generally, Si reduces the translocation of Cd from roots to shoots or inhibits the uptake of Cd in the plants. Wu et al. (2015) showed that in tomato, Si supply decreased root-to-shoot Cd transport; while in cucumber, Si supply reduced the Cd uptake by roots. Previous studies showed that Si supplementation decreased the accumulation of Cd in roots and shoots of Cd-stressed pea plants when supplied to hydroponics (Cruzado-Tafur et al. 2023) or after foliar Si application (Batool et al. 2022; El-Okkiah et al. 2022). In this way, Si can also limit the accumulation of Cd in edible parts of plants such as fruits, reducing human Cd consumption (Batool et al. 2022).
Furthermore, our research hypothesis suggests that Cd binds to a variety of compounds within plant roots, characterized by diverse molar masses and functional groups containing oxygen (O), nitrogen (N), and sulfur (S). The binding affinity and capacity of Cd depend significantly on whether these compounds are hydrophilic or hydrophobic. Si appears to influence these interactions, potentially by increasing both the amount of pectins within the cell walls and the activity of pectin methylesterase (PME). This suggests that Si supplementation not only affects the physical retention of Cd but also may alter the biochemical pathways that govern Cd sequestration in plant roots. Thus, role of Si extends beyond simple physical barrier enhancement to include dynamic changes in the biochemical interactions within the root environment. Cd was found in the stele where the vascular tissue responsible for the upward conduction of water and nutrients from the roots is present. It has been shown that Cd can be sequestered in the roots by cytosolic binding partners for Cd ions in plants, e.g., glutathione (GSH) and GSH-derived peptides called PCs, and/or transported through the xylem to the shoot (Luo and Zhang 2021). The cell wall plays an important role in Cd accumulation in the roots of metal-tolerant plants, e.g., rice roots (Yu et al. 2020). Pectins are one of the cell wall polysaccharides and play an important role in the binding of Cd to the roots of plants. They are characterized by high Cd accumulation (Wei et al. 2021). Yu et al. (2020) indicated that pectins in root cell walls are the main site of Cd binding (accumulation of up to 65% of the Cd taken up). Furthermore, pectins affect the uptake and distribution of Cd in plant tissues. Plants may have different pectin structures and metabolism adaptations to cope with Cd stress (Wei et al. 2021). For example, parsnips increase their pectin content and degree of methylation, while celeriac decreases them in response to Cd exposure (Szerement and Szatanik-Kloc 2022). Our research using pea plants is a second strategy for adapting plants to Cd stress (reduction of pectin content by 9%). However, Si supplementation eliminates the effect of Cd, making the pectin content comparable to the content in control roots. Głazowska et al. (2018) proved that Si affects the composition of cell walls by changing the bonds of non-cellulosic polymers and lignins. The changes generated by Si are related to the cell wall remodelling process and affect its components. The reaction of pectin demethylation occurs thanks to PME. During this reaction, significant amounts of free carboxyl groups, which bind metal cations in cell walls, are produced (Paynel et al. 2009). PME enzyme activity increases in Cd-treated plants and is higher after Si application in Cd-stressed plants. Increased PME activity has been observed in response to Cd stress in various plant species, such as Linum usitatissimum, Pseudotsuga menziesii, Arabidopsis thaliana and Brassica chinensis (Astier et al. 2014; Douchiche et al. 2010; Wang et al. 2020; Zhu et al. 2012). An increase in the activity of the pectin PME correlates with a rise in of low methyl-esterified pectins because it releases of its free carboxyl groups (-COOH) that can bind Cd2+. It is well established that increased expression and activity of PME boost the capacity of the cell wall to bind Cd, thus reducing the entry of Cd²⁺ into plant cells (Riaz et al. 2021).
In order to determine the mobility of Cd compounds/complexes in the root sample and to assess the hydrophilic/hydrophobic properties of these complexes, we used four solvents with different physicochemical properties to leach these compounds from the samples. The highest efficiency was recorded for extraction with using the enzymes: pectinase and cellulase. This may mean that these selective degradations of initial compounds aid in the leaching of Cd-containing compounds in the sample. This may be due to the fact that Cd was bound to chemical compounds rich in pectins and cellulose. Pectins and cellulose typically occurred biopolymers found in the structure of plant cell walls, and their Cd-binding properties are due to the presence of functional groups such as carboxyl, hydroxyl, and other O-donor groups. The individual solvents for the selective elution of Cd from a sample can be generally characterised. Water could be included in the group of solvents with the weakest properties to solubilise and extract the hydrophilic compound. The ammonium acetate solution was reported as a solution dedicated to the extraction of small organic compounds especially, and its extraction efficiency can be optimised by a change of pH and buffer concentration. Pectinase and cellulase are enzymes dedicated to degradation of water insoluble residue mostly, such as pectic and cellulose type compounds in cell wall of plants (Ruzik and Dyoniziak 2022).
In the study, the observed uniform extraction efficiencies of ammonium acetate, pectinase, and cellulase across the experiments highlight intriguing aspects of the chemical nature and cellular interactions of Cd within plant systems. In particular, the extraction efficiency for Cd did not exceed 20% for any of the solvents used, which is consistent with previous findings where similar low recovery rates were documented (Mounicou et al. 2002a, b, 2003). This phenomenon is indicative of the intrinsic challenges associated with extracting Cd from complex biological matrices where Cd is tightly bound within the cellular structure. The similarity in extraction efficiencies across diverse solvents can be primarily attributed to the hydrophilic nature of the Cd complexes formed within plant tissues. These complexes are likely composed of Cd bound to polysaccharide components of the cell wall, such as pectins and cellulose, which are inherently hydrophilic. Despite the enzymatic capability of pectinase and cellulase to degrade these polysaccharides, their extraction efficiency was not significantly higher than that of ammonium acetate, a simple salt solution. This outcome suggests that the binding of Cd may involve more stable or inaccessible complexes within the cell wall matrix, potentially limiting the effectiveness of enzymatic breakdown. Additionally, the treatment with Si could have further influenced the structure and accessibility of these Cd-binding sites. Si is known to strengthen the plant cell wall, and its presence could modify the physical properties of cellulose and pectins, potentially making them less amenable to enzymatic degradation (Szpunar et al. 1999). This modification may contribute to the overall resistance of Cd complexes to extraction, regardless of the solvent used. Moreover, the requirement to maintain mild extraction conditions to prevent the degradation of complex Cd species further complicates the extraction process. Cd forms complex-type compounds that are sensitive to harsh extraction conditions, which could lead to the breakdown of the metal complexes, thus reducing the efficiency of Cd recovery. This necessitates the use of milder conditions, which may be less effective but are essential to preserve the integrity of the complexes.
Each of the solvents used is capable of extracting hydrophilic compounds; however, the use of enzymes should result in the dissolution of the cell wall matrix (pectins, cellulose, hemicellulose) (Mounicou et al. 2003). Hence, one would expect higher efficiencies for extraction enzyme systems in this case. However, the samples come from an environment badly contaminated with Cd and the total Cd content significantly exceeds the toxic concentration thresholds. The plant counteracts stress by inducing a series of natural actions that detoxify Cd. One of them is complexing with bioligands. An example could be the increase in the production of pectins (as well as de-esterification) and PCs (Nagayama et al. 2019). Both compounds are capable of binding Cd in the cell wall (pectins) and cytoplasm (PCs) and complexing to a non-active toxic form, respectively. The increased amount of pectins in the cell wall through the multiplied number of carboxyl groups (and reduced number of esterified groups) capable of binding Cd in the ionic form is another mechanism of detoxification. In turn, the increased content of pectins in the material affects the biophysical changes of the cell wall (Shin et al. 2021). Hence, the result may be the reduced cell wall permeability and, consequently, a lower-than-expected value of recovery of Cd extraction from this type of sample (from enzymatically assisted extractions). In addition, these samples contain Si also incorporated into the structures of the cell wall, and as a result the cell wall becomes even more inert to the leaching of the components present in the sample (Głazowska et al. 2018; Guerriero et al. 2016). The participation of Si in the Cd binding process is postulated (Greger et al. 2016; Liu et al. 2013). Comparing the values from these experiments, it can be concluded that the remaining portion of Cd is still not solubilised and that it creates an insoluble fraction (as well as a crystalline form and bound to Si-based compounds). The analysis of Cd speciation combined with the untargeted analysis of the identification of compounds - ligands for solutions differing in the type of solvents and the non-supplemented and Si-supplemented samples shows significant differences and the influence of these variables.
The effect of the extraction solvent on the distribution of Cd compounds is observable and significant. Likewise, for samples initially containing Si, the influence of the solvent on the distribution of Cd is noticeable, especially in the extraction carried out with water and pectinase, which may indicate that Si participates in the binding of Cd, and therefore Si may act as a kind of linkage between pectins and Cd. Cd is heterogeneously distributed between species differing in molar mass and eluted with several fractions, depending on the extraction solvent used (with the exception of ammonium acetate) and connected with high molar mass species (higher than 85 kDa). In separations with water as a mobile phase, peaks are primarily close to void volume. This relationship suggests a lack of interaction of species with the stationary phase and, simultaneously, low resolution for bigger particles. For extraction using water and pectinase, a change in the distribution of Cd compounds and the Cd content in these fractions was recorded compared to plant samples supplemented with Si compounds and control plants. This may suggest that Si compounds bioaccumulated in the plant influence and/or participate in the active binding of Cd with compounds originally present in the plant, or that Si compounds themselves may be ligands as a result of the interaction between these compounds. However, no silicon-containing compound was directly detected in the cadmium-containing samples analysed. This may be due, on the one hand, to the lack of such direct Cd binding from Si bonding or overlap the degradation of the complexes during the sample preparation stage. Cd complexes could degrade during and after acid addition at the sample preparation step (Bramanti et al. 2006) or they may be attributed to the MALDI ionisation technique used, which suffers from limited possibilities of detecting low-molecular compounds, because low-mass compounds were expected.
Based on the identification carried out, it is possible to indicate compounds that were related to Cd in the analysed fractions: (i) interaction with oxygen through the carboxylic group: galacturonic acid is part of a monomeric unit of a pectin biopolymer (Voragen et al. 2009); sialic (Kallolimath et al. 2016), glutamic and aspartic acids, which could be part of the protein backbone of the biopolymer as well as monomeric units of different protein, (ii) interaction with sulphur contain bioligands: methionine and cysteine could be monomeric parts of many biopolymers (Ravanel et al. 1998), (iii) interaction with nitrogen contain bioligands: serine amino acid could create stabile complexes with Cd cations (Sóvágó and Várnagy 2013). The main biopolymer responsible for Cd binding were pectins. It is with accordance with other conducted studies, in which the fractionation approach was utilized (Uddin et al. 2020). Extraction with ammonium acetate primarily isolates pectin as the dominant compound, along with 4-carboxyglutamic acid (Gomord and Faye 2004), a compound absent from previous plant tissue analyses but known to bind divalent metals (Burnier et al. 1981). Furthermore, 2-aminobutyric acid was identified, which serves as a stressor and signalling molecule within the plant (Yao et al. 2020).
5 Conclusions
This study integrates physiological and analytical methodologies to elucidate the mechanisms of cadmium binding within the root cell walls of pea plants, focussing in particular on the role of silicon in modulating these processes. Our findings confirm that silicon supplementation significantly alters the composition of the cell wall, notably by enhancing the levels of pectin and the activity of pectin methylesterase. These changes facilitate the formation of cadmium complexes with the carboxyl groups of pectins, predominantly through the interaction with galacturonic acid residues, thereby increasing the density of active binding sites (Eq. 1, Fig. 5). The speciation analysis further demonstrates that silicon affects not only the quantity and quality of cadmium binding but also its distribution within the root cell walls, particularly in treatments with water and pectinase, where enhanced binding was observed. These results suggest a dual function of silicon in both enhancing the detoxification capacity of plants and altering the biophysical properties of the cell wall to retain more cadmium. By delineating the specific interactions between cadmium ions and bioligands, such as pectins, this research contributes to a deeper understanding of the physiological pathways involved in cadmium retention and highlights the potential of silicon as a mitigative agent against cadmium stress in plants. Overall, the study provides comprehensive insights into the dynamic interactions within the cell walls of pea roots, presenting a robust model of cadmium binding that underscores the critical role of silicon in enhancing the plant’s natural defence mechanisms against cadmium toxicity.
References
Astier C, Gloaguen V, Faugeron C (2014) Phytoremediation of cadmium-contaminated soils by young Douglas fir trees: effects of cadmium exposure on cell wall composition. Int J Phytorem 16:790–803. https://doi.org/10.1080/15226514.2013.856849
Baldwin L, Domon J-M, Klimek JF, Fournet F, Sellier H, Gillet F, Pelloux J, Lejeune-Hénaut I, Carpita NC, Rayon C (2014) Structural alteration of cell wall pectins accompanies pea development in response to cold. Phytochemistry 104:37–47. https://doi.org/10.1016/j.phytochem.2014.04.011
Barałkiewicz D, Kózka M, Piechalak A, Tomaszewska B, Sobczak P (2009) Determination of cadmium and lead species and phytochelatins in pea (Pisum sativum) by HPLC-ICP-MS and HPLC-ESI-MSn. Talanta 79:493–498. https://doi.org/10.1016/j.talanta.2009.04.026
Bari MA, Prity SA, Das U, Akther MS, Sajib SA, Reza MA, Kabir AH (2020) Silicon induces phytochelatin and ROS scavengers facilitating cadmium detoxification in rice. Plant Biol (Stuttg) 22:472–479. https://doi.org/10.1111/plb.13090
Batool T, Javied S, Ashraf K, Sultan K, Zaman QU, Haider FU (2022) Alleviation of cadmium stress by Silicon Supplementation in peas by the Modulation of Morpho-Physio-Biochemical Variables and Health Risk Assessment. Life (Basel) 12. https://doi.org/10.3390/life12101479
Bernabeu de Maria M, Lamarche J, Ronga L, Messori L, Szpunar J, Lobinski R (2023) Selenol (-SeH) as a target for mercury and gold in biological systems: contributions of mass spectrometry and atomic spectroscopy. Coord Chem Rev 474:214836. https://doi.org/10.1016/j.ccr.2022.214836
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Bramanti E, Toncelli D, Morelli E, Lampugnani L, Zamboni R, Miller KE, Zemetra J, D’Ulivo A (2006) Determination and characterization of phytochelatins by liquid chromatography coupled with on line chemical vapour generation and atomic fluorescence spectrometric detection. J Chromatogr A 1133:195–203. https://doi.org/10.1016/j.chroma.2006.08.045
Burnier JP, Borowski M, Furie BC, Furie B (1981) Gamma-carboxyglutamic acid. Mol Cell Biochem 39:191–207. https://doi.org/10.1007/BF00232574
Clemens S (2006) Toxic metal accumulation, responses to exposure and mechanisms of tolerance in plants. Biochimie 88:1707–1719. https://doi.org/10.1016/j.biochi.2006.07.003
Cobbett CS (2000) Phytochelatins and their roles in heavy metal detoxification. Plant Physiol 123:825–832. https://doi.org/10.1104/pp.123.3.825
Cruzado-Tafur E, Orzoł A, Gołębiowski A, Pomastowski P, Cichorek M, Olszewski J, Walczak-Skierska J, Buszewski B, Szultka-Młyńska M, Głowacka K (2023) Metal tolerance and cd phytoremoval ability in Pisum sativum grown in spiked nutrient solution. J Plant Res 136:931–945. https://doi.org/10.1007/s10265-023-01493-1
Douchiche O, Driouich A, Morvan C (2010) Spatial regulation of cell-wall structure in response to heavy metal stress: cadmium-induced alteration of the methyl-esterification pattern of homogalacturonans. Ann Bot 105:481–491. https://doi.org/10.1093/aob/mcp306
Dutta A, Patra A, Singh Jatav H, Singh Jatav S, Kumar Singh S, Sathyanarayana E, Verma S, Singh P (2021) Toxicity of Cadmium in Soil-Plant-Human Continuum and Its Bioremediation Techniques. In: L. Larramendy M, Soloneski S (eds) Soil Contamination - Threats and Sustainable Solutions. IntechOpen
El Rasafi T, Oukarroum A, Haddioui A, Song H, Kwon EE, Bolan N, Tack FMG, Sebastian A, Prasad MNV, Rinklebe J (2022) Cadmium stress in plants: a critical review of the effects, mechanisms, and tolerance strategies. Crit Rev Environ Sci Technol 52:675–726. https://doi.org/10.1080/10643389.2020.1835435
El-Okkiah SAF, El-Tahan AM, Ibrahim OM, Taha MA, Korany SM, Alsherif EA, AbdElgawad H, Abo Sen EZF, Sharaf-Eldin MA (2022) Under cadmium stress, silicon has a defensive effect on the morphology, physiology, and anatomy of pea (Pisum sativum L.) plants. Front Plant Sci 13:997475. https://doi.org/10.3389/fpls.2022.997475
Gill SS, Tuteja N (2011) Cadmium stress tolerance in crop plants: probing the role of sulfur. Plant Signal Behav 6:215–222. https://doi.org/10.4161/psb.6.2.14880
Głazowska S, Baldwin L, Mravec J, Bukh C, Hansen TH, Jensen MM, Fangel JU, Willats WGT, Glasius M, Felby C, Schjoerring JK (2018) The impact of silicon on cell wall composition and enzymatic saccharification of Brachypodium distachyon. Biotechnol Biofuels 11:171. https://doi.org/10.1186/s13068-018-1166-0
Głowacka K, Olszewski J, Sowiński P, Kalisz B, Najdzion J (2022) Developmental and physiological responses of Pisum sativum L. after short- and long-time cadmium exposure. Agriculture 12:637. https://doi.org/10.3390/agriculture12050637
Gomord V, Faye L (2004) Posttranslational modification of therapeutic proteins in plants. Curr Opin Plant Biol 7:171–181. https://doi.org/10.1016/j.pbi.2004.01.015
Greger M, Kabir AH, Landberg T, Maity PJ, Lindberg S (2016) Silicate reduces cadmium uptake into cells of wheat. Environ Pollut 211:90–97. https://doi.org/10.1016/j.envpol.2015.12.027
Guerriero G, Hausman J-F, Legay S (2016) Silicon and the Plant Extracellular Matrix. Front Plant Sci 7:463. https://doi.org/10.3389/fpls.2016.00463
Guo X, Luo J, Du Y, Li J, Liu Y, Liang Y, Li T (2021) Coordination between root cell wall thickening and pectin modification is involved in cadmium accumulation in Sedum Alfredii. Environ Pollut 268:115665. https://doi.org/10.1016/j.envpol.2020.115665
He J, Zhou J, Wan H, Zhuang X, Li H, Qin S, Lyu D (2020) Rootstock-Scion Interaction affects Cadmium Accumulation and Tolerance of Malus. Front Plant Sci 11:1264. https://doi.org/10.3389/fpls.2020.01264
Hoagland DR, Arnon DI (1950) The Water Culture Method for Growing Plants without Soil. 347
Jia H, Wang X, Wei T, Zhou R, Muhammad H, Hua L, Ren X, Guo J, Ding Y (2019) Accumulation and fixation of cd by tomato cell wall pectin under cd stress. Environ Exp Bot 167:103829. https://doi.org/10.1016/j.envexpbot.2019.103829
Kallolimath S, Castilho A, Strasser R, Grünwald-Gruber C, Altmann F, Strubl S, Galuska CE, Zlatina K, Galuska SP, Werner S, Thiesler H, Werneburg S, Hildebrandt H, Gerardy-Schahn R, Steinkellner H (2016) Engineering of complex protein sialylation in plants. Proc Natl Acad Sci U S A 113:9498–9503. https://doi.org/10.1073/pnas.1604371113
Khalid H, Zia-ur-Rehman M, Naeem A, Khalid MU, Rizwan M, Ali S, Umair M, Sohail MI (2019) Solanum nigrum L.: a Novel Hyperaccumulator for the Phyto-Management of Cadmium Contaminated soils. Cadmium toxicity and tolerance in plants. Elsevier, pp 451–477
Li X, Li Y, Qu M, Xiao H, Feng Y, Liu J, Wu L, Yu M (2016) Cell Wall Pectin and its Methyl-esterification in Transition Zone Determine Al Resistance in cultivars of pea (Pisum sativum). Front Plant Sci 7:39. https://doi.org/10.3389/fpls.2016.00039
Li D, Shu Z, Ye X, Zhu J, Pan J, Wang W, Chang P, Cui C, Shen J, Fang W, Zhu X, Wang Y (2017) Cell wall pectin methyl-esterification and organic acids of root tips involve in aluminum tolerance in Camellia sinensis. Plant Physiol Biochem 119:265–274. https://doi.org/10.1016/j.plaphy.2017.09.002
Liu J, Ma J, He C, Li X, Zhang W, Xu F, Lin Y, Wang L (2013) Inhibition of cadmium ion uptake in rice (Oryza sativa) cells by a wall-bound form of silicon. New Phytol 200:691–699. https://doi.org/10.1111/nph.12494
Liu J, Shao Y, Feng X, Otie V, Matsuura A, Irshad M, Zheng Y, An P (2022) Cell Wall Components and Extensibility regulate Root Growth in Suaeda salsa and Spinacia oleracea under Salinity. Plants (Basel) 11. https://doi.org/10.3390/plants11070900
Luo J-S, Zhang Z (2021) Mechanisms of cadmium phytoremediation and detoxification in plants. Crop J 9:521–529. https://doi.org/10.1016/j.cj.2021.02.001
Ma J, Cai H, He C, Zhang W, Wang L (2015) A hemicellulose-bound form of silicon inhibits cadmium ion uptake in rice (Oryza sativa) cells. New Phytol 206:1063–1074. https://doi.org/10.1111/nph.13276
Mounicou S, Szpunar J, Lobinski R, Andrey D, Blake C-J (2002a) Bioavailability of cadmium and lead in cocoa: comparison of extraction procedures prior to size-exclusion fast-flow liquid chromatography with inductively coupled plasma mass spectrometric detection (SEC-ICP-MS). J Anal Spectrom 17:880–886. https://doi.org/10.1039/b201639g
Mounicou S, Szpunar J, Andrey D, Blake C, Lobinski R (2002b) Development of a sequential enzymolysis approach for the evaluation of the bioaccessibility of cd and pb from cocoa. Analyst 127:1638–1641. https://doi.org/10.1039/b207427n
Mounicou S, Szpunar J, Andrey D, Blake C, Lobinski R (2003) Concentrations and bioavailability of cadmium and lead in cocoa powder and related products. Food Addit Contam 20:343–352. https://doi.org/10.1080/0265203031000077888
Nagayama T, Nakamura A, Yamaji N, Satoh S, Furukawa J, Iwai H (2019) Changes in the distribution of pectin in Root Border cells under aluminum stress. Front Plant Sci 10:1216. https://doi.org/10.3389/fpls.2019.01216
Orzoł A, Gołębiowski A, Szultka-Młyńska M, Głowacka K, Pomastowski P, Buszewski B (2022) ICP-MS analysis of Cadmium Bioaccumulation and its effect on pea plants (Pisum sativum L). Pol J Environ Stud 31:4779–4787. https://doi.org/10.15244/pjoes/149259
Orzoł A, Szultka-Młyńska M, Głowacka K, Krakowska-Sieprawska A, Złoch M, Buszewski B (2023) Changes in glutathione content and glutathione reductase activity in silicon supplementation during cadmium stress in pea (Pisum sativum L). In: Kalbarczyk K, Danielewska A (eds) Interdyscyplinarne badania w naukach przyrodniczych– innowacje, analizy i perspektywy (1). Wydawnictwo Naukowe TYGIEL Sp. z o. o. Lublin, Poland, pp 45–60
Paynel F, Schaumann A, Arkoun M, Douchiche O, Morvan C (2009) Temporal regulation of cell-wall pectin methylesterase and peroxidase isoforms in cadmium-treated flax hypocotyl. Ann Bot 104:1363–1372. https://doi.org/10.1093/aob/mcp254
Piechalak A, Tomaszewska B, Baralkiewicz D, Malecka A (2002) Accumulation and detoxification of lead ions in legumes. Phytochemistry 60:153–162. https://doi.org/10.1016/s0031-9422(02)00067-5
Pons M-L, Collin B, Doelsch E, Chaurand P, Fehlauer T, Levard C, Keller C, Rose J (2021) X-ray absorption spectroscopy evidence of sulfur-bound cadmium in the Cd-hyperaccumulator Solanum nigrum and the non-accumulator Solanum melongena. Environ Pollut 279:116897. https://doi.org/10.1016/j.envpol.2021.116897
Rahman MF, Ghosal A, Alam MF, Kabir AH (2017) Remediation of cadmium toxicity in field peas (Pisum sativum L.) through exogenous silicon. Ecotoxicol Environ Saf 135:165–172. https://doi.org/10.1016/j.ecoenv.2016.09.019
Ravanel S, Gakière B, Job D, Douce R (1998) The specific features of methionine biosynthesis and metabolism in plants. Proc Natl Acad Sci U S A 95:7805–7812. https://doi.org/10.1073/pnas.95.13.7805
Riaz M, Kamran M, Rizwan M, Ali S, Parveen A, Malik Z, Wang X (2021) Cadmium uptake and translocation: selenium and silicon roles in cd detoxification for the production of low cd crops: a critical review. Chemosphere 273:129690. https://doi.org/10.1016/j.chemosphere.2021.129690
Ruzik L, Dyoniziak A (2022) Natural deep Eutectic solvents as a Key Metal Extractant for Fractionation in Speciation Analysis. Molecules 27. https://doi.org/10.3390/molecules27031063
Schmohl N, Horst WJ (2000) Cell wall pectin content modulates aluminium sensitivity of Zea mays (L.) cells grown in suspension culture. Plant Cell Environ 23:735–742. https://doi.org/10.1046/j.1365-3040.2000.00591.x
Seregin IV, Ivanov VB (1997) Histochemical investigation of cadmium and lead distribution in plants. Russ J Plant Physiol 44:791–796
Shin Y, Chane A, Jung M, Lee Y (2021) Recent advances in understanding the roles of Pectin as an active participant in Plant Signaling Networks. Plants (Basel) 10. https://doi.org/10.3390/plants10081712
Sóvágó I, Várnagy K (2013) Cadmium(II) complexes of amino acids and peptides. Met Ions Life Sci 11:275–302. https://doi.org/10.1007/978-94-007-5179-8_9
Szerement J, Szatanik-Kloc A (2022) Cell-wall pectins in the roots of Apiaceae plants: adaptations to cd stress. Acta Physiol Plant 44. https://doi.org/10.1007/s11738-022-03386-7
Szpunar J, Pellerin P, Makarov A, Doco T, Williams P, Łobiński R (1999) Speciation of metal-carbohydrate complexes in fruit and vegetable samples by size-exclusion HPLC-ICP-MS. J Anal Spectrom 14:639–644. https://doi.org/10.1039/A808231F
Uddin MM, Chen Z, Huang L (2020) Cadmium accumulation, subcellular distribution and chemical fractionation in hydroponically grown Sesuvium portulacastrum [Aizoaceae]. PLoS ONE 15:e0244085. https://doi.org/10.1371/journal.pone.0244085
Ueno D, Ma JF, Iwashita T, Zhao F-J, McGrath SP (2005) Identification of the form of cd in the leaves of a superior Cd-accumulating ecotype of Thlaspi caerulescens using 113Cd-NMR. Planta 221:928–936. https://doi.org/10.1007/s00425-005-1491-y
Vacchina V, Połeć K, Szpunar J (1999) Speciation of cadmium in plant tissues by size-exclusion chromatography with ICP-MS detection. J Anal Spectrom 14:1557–1566. https://doi.org/10.1039/A904845F
Vaculík M, Landberg T, Greger M, Luxová M, Stoláriková M, Lux A (2012) Silicon modifies root anatomy, and uptake and subcellular distribution of cadmium in young maize plants. Ann Bot 110:433–443. https://doi.org/10.1093/aob/mcs039
Voragen AGJ, Coenen G-J, Verhoef RP, Schols HA (2009) Pectin, a versatile polysaccharide present in plant cell walls. Struct Chem 20:263–275. https://doi.org/10.1007/s11224-009-9442-z
Wang L, Li R, Yan X, Liang X, Sun Y, Xu Y (2020) Pivotal role for root cell wall polysaccharides in cultivar-dependent cadmium accumulation in Brassica chinensis L. Ecotoxicol Environ Saf 194:110369. https://doi.org/10.1016/j.ecoenv.2020.110369
Wei W, Peng H, Xie Y, Wang X, Huang R, Chen H, Ji X (2021) The role of silicon in cadmium alleviation by rice root cell wall retention and vacuole compartmentalization under different durations of cd exposure. Ecotoxicol Environ Saf 226:112810. https://doi.org/10.1016/j.ecoenv.2021.112810
White PJ, Brown PH (2010) Plant nutrition for sustainable development and global health. Ann Bot 105:1073–1080. https://doi.org/10.1093/aob/mcq085
Wu J, Guo J, Hu Y, Gong H (2015) Distinct physiological responses of tomato and cucumber plants in silicon-mediated alleviation of cadmium stress. Front Plant Sci 6:453. https://doi.org/10.3389/fpls.2015.00453
Yao L, Zhong Y, Wang B, Yan J, Wu T (2020) BABA application improves soybean resistance to aphid through activation of phenylpropanoid metabolism and callose deposition. Pest Manag Sci 76:384–394. https://doi.org/10.1002/ps.5526
Yu H, Wu Y, Huang H, Zhan J, Wang K, Li T (2020) The predominant role of pectin in binding cd in the root cell wall of a high cd accumulating rice line (Oryza sativa L). Ecotoxicol Environ Saf 206:111210. https://doi.org/10.1016/j.ecoenv.2020.111210
Zhang H, Zhou W, Chen Y, Xu H, Hou D, Lv S, Sun X, Wang F, Yang L (2022) The tolerance, absorption, and Transport Characteristics of Macleaya cordata in relation to lead, zinc, Cadmium, and copper under Hydroponic conditions. Appl Sci 12:9598. https://doi.org/10.3390/app12199598
Zhu XF, Lei GJ, Jiang T, Liu Y, Li GX, Zheng SJ (2012) Cell wall polysaccharides are involved in P-deficiency-induced cd exclusion in Arabidopsis thaliana. Planta 236:989–997. https://doi.org/10.1007/s00425-012-1652-8
Acknowledgements
This work was supported by research project Opus 18 No. 2019/35/B/ST4/02791(2020–2024) from the National Science Centre (Kraków, Poland). Małgorzata Szultka-Młyńska, Paweł Pomastowski and Katarzyna Rafińska are members of Toruń Center of Excellence “Towards Personalized Medicine” operating under Excellence Initiative-Research University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gołębiowski, A., Szultka-Młyńska, M., Pomastowski, P. et al. Role of Silicon in Counteracting Cadmium Stress in Pea Plants (Pisum sativum L.): Insights Into Cadmium Binding Mechanisms and Pectin Methylesterase Activity. J Soil Sci Plant Nutr (2024). https://doi.org/10.1007/s42729-024-01929-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42729-024-01929-0