Abstract
Sustainable agriculture aims to provide food needs while improving soil health and protecting it from degradation and contamination from excessive chemical fertilizer use. Sandy-textured soils have low fertility and water-holding capacity. This study assessed the integrated impact of super absorbent polymers (SAPs) and biofertilizer application on the soil chemical characteristics and wheat growth parameters in sandy loam soil. Two super absorbent polymers (SAPs) included Barbary plant G3 (P1) and Aqua Gool polymer (P2), and four microbial inoculations (Trichoderma harzianum (T), Actinomycetes (Streptomyces rochei and Streptomyces atrovirens) (AC1 and AC2), and Bacillus subtilis (B)) as biofertilizers were used in our pot experiment. The SAPs were applied to soil at a level of 0.2% (w/w), while biofertilizers were applied in the form of microbial cell suspensions (50 ml per pot) in addition to treating wheat seed with these suspensions during cultivation. Wheat plants were irrigated every 8 days to field capacity level. Amending soil with super absorbent polymers and microbes either individually or in combination significantly reduced pH and EC, increased organic matter level, and the availability of macro- and micronutrients in soil. Wheat growth metrics, including shoot length, tiller number, biomass accumulation, leaf area, and grain yield, exhibited considerable enhancements relative to the plants of the control treatment. The interaction between P1 polymer and Streptomyces atrovirens (AC2) showed the highest performance in improving the almost studied parameters. The application of SAPs with microbial biofertilizers offers a promising eco-friendly method for enhancing soil health and wheat yield.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Ensuring food security is an enormous challenge for the world due to insufficient crop yields for direct human consumption, diminished water supplies, and the deterioration of agricultural lands (Hemathilake and Gunathilake 2022). The agricultural economy has been significantly impacted by climate change in recent years due to water scarcity and the resulting desertification especially in dry and semi-dry regions (Oladosu et al. 2022; Junaid and Gokce 2024). Food security depends greatly on wheat cultivation. Wheat is considered a strategic crop that contributes to bridging the food gap for the world population. For approximately 35% of the world’s population, wheat is the staple crop, and more than two thirds of the wheat produced worldwide is consumed for food (Grote et al. 2021). Wheat is an important food crop next to rice in terms of consumption (Senbeta and Worku 2023). Nowadays, wheat is cultivated more extensively than any other crop, accounting for 20% of our protein and carbohydrates and over 25% of the world’s total output (Langridge et al. 2022). In Egypt, it is necessary to expand wheat cultivation on newly reclaimed soils to cope with population growth. The soils of most newly reclaimed areas are coarse-textured and have a higher proportion of sand fraction comparing to the other fractions (silt and clay). These soils are generally characterized by poor physical and chemical properties such as rapid permeability, low capacity of water holding, low cation exchange capacity (CEC), inherent poor fertility, low content of organic matter (OM), and low productivity (Huang and Hartemink 2020). Various soil amendments could be added these characteristics. Super absorbent polymers (SAPs) and biofertilizers have become significant agricultural amendments for enhancing the soil quality and health as well as crop productivity (Zhang et al. 2018; Zheng et al. 2023).
As soil conditioners, Superabsorbent polymers (SAPs) are indeed a type of hydrogel, they consist of hydrophilic polymers, such as polyacrylic acid (PAA) and polyacrylamide, which are 3D crosslinked to form a network structure (Takahashi et al. 2023). The crosslinking agents used in SAPs can be polycations or other chemicals that help to create a stable network structure (Lee et al. 2020). SAPs have the ability to absorb and retain large volumes of water. Through their water retention and controlled release properties, SAPs help reduce irrigation requirements, enhance water use efficiency, and stop rapid water loss through evaporation and drainage (Dingley et al. 2024). As the soil dries, the water that has been held in the SAPs is released gradually through a diffusion mechanism, allowing the soil to stay wet for a longer period of time, thereby promoting plant growth and soil microbial activity (Khodadadi Dehkordi 2018; Albalasmeh et al. 2022). By adding SAPs to the soil, aggregate stability and soil structure are improved. This has the benefit of increasing soil porosity, which improves soil microbial and plant root oxygen circulation (Oladosu et al. 2022). SAPs work as agents for slow-release fertilizers, improve soil nutrient retention, and raise plant nutrient availability. Water-soluble nutrients are more readily available for plant uptake due to SAPs’ superior ability to absorb and retain water. This reduces nutrient loss by leaching in the soil and minimizes the groundwater contamination risk (Singh et al. 2021). In order to increase sustainable agricultural output in the event of soil water challenges and as a soil conditioner, using hydrogel will be a productive choice (Sri et al. 2019).
Due to the increased awareness of the usage of sustainable tools that pose less risk in the agricultural sector, biofertilizers have emerged as a safe and efficient alternative to the chemically manufactured fertilizers in recent times (Sharma et al. 2023). Biofertilizers are preparations in laboratory containing beneficial and efficient strains of living microorganisms such as bacteria, fungi and actinomycetes (Zambrano-Mendoza et al. 2021; Farhad et al. 2023). These microorganisms are applied to soil, seeds, or plants, they play an important role in the sustainable agriculture by their ability to colonize the rhizosphere or the inside of plants, promoting nutrient mobilization, improving soil fertility and plant growth (Khan et al. 2023). Biofertilizers can contain one or more microbial strains and are added in a variety of formulations, including liquids (suspensions), powders, and granules (Díez-Méndez et al. 2024). They can improve plant nutrient availability, increase productivity, enhance the agroecological environment, replenish the soil’s beneficial bacteria and microbial population (Zhang et al. 2018; Fasusi et al. 2021; Nosheen et al. 2021). Biofertilizers based on Bacillus sp., Trichoderma sp., and Actinomycetes sp. have shown significant benefits in agriculture. They can nourish plants and protect them from a variety of plant diseases and stressful environments through natural processes such as nitrogen fixation, hormone generation, siderophore synthesis, potassium and phosphorus solubilization, and the development of several hydrolytic enzymes (Zambrano-Mendoza et al. 2021; Chaudhary et al. 2022; Shankar and Prasad 2023).
In Egypt, the use of biofertilizers as a cheap and clean substitutional to chemical fertilizers is still not widespread, especially in the recently reclaimed soils of arid and semi-arid regions that suffer from coarse texture, low moisture holding ability, high temperatures, and evaporation rates, as these factors together constitute a threat to the survival and functioning of biofertilizer microbes. This study was carried out in the research farm of Sohag University, New Sohag City, southwest of Sohag governorate in Egypt. Climatically, Sohag Governorate is a part of the arid belt of Egypt which has warm winter with scarce or no rainfall and hot summer (Elbeih et al. 2011; Omran 2008). Our hypothesis is that when using biofertilizers in the coarse-textured soils of the dry and semi-dry regions, adding polymers increases the efficiency and effectiveness of biofertilizer microbes because these polymers increase water retention in the soil for a longer period, which is a crucial factor for the activity and proliferation of beneficial microbes. In addition to the role of these polymers in improving soil structure and aeration, which reflect positively on the performance of those microbes. This study aimed to investigate the effect of super absorbent polymers addition in combination with some biofertilizers such as Trichoderma sp., Actinomycetes sp. and Bacillus sp. on soil chemical characteristics and fertility as well as the wheat’s growth and productivity in sandy loam soil.
2 Materials and Methods
2.1 Soil Characterization
The experiment was carried out in the Sohag University’s agricultural farm in New Sohag City, which is situated at the western boundary of the Nile valley, approximately 15 km southwest of Sohag City (26°28’ 13” N and 31°40’ 20” E). The used soil air dried, grinded in a wooden mortar, and then sieved using 2 mm sieve. The sieved soil was analyzed for some properties as follows; the particle size distribution was determined based on the international pipette procedure (Piper 1950). The pressure plate membrane apparatus (Soilmoisture Equipment Corp, USA) was used for field capacity (FC%) and wilting point (WP%) determination in the studied soil (Black 1965), and the available water (AW%) calculated by subtracting the WP percentage from FC percentage. A 1:2.5 soil to water suspension was prepared for measuring soil pH by using pH meter (pH 211 microprocessor, HANNA Instruments, UK) and a glass electrode (McLean 1982). The electrical conductivity (EC) of soil was determined using the EC meter (Orion 150, USA) in a 1:5 soil to water extract (Jackson 1973). The organic matter (OM) % in the studied soil was determined according to the wet oxidation of Walkley and Black procedure (Jackson 1973). The alkaline permanganate method (Subbiah and Asija 1956) was used for available nitrogen (N) determination by Kjeldhal equipment (Automatic distillation system Rapidstill II, Labconco, USA). The sodium bicarbonate (0.5 M, with pH = 8.50) solution used for extraction the available phosphorus (P), and the extractable P was analyzed calorimetrically using the spectrophotometer (Jenway 7305 Bibby Scientific Ltd, UK) according to Olsen et al. (1954) method. The ammonium acetate (NH4OAc) solution (1 M) was used for extraction the available potassium (K) (Carson 1980), the extractable K was measured using the flame photometer (Clinical PFP7 Buck Scientific, USA). The available micro-nutrients (Fe, Mn, Zn, and Cu) were extracted by using the DTPA solution (DTPA 0.005 M, 0.1 M triethanolamine, 0.01 M CaCl2 at pH = 7.30) (Lindsay and Norvell 1978) and measured using atomic absorption spectroscopy (210VGP, Buck Scientific, Inc., USA). The characterization of the studied soil is shown in Table 1.
2.2 Soil Amendments
2.2.1 Superabsorbent Polymers (SAPs)
Two types of superabsorbent polymers (P) were employed in this study. The first type (P1), named “Barbary Plant G3”, obtained from Lucky Star TG, Egypt. Barbary Plant G3 is composed of hydro polymer enriched with macro-nutrients (N, P, and K) and micro-nutrients (Fe, Mn, Zn, Cu, Br, and Mo), and growth regulators. The second type (P2) named Aqua Gool (AG), it is a Russian production and composed of hydro potassium polymer (90%) and polyacrylamide allylamine hydrochloride. Table 2 shows the characteristics of these polymers. These polymers were thoroughly mixed with the studied soil at a level of 0.2% (w/w) in our pot experiment.
2.2.2 The Studied Biofertilizers
Four microbial isolates, selected as biofertilizers, were obtained from the stock cultures of the Plant Pathology Department, Faculty of Agriculture, Sohag University, Egypt. These isolates included one strain of the fungus Trichoderma (Trichoderma harzianum Rifai), two strains of Actinomycetes sp. (Streptomyces rochei and Streptomyces atrovirens), and one strain of the bacterium Bacillus (Bacillus subtilis).
2.2.3 Preparation of Microbial Inoculants
The microbial inoculants preparation involved growing the microbial isolates in 250 ml conical flasks containing 200 ml of specific nutrient media. For Bacillus subtilis and Actinomycetes sp. (Streptomyces rochei and Streptomyces atrovirens), nutrient glucose agar (NGA) broth medium (5.0 g pepton, 3.0 g beef extract, 5.0 g glucose, 20.0 g agar in 1 L of distilled water, pH 7.0) was used (Dowson 1957), while Trichoderma harzianum was grown in potato dextrose agar (PDA) broth medium (200.0 g potato infusion, 20.0 g dextrose, 15.0 g agar in 1 L of distilled water, pH 6.0) according to Anonymous (1968). Incubation was carried out on a rotary shaker (150 rpm) at controlled temperatures and durations (25 °C and 3 days for Bacillus and Actinomycetes, 28 °C and 14 days for Trichoderma). After incubation, the resulting microbial suspensions were adjusted to specific concentration (5 × 104 CFU ml− 1 for Trichoderma and 5 × 106 CFU ml− 1 for Actinomycetes strains and Bacillus) using sterilized distilled water (SDW) and a hemocytometer (Precicolor HBG, Germany).
2.3 Pot Experiment
The pot experiment was carried out in a completely randomized design with three replicates. Plastic pots of 35 cm (diameter) and 30 cm (depth) were utilized. Every pot was filled with 8.5 kg of the studied dried soil. The experiment consisted of 15 treatments in 3 replicates, these treatments are summarized as the following:
-
1.
Control soil (CK).
-
2.
Soil + Polymer 1 (P1).
-
3.
Soil + Polymer 2 (P2).
-
4.
Soil + Trichoderma (Trichoderma harzianum) (T).
-
5.
Soil + Actynomicetes1 (Streptomyces rochei) (AC1).
-
6.
Soil + Actinomycetes 2 (Streptomyces atrovirens) (AC2).
-
7.
Soil + Bacillus (Bacillus subtilis) (B).
-
8.
Soil + Polymer1 + Trichoderma (Trichoderma harzianum) (P1T).
-
9.
Soil + Polymer1 + Actynomicetes1 (Streptomyces rochei) (P1AC1).
-
10.
Soil + Polymer1 + Actinomycetes 2 (Streptomyces atrovirens) (P1AC2).
-
11.
Soil + Polymer1 + Bacillus (Bacillus subtilis) (P2B).
-
12.
Soil + Polymer 2 + Trichoderma (Trichoderma Harzianum) (P2T).
-
13.
Soil + Polymer 2 + Actynomicetes1 (Streptomyces rochei) (P2AC1).
-
14.
Soil + Polymer 2 + Actinomycetes 2 (Streptomyces atrovirens) (P2AC2).
-
15.
Soil + Polymer 2 + Bacillus (Bacillus subtilis) (P2B).
The studied polymers thoroughly mixed with soil before cultivation at a level of 0.2% (w/w). The wheat seeds treated with the prepared microbial suspensions, and then another 50 ml of each suspension was added per pot during cultivation in all treatments that received microbes. Wheat seeds (Shandaweel 1 local variety) were sown in each pot on November 29, 2021. After 10 days from sowing, the wheat plants were thinned to uniform and healthy 10 plants in each pot and irrigated every 8-day intervals to the field capacity moisture level until the experiment end. After 90 days from cultivation, three plants were taken from each pot for measuring the growth parameters, including shoot length, plant fresh and dry weights, tillers and leaves number per plant, and leaf area index per plant. After that, the remained wheat plants in the pots were harvested after 160 days from cultivation and the weight of 1000 grains was determined. As regard to the climatic conditions during the growing season of the pot experiment, the average maximum temperature recorded in May 2022 was 35 °C, while the lowest recorded temperature in January 2022 was 19 °C with almost no precipitations (0.0 to 0.1 mm as monthly average). Wind speeds reaching 9 knots by May 2022 after averaging 6.6 knots in December 2021. The relative humidity decreased from 51% in January 2022 to 19% in May 2022 (https://weatherspark.com/).
2.4 Plant Analysis
The plants that taken after 90 days after cultivation were oven-dried at 70 °C until reaching a constant weight. The dried plants were grounded by using a stainless-steel mill. A 0.5 g of the grounded straw was digested using sulfuric and perchloric acid according to Chapman and Pratt (1961), then the macronutrients (N, P, and K) and micronutrients (Fe, Mn, Zn, and Cu) contents were analyzed for all treatments, following the methods outlined by Jackson (1973), to assess the effects of different applied amendments on the wheat’s nutritional status.
2.5 Soil Analysis After Wheat Harvest
After harvesting, the soil of each pot was well mixed and a soil sample was collected from each pot. Then, these collected soil samples were analyzed. The assessed soil parameters included soil pH in 1:2.5 soil to water suspension, electrical conductivity (EC) in 1:5 soil: water extract, organic matter (OM) percent, available N, P and K contents, and the DTPA-extractable micronutrients were determined in all soil samples according to the above-mentioned methods. All soil and plant analyses were carried out in soil and water department laboratory, Faculty of Agriculture, Sohag, Egypt.
2.6 Statistical Analysis
Using SPSS version 27 software, the analysis of variance (ANOVA) was performed on all of the obtained results. For comparing between means in the studied treatments, post-hoc comparisons using Duncan’s Multiple Range test was performed at a significance level of p < 0.05 (Gomez and Gomez 1984).
3 Results
3.1 Soil Chemical Properties
3.1.1 Soil pH
Data in Table 3 demonstrates that the application of polymers and microorganisms, whether individual or in combination, had considerable effect on the chemical properties of the investigated soil. Soil pH decreased in all treatment comparing with the untreated control. The highest value of 8.45 was recorded in the control (CK) treatment which significantly decreased to 8.20 and 8.36 with applying P1 and P2 treatments individually. Similarly, inoculation of soil with T, AC1, AC2, and B microbes had a clear and significant effect in reducing the pH value to 8.12, 8.27, 8.01 and 8.20, respectively compared to the control. The lowest pH value of 7.77 was observed in the P1AC2 interaction treatment.
3.1.2 Soil EC
The effect of super absorbent polymers and biofertilization on soil electrical conductivity (EC) was illustrated in Table 3. It is noticeable that soil EC decreased throughout all treatments in comparison with the control soil (CK). The control treatment had the highest soil EC value of 0.61 dS m− 1. Addition of P1 and P2 polymers alone decreased the EC value significantly to 0.48 and 0.50 dS m− 1, respectively. While, amending soil with T, AC1, AC2, and B microbes only significantly reduced EC to 0.53, 0.52, 0.51, and 0.52 dS m− 1, respectively, relative to the untreated control (0.61dS m− 1). The lowest soil EC of 0.48 dS m− 1 with obtained from P1-treated soil.
3.1.3 Soil Organic Matter (SOM)
Regarding soil organic matter (SOM) content results in Table 3, a considerable enhancement was observed in SOM content as a result of polymers and micro-organisms application comparing with the control. The soil organic matter percentage increased significantly from 0.48% in control soil to 1.02 and 0.96% in P1 and P2-amended soils, and to 0.90, 0.92, 0.87 and 0.75% in T, AC1, AC2 and B inoculated soil with, respectively. For the interaction effect, treating soil with polymer P1 and Actinomycetes AC2 (P1AC2) resulted in the highest content of SOM (1.22%).
3.1.4 Available N Content in soil
The data illustrated in Table 4 showed that the hydro-polymers and biofertilizers application caused a significant improvement in the soil available N content, addition of polymers P1 and P2 only raised the average available nitrogen to 24.73 and 20.53 mg kg− 1, respectively, compared to 18.03 mg kg− 1 in the control treatment. Similarly, the T, AC1, AC2 and B microbes enhanced the available N to 21.67, 23.33, 24.10 and 19.60 mg kg− 1 by 20.19, 29.40, 33.67 and 8.71%, respectively more than the control soil. The maximum content of available N (28.03 mg kg− 1) was observed in P1AC2 treatment.
3.1.5 Available P Content in Soil
Concerning the effect of hydro-polymers and the different biofertilizers addition on the content of available phosphorus in the investigated sandy loam soil, the data in Table 4 illustrated a significant improvement in the available P status in all treated soils compared to the control soil that didn’t receive polymers or microbes. The maximum available P content (6.09 mg kg− 1) was observed in P1AC2-treated soil, while the lowest one (3.15 mg kg− 1) was recorded in control soil. Regarding to the individual effects of the studied treatments and in comparison with control, the soil available P significantly increased to 4.70 and 3.97 mg kg− 1 in P1 and P2-amended soils, respectively, and to 4.08, 3.69, 3.97, and 3.22 mg kg− 1 as a result of T, AC1, AC2, and B microbes application, respectively.
3.1.6 Available K Content in Soil
A significant increase occurred in the soil available potassium content from 84.67 mg kg− 1 in control (CK) to 120.0 and 113.00 mg kg− 1 after amending soil with the studied P1 and P2 super absorbent polymers. While, the inoculated soils with T, AC1, AC2, and B microbial strains improved the soil available K to 101.00, 97.67, 102.67, and 92.00 mg kg− 1, respectively. As interaction, the treated soil with P1 and AC2 strain recorded the highest content of available K (186.67 mg kg− 1) comparing with other treatments (Table 4).
3.1.7 Available Micronutrients (Fe, Mn, Zn, and Cu) in Soil
Data presented in Table 4 indicated that the available micronutrients (Fe, Mn, Zn, and Cu) which extracted by DTPA solution from the studied soil significantly increased by adding all polymers and microbial treatments either individually or in combination compared to the control treatment. The treatment of the interaction between P1 polymer and AC2 actinomycetes strain gave the highest available micronutrients contents (6.07 mg Kg− 1 for Fe, 3.99 mg kg− 1 for Mn, 1.09 mg kg− 1 for Zn, and 0.47 mg kg− 1 for Cu), while the lowest ones were recorded in the control treatment (4.33 mg kg− 1 for iron (Fe), 2.42 mg kg− 1 for manganese (Mn), 0.39 mg kg− 1 for zinc (Zn), and 0.21 mg kg− 1 for copper (Cu)).
3.2 Wheat Growth Parameters
3.2.1 Shoot Length
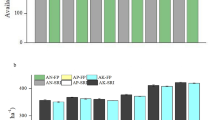
The Plant shoot length increased significantly with applications of polymers and biofertilizers compared to the unamended control (CK) soil (Fig. 1). Amending the sandy loam soil with P1 and P2 polymers improved the wheat shoot length from 61.17 cm plant− 1 in control to 70.57 and 69.67 cm plant− 1; by 15.37 and 13.90%, respectively. The integration between polymer P1 and Actinomycetes AC2 strain gave the best shoot length at all (75.17 cm plant− 1).
Effect of applied super absorbent polymers (SAPs) and microbial treatments on wheat shoot length. CK: control, P1: polymer1, P2: polymer2, T: Trichoderma (Trichoderma harzianum), AC1: Actynomicetes1 (Streptomyces rochei), AC2: Actinomycetes 2 (Streptomyces atrovirens), B: Bacillus (Bacillus subtilis), P1T: Polymer1 + Trichoderma (Trichoderma harzianum), P1AC1: Polymer1 + Actynomicetes1 (Streptomyces rochei), P1AC2: Polymer1 + Actinomycetes 2 (Streptomyces atrovirens), P1B: Polymer1 + Bacillus (Bacillus subtilis), P2T: Polymer 2 + Trichoderma (Trichoderma harzianum), P2AC1: Polymer 2 + Actynomicetes1 (Streptomyces rochei), P2AC2: Polymer 2 + Actinomycetes 2 (Streptomyces atrovirens) (P2AC2), P2B: Polymer 2 + Bacillus (Bacillus subtilis). Different lowercase letters denote significant differences (based on Duncan’s Multiple Range test at p < 0.05), and vertical bars show the standard errors (SE) of means
3.2.2 Number of Tillers and Leaves
Polymers and micro-organisms application considerably affected the average of tillers and leaves number per plant in wheat compared to untreated control (CK) (Figs. 2 and 3). The number of tillers increased from 4.00 in CK treatment to 7.33 and 6.33 with P1 and P2 addition by 83% and 58%, and to 4.67, 5.33, 5.67 and 4.33 after treating soil with T, AC1, AC2 and B isolates, respectively by 17–42%. As regard to the interaction, the P1AC2 treatment showed the highest number of tillers (9.00 per plant). Regarding to leaves number and comparing with control (CK) treatment, there was about 28 and 43% increase in the average of leaves number as a result of polymers P1 and P2 application, whereas the microbes contributed to 6–29% more leaves. Also, the P1AC2 showed the best effect in enhancement of leaves number average (7.33 per plant).
Effect of applied super absorbent polymers and microbial treatments on the tillers number per plant. CK: control, P1: polymer1, P2: polymer2, T: Trichoderma (Trichoderma harzianum), AC1: Actynomicetes1 (Streptomyces rochei), AC2: Actinomycetes 2 (Streptomyces atrovirens), B: Bacillus (Bacillus subtilis), P1T: Polymer1 + Trichoderma (Trichoderma harzianum), P1AC1: Polymer1 + Actynomicetes1 (Streptomyces rochei), P1AC2: Polymer1 + Actinomycetes 2 (Streptomyces atrovirens), P1B: Polymer1 + Bacillus (Bacillus subtilis), P2T: Polymer 2 + Trichoderma (Trichoderma harzianum), P2AC1: Polymer 2 + Actynomicetes1 (Streptomyces rochei), P2AC2: Polymer 2 + Actinomycetes 2 (Streptomyces atrovirens) (P2AC2), P2B: Polymer 2 + Bacillus (Bacillus subtilis). Different lowercase letters denote significant differences (based on Duncan’s Multiple Range test at p < 0.05), and vertical bars show the standard errors (SE) of means
Effect of applied super absorbent polymers and microbial treatments on the leaves number per plant. CK: control, P1: polymer1, P2: polymer2, T: Trichoderma (Trichoderma harzianum), AC1: Actynomicetes1 (Streptomyces rochei), AC2: Actinomycetes 2 (Streptomyces atrovirens), B: Bacillus (Bacillus subtilis), P1T: Polymer1 + Trichoderma (Trichoderma harzianum), P1AC1: Polymer1 + Actynomicetes1 (Streptomyces rochei), P1AC2: Polymer1 + Actinomycetes 2 (Streptomyces atrovirens), P1B: Polymer1 + Bacillus (Bacillus subtilis), P2T: Polymer 2 + Trichoderma (Trichoderma harzianum), P2AC1: Polymer 2 + Actynomicetes1 (Streptomyces rochei), P2AC2: Polymer 2 + Actinomycetes 2 (Streptomyces atrovirens) (P2AC2), P2B: Polymer 2 + Bacillus (Bacillus subtilis). Different lowercase letters denote significant differences (based on Duncan’s Multiple Range test at p < 0.05), and vertical bars show the standard errors (SE) of means
3.2.3 Fresh and Dry Weight
The data in Fig. 4 showed an increase in the plant fresh and dry weight as a result of amending soil with super absorbent polymers and microorganisms and their interactions as compared to the control. As an individual effect, the application of P1 and P2 boosted the average of fresh weight by 68% and 65%, while the soil biofertilization with T, AC1, AC2 and B microbial strains elevated it by 34–45% over the control plant (16.33 g plant− 1 as an average). Based on the interaction between the applied treatments, the plant fresh weight average was maximum (42.03 g plant− 1) in P1AC2 treatment. The plant dry weight showed similar trends (Fig. 5), polymers application increased the plant dry weight by 50%, while amending soil with different microbial treatments improved the plant DW by 21–39%. The P1AC2 treatment showed the maximum plant DW (21.33 g plant− 1) and the lowest one was noticed in control (CK) plants (10.27 g plant− 1).
Effect of applied super absorbent polymers and microbial treatments on plant fresh weight. CK: control, P1: polymer1, P2: polymer2, T: Trichoderma (Trichoderma harzianum), AC1: Actynomicetes1 (Streptomyces rochei), AC2: Actinomycetes 2 (Streptomyces atrovirens), B: Bacillus (Bacillus subtilis), P1T: Polymer1 + Trichoderma (Trichoderma harzianum), P1AC1: Polymer1 + Actynomicetes1 (Streptomyces rochei), P1AC2: Polymer1 + Actinomycetes 2 (Streptomyces atrovirens), P1B: Polymer1 + Bacillus (Bacillus subtilis), P2T: Polymer 2 + Trichoderma (Trichoderma harzianum), P2AC1: Polymer 2 + Actynomicetes1 (Streptomyces rochei), P2AC2: Polymer 2 + Actinomycetes 2 (Streptomyces atrovirens) (P2AC2), P2B: Polymer 2 + Bacillus (Bacillus subtilis). Different lowercase letters denote significant differences (based on Duncan’s Multiple Range test at p < 0.05), and vertical bars show the standard errors (SE) of means
Effect of applied super absorbent polymers and microbial treatments on plant dry weight. CK: control, P1: polymer1, P2: polymer2, T: Trichoderma (Trichoderma harzianum), AC1: Actynomicetes1 (Streptomyces rochei), AC2: Actinomycetes 2 (Streptomyces atrovirens), B: Bacillus (Bacillus subtilis), P1T: Polymer1 + Trichoderma (Trichoderma harzianum), P1AC1: Polymer1 + Actynomicetes1 (Streptomyces rochei), P1AC2: Polymer1 + Actinomycetes 2 (Streptomyces atrovirens), P1B: Polymer1 + Bacillus (Bacillus subtilis), P2T: Polymer 2 + Trichoderma (Trichoderma harzianum), P2AC1: Polymer 2 + Actynomicetes1 (Streptomyces rochei), P2AC2: Polymer 2 + Actinomycetes 2 (Streptomyces atrovirens) (P2AC2), P2B: Polymer 2 + Bacillus (Bacillus subtilis). Different lowercase letters denote significant differences (based on Duncan’s Multiple Range test at p < 0.05), and vertical bars show the standard errors (SE) of means
3.2.4 Leaf Area Index
Results in Fig. 6 illustrated that the plant Leaf area index positively affected by adding of polymers and micro-organisms as well as their interactions. The leaf area index increased by 48% each over the unamended control soil for P1 and P2-amended soils, respectively. Adding T, AC1, AC1 and B microbes increased the leaf area index significantly from 12.97 cm2 in unamended control soil to 11.60, 16.76, 15.06 and 15.26 cm2, respectively. Polymers application with the microbial isolates together gave the highest average of leaf area index (22.57 cm2) in P2AC2 treatment.
Effect of applied super absorbent polymers and microbial treatments on plant leaf area index. CK: control, P1: polymer1, P2: polymer2, T: Trichoderma (Trichoderma harzianum), AC1: Actynomicetes1 (Streptomyces rochei), AC2: Actinomycetes 2 (Streptomyces atrovirens), B: Bacillus (Bacillus subtilis), P1T: Polymer1 + Trichoderma (Trichoderma harzianum), P1AC1: Polymer1 + Actynomicetes1 (Streptomyces rochei), P1AC2: Polymer1 + Actinomycetes 2 (Streptomyces atrovirens), P1B: Polymer1 + Bacillus (Bacillus subtilis), P2T: Polymer 2 + Trichoderma (Trichoderma harzianum), P2AC1: Polymer 2 + Actynomicetes1 (Streptomyces rochei), P2AC2: Polymer 2 + Actinomycetes 2 (Streptomyces atrovirens) (P2AC2), P2B: Polymer 2 + Bacillus (Bacillus subtilis). Different lowercase letters denote significant differences (based on Duncan’s Multiple Range test at p < 0.05), and vertical bars show the standard errors (SE) of means
3.2.5 Weight of Thousand Grains
The weight of the thousand grains is a crucial factor that directly raises the economic yield. The data presented in Fig. 7 indicated that applying of polymers and microorganisms to the studied sandy loam soil both individually and in combination had a positive impact on the weight of 1000 grains per pot in wheat plants. The 1000 grains weight was the highest (47.51 g pot− 1) in P1AC2 treatment, while the lowest one (33.52 g pot− 1) was observed in the unamended control (CK) soil.
Effect of applied super absorbent polymers (SAPs) and microbial treatments on weight of 1000 grains in wheat plants. CK: control, P1: polymer1, P2: polymer2, T: Trichoderma (Trichoderma harzianum), AC1: Actynomicetes1 (Streptomyces rochei), AC2: Actinomycetes 2 (Streptomyces atrovirens), B: Bacillus (Bacillus subtilis), P1T: Polymer1 + Trichoderma (Trichoderma harzianum), P1AC1: Polymer1 + Actynomicetes1 (Streptomyces rochei), P1AC2: Polymer1 + Actinomycetes 2 (Streptomyces atrovirens), P1B: Polymer1 + Bacillus (Bacillus subtilis), P2T: Polymer 2 + Trichoderma (Trichoderma harzianum), P2AC1: Polymer 2 + Actynomicetes1 (Streptomyces rochei), P2AC2: Polymer 2 + Actinomycetes 2 (Streptomyces atrovirens) (P2AC2), P2B: Polymer 2 + Bacillus (Bacillus subtilis). Different lowercase letters denote significant differences (based on Duncan’s Multiple Range test at p < 0.05), and vertical bars show the standard errors (SE) of means
3.3 Macronutrients (N, P, and K) Content in Wheat Plants
3.3.1 Nitrogen (N) Content
The wheat plants in the unamended control soil (CK) contained the lowest content of nitrogen (1.67%) (Table 5). Adding the super absorbent polymers P1 and P2 significantly improved the nitrogen content by 55% and 49%, respectively, over the control. The inoculation with T, AC1, AC2, and B microbial isolates enhanced the N content by 15–35% compared to the control plants. Based on the interaction effect, the N content reached a maximum of 3.17% in plants of P1AC2 treatment (Table 5).
3.3.2 Phosphorus (P) Content
Comparing with the unamended soil (CK), polymers and micro-organisms application resulted in a significant increase in P content of wheat plants (Table 5). The maximum content of phosphorus in wheat plants (0.44%) was observed under application of P1AC2 treatment.
3.3.3 Potassium Content
The presented results in Table 5 indicated that almost all treatments increased the potassium content (%) in wheat plants. polymers applications caused an increase in phosphorus content in plant from 1.70% (unamended soil) to 2.65 and 2.80% for P1 and P2 treated soil, respectively. Amending soil with T, AC1, AC2 and B microorganisms raised P content to 1.85, 1.77, 2.32 and 2.01%, respectively. The P1 and AC2 combination also maximized potassium levels to 3.18%.
3.4 Micronutrients (Fe, Ma, Zn, and Cu) Content
A clear enhancement was noticed in the micronutrients content of wheat plants under application of polymers and microbes (Table 5). The Fe content increased significantly from 25.10 mg kg− 1 in control treatment to 36.67 and 32.00 mg kg− 1 by addition of P1 and P2 polymers, respectively and to 27.67, 26.67, 31.68 and 26.68 mg kg− 1 with applying T, AC1, AC2 and B biofertilizers only. Similarly, the application of polymers and microbes had a significant influence on the Mn content, the lowest value of Mn content (22.33 mg kg− 1) was recorded for the control treatment. Applying polymers increased the plant content of Mn to 31.00 and 28.00 mg kg− 1 with P1 and P2, respectively. Moreover, the incorporation of various microorganisms (T, AC1, AC2, and B) into the soil increased the content of Mn to 26.67, 24, 26.67 and 23.33 mg kg− 1, respectively. The Zn content in wheat plants significantly increased from 10.67 mg kg− 1 in the unamended control soil to 15.67 and 15.33 mg kg− 1 in P1 and P2-amended soils, respectively. Additionally, the different microorganisms (T, AC1, AC2, and B) addition also improved the plant content of Zn to 14.00, 13.67, 14.23 and 13.33 mg kg− 1, respectively. Also, the wheat plants content from Cu enhanced significantly from 6.33 mg kg− 1 in control treatment to 11.67 and 10.83 mg kg− 1 in the amended soils with P1 and P2 polymers, respectively. On the other hand, the biofertilization with T, AC1, AC2, and B microbes only increased the plant content of Cu significantly to 11.33, 13.00, 12.33, and 10.00 mg kg− 1, respectively.
Regarding to the effect of interaction between the applied polymers and microbes, the obtained results indicated that the plants of P1AC2 treatment recorded the highest Fe, Mn, Zn and Cu contents (43.67, 39.76, 31.33, and 15.33 mg kg− 1, respectively).
4 Discussion
The use of SAP materials and biofertilizers has been found to be effective in improving the chemical properties of the studied sandy loam soil, including pH, EC, and SOM percentage. Soil pH is a critical factor that influences the availability of nutrients for plant growth; most of the necessary nutrients are available to plants at a slightly acidic to neutral pH (6.0-7.5). A reduction in soil pH was observed after application of SAPs and biofertilizers, that may be related to the acidifying effect of the polymers’ carboxylate ions, these findings are matched with those of Ostrand et al. (2020), Ali et al. (2023) and Mahgoub (2020), who discovered that when H+ dissociates from hydrogel and other cation-cation like functional groups to the surface, negative charge is produced. This neutralizes the cation exchange sites and raises the acidity of the soil. In addition to the organic acids produced by Trichoderma sp., Bacillus sp. and Actinomycetes sp. such as fumaric, citric, and gluconic acids, which have an effective role in reducing the soil pH. Furthermore, Bacillus sp. break down complex organic compounds, releasing organic acids contributing to soil acidification and decreased pH levels. Similar explanations were obtained by Bononi et al. 2020; Saxena et al. (2020), Abdullah et al. (2021), Fu et al. (2021), Conte et al. (2022) and Singh et al. (2023).
Soil electrical conductivity (EC) has been mostly used as a measure of soil salinity. The obtained decrease in soil EC in response to SAPs and microorganisms’ application could be related to the salt ions binding in the long chain structure of the polymer, thereby reducing the pore water’s salt content (Malik et al. 2023). Additionally, the high-water holding of SAPs which is reliant on the forming of hydrogen bonds between molecules of water and hydrophilic groups which reduces the solution ionic strength and soil EC values (Zhang et al. 2021). Also, SAPs can adsorb Na+, K+, Mg+ 2 and Ca+ 2 cations due to their high CEC, thus decrease the soil EC value (Ali et al. 2023). Moreover, Actinomycetes sp., Trichoderma sp., and Bacillus sp. significantly influence soil electrical conductivity (EC) through producing organic acids that reduce the electrical conductivity of the soil by chelating metallic ions (Farhad et al. 2023). The enzymatic activities of Bacillus sp. collectively contribute to the reduction of EC in the soil (Bhatti et al. 2017; Gul et al. 2023). Moreover, Bacillus bacteria can promote the growth of plants, which can lead to increased water uptake and improved nutrient uptake, thus diluting the concentration of salts in the soil.
The percentage of soil organic matter (SOM) is an important measure of soil health and is essential to many ecological functions. It has been demonstrated that high concentrations of SOM increase crop yields and enhance soil physiochemical characteristics. The results of this study indicated that SOM content was positively influenced by amending soil with SAPs and biofertilizers. Superabsorbent polymers (SAPs) have the potential benefit of improving soil aggregate stability. The SAP structures’ hydrophilic groups enhance the adsorption and flocculation of soil particles (Ji et al. 2022 & Mamedov et al. 2017), and this gives the SAPs the benefit of helping in SOM retention in the soil by incorporating it into aggregates where it can be preserved from decomposition (Sroka and Sroka 2024), that explains the enhancement in SOC content after SAPs addition. Also, Li et al. (2019), and Kumar et al. (2020) demonstrated the positive impact of SAPs on SOM accumulation and soil fertility. Additionally, the microorganism’s application also played a vital role in improving SOM content because they enhance the populations of living soil microorganisms and the microbial communities, which are a vital part of soil organic matter and responsible for SOM accumulation. In addition to the fact of soil microbes like Bacillus bacteria and Actinomycetes can produce polysaccharides that are considered important binding agents, which can enhance soil aggregation and soil structure by soil particles adhering to form micro aggregates (Ren et al. 2022), thus increasing its capacity to protect SOM from decomposition. So, the interaction between SAPs and microbes’ application in our study confirming their effectiveness in enhancing SOM content. The biofertilizer also improves the population of beneficial microbes in soil. These findings are matched with Shukla et al. (2022) and Gul et al. (2023). Other studies highlighted the effectiveness of SAPs and microorganisms in enhancing SOM content for instance, Tahir et al. (2018), Zhang et al. (2018), and Yaseen et al. (2020).
The availability of both essential macro and micro-nutrients is a determinant of soil quality and the development of plants growing in it. The polymers’ abilities to decrease nutrient leaching and the microorganisms’ impacts on chemical properties and nutrient solubilization seemingly increased nutrient retention and availability in the treated soil. These thorough findings coincide with past studies on nutrient availability responses to polymer and microbial amendments (Ali et al., 2019). The super absorbent polymers such as Hydrogels can also be used as materials for nutrients slow release in soil (Adjuik et al. 2022). Hydrogels can increase the soil content of nutrients by acting as a nutrient mobilizer and water-holding reservoir when used in the soil (Palanivelu et al. 2022). Our findings are matched with Dahri et al. (2019), Ali et al. (2023), and Wu et al. (2023) who suggested that SAP incorporation has been found to increase levels of OM, available nitrogen, phosphorus, and potassium in the amended soil. In this study, application of P1 and P2 polymers improved the soil content of the available nutrients because of their high-water holding capacity, in addition, the first polymer (Barbary plant g3, P1), in particular, contains a reasonable content of N, P, and K (6.5%, 4.80%, and 8.2% K). Accordingly, incorporating sustainable polymers into soils can be effective approach to improve nutrients retention and availability.
The added microbes had a significant impact on enhancement of macro- and micro-nutrients in the studied soil. It has been demonstrated that Actinomycetes participate in the atmospheric nitrogen fixation process in a variety of legumes and non-legumes without nodules forming (Kontro et al. 2022). Thus, Actinomycetes have an important influence on increasing the amount and availability of nitrogen in the plant-soil system (Nosheen et al. 2021). Furthermore, Actinomycetes can affect photosynthesis process, carbon, and nitrogen metabolism. Moreover, Through the production of acidic or alkaline phosphatase enzymes, Actinomycetes can improve the mineralization of the organic phosphates and increase the P availability in soil (AbdElgawad et al. 2020). Furthermore, the chelating qualities of the flavonoids and phenolics released by the Actinomycetes may account for the observed rise in soil nutrients content following enrichment with actinomycetes. Actinomycetes enrich soil rhizosphere, they have a direct impact on plant growth through excreting plant growth-promoting substances, mineral nutrients, and enhancing the soil content of essential micro- and macro-elements. In similar with our results, AbdElgawad et al. (2020) indicated the positive effect of Actinomycetes in increasing the availability of elements like nitrogen, phosphorus, and magnesium that are necessary for biosynthesis of chlorophyll, and for the effective functioning of the photosynthetic machinery.
Certain Trichoderma strains have the ability to produce organic acids, and acidify the surrounding environment. This process allows the solubilization of P ions, micronutrients, and mineral cations, such as Fe+ 3, Mg+ 2, and Mn+ 4 which be then available for plant uptake in the soil (Kamal et al. 2018; Nosheen et al. 2021). According to Bader et al. (2020) on the effect of Trichoderma atroviride on Chinese cabbage, irrigation with this biofertilizer increased soil enzyme activity, yield by 37%, and increased the concentration of inorganic nitrogen and phosphorus content of the soil. Bacillus species can fix atmospheric N2 and provide it to plants, enhancing plant growth and yield (Radhakrishnan et al. 2017). Sun et al. (2020) indicated that by using Bacillus subtilis as a biofertilizer, the proportion of ammonia emissions was lowered by 44%, increasing the amount of residual N in the soil. Bacillus species can help dissolve inorganic phosphate, potassium, zinc, and other nutrients, making them more available for plant absorption (Prakash and Arora 2019). Bacillus bacteria can produce organic acids that help break down silicates and remove metals, allowing the potassium ions in the silicates to become soluble and available to plants. They move potassium from the inaccessible forms found in the soil (Sattar et al. 2019). Also, the biofertilizers especially Bacillus bacteria SP. have an effective role in improving the micronutrients (Fe+ 2, Mn+ 2, Zn+ 2, Cu+ 2) availability in soil through oxidoreductive systems, chelated ligands, protonation, and acidification actions that dissolve the micronutrients in the soil (Daniel et al. 2022). These mechanisms attribute the increase in the soil nutrients availability that we found in response to application of these microbes to the studied sandy loam soil.
Plant growth involves a complex process of cell division, expansion, and differentiation, leading to an increase in size and biomass. soil quality directly influences plant growth by providing water, essential nutrients, and good aeration. Application of soil amendments aid in enhancement of soil quality and hence plant growth and development. The study showed an improvement in the wheat growth parameters and the 1000-grain weight in response to the studied polymers application. This could be explained by the hydrogel’s ability to increase the amount of water available in the root zone and the efficiency of nutrient uptake. These factors boost the photosynthesis rate and the carbohydrates production which are then transported and deposited in the estuaries, increasing the weight of a thousand grains (Roy et al. 2019) or attributed to the hydrogel’s ability to retain and store water. It is used for extended periods of time before being gradually released to allow the plant to carry out all necessary functions, including photosynthesis and the production of dry matter. Additionally, the process of cell division and elongation leads to plant growth and increases the area of the flag leaf (which acts as a light intercepting area), which in turn affects the production of more dry materials and transporting them to the outfalls (grains) and thus gain in the weight of 1000 grains in wheat. These results coincide with prior findings of 15–20% greater fresh and dry weights in wheat with superabsorbent polymers (Bandak et al. 2021; Yang et al. 2022), and 10–30% increased wheat height with hydrogels and vermicompost (Kumar et al. 2022). Albalasmeh et al. (2022) observed that maize plant height improved by 26.3% in comparison with control plants in the silty clay loam soil treated with 0.5% (w/w) hydrogel polymer, while the increase was by 110.7% for plants that were cultivated in sandy soil treated with 1% (w/w) hydrogel polymer. Similar study by Benard et al. (2022) reported that the application of SAP at a rate of 0.1% increased cowpea plant height, number of mature leaves, leaf area, and dry weight/plant by 24%, 11.1%, 11.7%, and 85.9%, respectively. Another study by Yang et al. (2020) found that the maize leaf area index increased with applications of SAP hydrogels, in addition to an increase up to 26% in the 1000-grain weight compared to control. Similar findings were indicated by Kumar et al. (2019); AbdAllah et al. (2021) and Zangana et al. (2023).
Microorganisms that live in the rhizosphere can directly or indirectly promote plant development. In this study, the applied microbes through biofertilization showed growth-promoting effects. Plant growth promotion is directly influenced by increased nutrient availability and phytohormone production (Santoyo et al. 2021). It has been shown that the synthesis and excretion of certain phytohormones, such as IAA, gibberellins (GA), and cytokinins, increase the surface area of root for greater plant nutrient adsorption from the soil and growth. Indirect mechanisms that lead to better plant health and crop productivity include the bioremediation of pollutants and contaminants, abiotic stress reduction, and disease suppression by biocontrol agents (Glick and Gamalero 2021; Zhang et al. 2021). Similar results were obtained by Akbari et al. (2020) who showed that Actinomycetes (Streptomyces strains) have exhibited excellent effects, encouraged shoot elongation and increased shoot and root fresh and dry weights of Wheat. Another study by Oljira et al. (2020) indicated that Trichoderma harzianum boosted increases in critical metrics related to wheat plant growth metrics, such as shoot dry matter, leaf area, and root dry matter. By forming a favorable environment and a symbiotic relationship with plants, Trichoderma enhances the general health of plants by releasing a variety of secondary metabolites, such as growth hormones, endochitinase, and proteolytic enzymes (Kamal et al. 2018). Trichoderma can improve root proliferation, create soil organic matter by releasing hormones that promote growth, and restore the soil’s nutrition cycle (Kumar et al. 2023). According to Djebaili et al. (2021), Triticum durum plants that were inoculated with Actinomycetes microbes showed a notable increase in biomass accumulation, root and shoot growth, and N and P content. Treatment with Actinomycetes has also been shown to enhance plant development by increasing the synthesis of IAA and gibberellic acid (Sharma et al. 2023). Similar to this, it has been observed that isolates of Streptomyces, the biggest genus of Actinomycetes, stimulate the development of maize and tomatoes because they may create siderophores (Warrad et al. 2020). In matching with our results, the Actinomycetes inoculants that applied to legumes soil, produced higher plant growth, seeds yield, and mineral content (AbdElgawad et al. 2020). And in another trial, Bacillus inoculants improved the wheat growth, straw weight and grain yield significantly compared to the nontreated wheat plants (Chandra et al. 2021). From the findings of our study, it has been demonstrated that combining SAP with biofertilizers increases plant growth more than utilizing SAP alone or biofertilizers only.
The noticed enhancement in the nutrient’s availability in the studied soil in response to polymers and biofertilizers application directly and positively affect plant macro- and micro-nutrients content in the cultivated wheat. SAPs can retain a large amount of water and nutrients once incorporated into the soil, slowly releasing them to improve the microbial activity and abundance, nutrients availability in soil, plant growth, plant nutrient content, and nutrient uptake efficiency (Malik et al. 2023). SAPs provide adequate moisture in soil and thus improve the uptake of nutrients by diffusion and root interaction. These findings align with previous results of Singh et al. (2017) who found that amending soil with hydrogel significantly improved the wheat content and uptake of nitrogen, phosphorus, and potassium compared to the non-treated soil. In the wheat plant, another study by Yaseen et al. (2020) approved that exopolysaccharide-producing bacteria and SAP application greatly enhanced maize’s uptake of NPK in both normal and drought stress conditions.
Our study indicated that the synergistic combination of polymers and Trichoderma, Actinomycetes, and Bacillus microbes resulted in a higher content of plant nutrients. The rhizosphere is the area of soil surrounding the root system and the nutrients absorption by plant occurs from it, plant roots secrete a variety of exudates which act as attractants for microbes. So, the applied beneficial microbes can colonize the rhizosphere and affect the nutrients uptake by plants. These beneficial microbes promote nutrient uptake, including P, K, N, Fe, Mn, Cu, and Zn. They achieve this through processes such as solubilization of P and K, fixing N, modification of soil pH, and production of organic and inorganic acids. Additionally, these microbes can create a microenvironment by synthesizing biofilms around microbial cells, which influences the dissolution of minerals and leads to release of available forms of nutrients that can be taken up by plants. Furthermore, they can increase the uptake and accumulation of plant nutrients through the production of volatile organic compounds that induce tolerance and systemic resistance in stressed plants (Saeed et al. 2021; Santoyo et al. 2021; Etesami et al. 2023). Using those beneficial microorganisms as biofertilizers increases the amount of nutrients that plants can absorb from the soil by boosting plant metabolism, which changes the root exudates composition, affects nutrient solubility and availability, and increases interactions with other soil microbes (Daniel et al. 2022). Our results agree with AbdElgawad et al. (2021) who indicated that the Plant tissues (roots and shoots) and seeds of legumes also had higher nitrogen and carbon contents, which was a result of the actinomycetes enriching the soils and increasing their N and C content. Actinomycetes stimulate the activity of enzymes in plants that metabolize nitrogen, such as glutamate dehydrogenase, nitrate reductase, glutamine synthetase, and glutamate synthase. This increases N content and promotes the metabolism and uptake of nitrogen in plants. They also found that all actinomycetes isolates increased the concentration of Fe, Cu, Zn, Mn, Ca, K, Mg, and P elements in the seeds of the studied legumes. The abiotic and biotic soil environment, ion absorption, mineral availability, minerals content, and root growth are all impacted by organic acids that are produced in the soil by Streptomycetes spp. (genus of Actinomycetes) (Javed et al. 2021). When added as a biofertilizer, Trichoderma improves both crop uptake and soil nutrient availability for crop absorption. Similar to our results, Metwally (2020) presented that the content of nitrogen, potassium, phosphorus in Onion plants (Allium cepa) was enhanced by the presence of Tricoderma viride in the soil. Inoculation of Mustard and Tomato soils with Trichoderma harzianum Improved nitrogen content and uptake as well as increased yield by 108 and 203% (Chaube and Pandey 2022). So, these beneficial microbial inoculants can supply the crop with nitrogen, phosphorus, potassium, and other plant micronutrients, enhancing the growth of plant and raising crop production without adding chemical fertilizers to the soil. Our research indicates that applying SAP and biofertilizers collectively has been shown to enhance the nutrient content of wheat plants.
5 Conclusion
In Egypt, the national economy and food security depend to a large extent on the agricultural sector. The steady population increase in Egypt creates the need to reclaim and cultivate more new lands to meet the population’s food needs and increase agricultural production. Almost all reclaimed soils in Egypt have poor physical, chemical, and fertility properties such as coarse texture, weak structure, low water holding capacity, high salinity, low organic matter content, low content, and low availability of plant nutrients. To obtain agricultural production from these soils, different amendments and fertilizers must be applied to improve their productivity. Using biofertilizers provides an environmentally friendly tool for plant nutrition compared with chemical fertilization. Many studies approved that biofertilizer application to the reclaimed soils did not achieve the desired goal, as the arid or semi-arid climatic conditions, low moisture content, and bad characteristics of these soils negatively affect the activity and proliferation of biofertilizer microbes. As soil amendments, superabsorbent polymers (SAPs) are distinguished by their ability to increase the water retention capacity of soil compared to other amendments. Our study focused on the impacts of integrated applications of superabsorbent polymers (SAPs) and biofertilizers on sandy loam soil properties and productivity. Our result indicated that using SAPs increased the efficiency of the applied biofertilizers, and the combination of these polymers and biofertilizers could be an effective tool for improving the sandy loam soil’s characteristics and the wheat growth that grows in it. This study produces a sustainable management strategy for improving soil fertility and crop yield in wheat cropping systems, especially in sandy-textured soils that are characterized by low fertility and low water holding capacity.
Data Availability
The corresponding author can provide the data supporting the study’s findings upon reasonable request.
References
AbdAllah AM, Mashaheet AM, Burkey KO (2021) Super absorbent polymers mitigate drought stress in corn (Zea mays L.) grown under rainfed conditions. Agr Water Manage 254:106946. https://doi.org/10.1016/j.agwat.2021.106946
AbdElgawad H, Abuelsoud W, Madany MM, Selim S, Zinta G, Mousa AS, Hozzein WN (2020) Actinomycetes enrich soil rhizosphere and improve seed quality as well as productivity of legumes by boosting nitrogen availability and metabolism. Biomolecules 10:1675. https://doi.org/10.3390/biom10121675
AbdElgawad H, Zinta G, Abuelsoud W, Hassan YM, Alkhalifah DHM, Hozzein WN, Zrieq R, Beemster GT, Schoenaers S (2021) An actinomycete strain of Nocardiopsis lucentensis reduces arsenic toxicity in barley and maize. J Hazard Mater 417:126055. https://doi.org/10.1016/j.jhazmat.2021.126055
Abdullah NS, Doni F, Mispan MS, Saiman MZ, Yusuf YM, Oke MA, Suhaimi NSM (2021) Harnessing Trichoderma in agriculture for productivity and sustainability. Agronomy 11:2559. https://doi.org/10.3390/agronomy11122559
Adjuik TA, Nokes SE, Montross MD, Wendroth O (2022) The impacts of bio-based and synthetic hydrogels on soil hydraulic properties: a review. Polymers 14:4721. https://doi.org/10.3390/polym14214721
Akbari A, Gharanjik S, Koobaz P, Sadeghi A (2020) Plant growth promoting Streptomyces strains are selectively interacting with the wheat cultivars especially in saline conditions. Heliyon 6:e03445. https://doi.org/10.1016/j.heliyon.2020.e03445
Albalasmeh AA, Mohawesh O, Gharaibeh MA, Alghamdi AG, Alajlouni MA, Alqudah AM (2022) Effect of hydrogel on corn growth, water use efficiency, and soil properties in a semi arid region. J Saudi Soc Agr Sci 21:518–524. https://doi.org/10.1016/j.jssas.2022.03.001
Ali AF, Salim HA, Alsaady MHM (2019) Response of two wheat cultivars to inoculation of Bacillus subtilis and Phosphorus fertilizer. J Physics: Conference Series 1294: 092036. IOP Publishing. https://doi.org/10.1088/1742-6596/1294/9/092036
Ali MA, Farag SG, Sillanpää M, Al-Farraj S, El-Sayed ME (2023) Efficiency of using Superabsorbent polymers in reducing Mineral Fertilizer Rates Applied in Autumn Royal vineyards. Horticulturae 9:451. https://doi.org/10.3390/horticulturae9040451
Anonymous (1968) Plant Pathologist’s Pocket Book. Commonwealth Mycological Institute, pp: 394–395
Bader AN, Salerno GL, Covacevich F, Consolo VF (2020) Native Trichoderma harzianum strains from Argentina produce indole-3 acetic acid and phosphorus solubilization, promote growth and control wilt disease on tomato (Solanum lycopersicum L). J King Saud Univ-Sci 32:867–873. https://doi.org/10.1016/j.jksus.2019.04.002
Bandak S, Naeini SARM, Zeinali E, Bandak I (2021) Effects of Superabsorbent Polymer A200 on soil characteristics and Rainfed Winter Wheat Growth (Triticum aestivum L). Arab J Geosci 14:712. https://doi.org/10.1007/s12517-021-06824-x
Benard DN, Obiero JPO, Mbuge DO (2022) Contribution of superabsorbent polymers to growth and yield of African leafy vegetables. Adv Agri 2022:8020938. https://doi.org/10.1155/2022/8020938
Bhatti AA, Haq S, Bhat RA (2017) Actinomycetes benefaction role in soil and plant health. Microb Pathogen 111:458–467. https://doi.org/10.1016/j.micpath.2017.09.036
Black CA (1965) Methods of Soil Analysis. Amer Soc Agro Inc., Madison, Wisconsim U. S. A.
Bononi L, Chiaramonte JB, Pansa CC, Moitinho MA, Melo IS (2020) Phosphorus-solubilizing Trichoderma spp. from Amazon soils improve soybean plant growth. Sci Rep 10:2858
Carson PL (1980) Recommended potassium test. In: Dahnke WC (ed) Recommended chemical test procedures for the North central region. NCR Publ. No. 221 (revised). North Dakota Agricultural Experimental Station. North Dakota State University, Fargo, North Dakota, USA, pp 17–18
Chandra P, Khobra R, Sundha P, Sharma RK, Jasrotia P, Chandra A, Singh DP, Singh GP (2021) Plant growth promoting Bacillus-based bio formulations improve wheat rhizosphere biological activity, nutrient uptake and growth of the plant. Acta Physiol Plant 43:1–12. https://doi.org/10.1007/s11738-021-03310-5
Chapman HD, Pratt PF (1961) Methods of soil, Plants and Water Analysis. University of California Division of Agricultural Sciences, pp 60–69
Chaube KS, Pandey S (2022) Tricoderma: a valuable multipurpose fungus for sustainable agriculture. Malaysian J Sustain Agri (MJSA) 6:97–100. https://doi.org/10.26480/mjsa.02.2022.97.100
Chaudhary P, Singh S, Chaudhary A, Sharma A, Kumar G (2022) Overview of biofertilizers in crop production and stress management for sustainable agriculture. Front Plant Sci 13:930340. https://doi.org/10.3389/fpls.2022.930340
Conte ED, Dal Magro T, Dal Bem LC, Dalmina JC, Matté JA, Schenkel VO, Schwambach J (2022) Use of Trichoderma spp. in no-tillage system: Effect on soil and soybean crop. Biol Control 171:104941. https://doi.org/10.1016/j.biocontrol.2022.104941
Dahri SH, Mangrio MA, Shaikh IA, Dahri SA, Steenbergen FV (2019) Effect of different forms of Super Absorbent polymers on Soil Physical & Chemical properties in Orchard field. World Acad J Eng Sci 6:12–20
Daniel AI, Fadaka AO, Gokul A, Bakare OO, Aina O, Fisher S, Burt AF, Mavumengwana V, Keyster M, Klein A (2022) Biofertilizer: the future of food security and food safety. Microorganisms 10:1220. https://doi.org/10.3390/microorganisms10061220
Díez-Méndez A, Marcos-García M, González-Dominici L, Peral-Aranega E, Saati-Santamaria Z, Garcia-Fraile P, Menéndez E (2024) Design and application of microbial biofertilizers. Microbial Technology for Agro-ecosystems. Academic, pp 21–40
Dingley C, Cass P, Adhikari B, Daver F (2024) Application of superabsorbent natural polymers in agriculture. Polym Renew Resou 15:210–255. https://doi.org/10.1177/20412479231226166
Djebaili R, Pellegrini M, Rossi M, Forni C, Smati M, Del Gallo M, Kitouni M (2021) Characterization of plant growth-promoting traits and inoculation effects on Triticum durum of actinomycetes isolates under salt stress conditions. Soil syst 5:26. https://doi.org/10.3390/soilsystems5020026
Dowson WJ (1957) Plant diseases due to bacteria, 2nd edn. Cambridge University Press, New York
Elbeih SF, Belal AAB, Zaghloul ESA (2011) Hazards mitigation and natural resources evaluation around Sohag–Safaga highway, Eastern Desert, Egypt. Egypt J Rem Sens Space Sci 14:15–28. https://doi.org/10.1016/j.ejrs.2011.01.001
Etesami H, Jeong BR, Glick BR (2023) Potential use of Bacillus spp. as an effective biostimulant against abiotic stresses in crops-A review. Curr Res Biotechno 5:100128. https://doi.org/10.1016/j.crbiot.2023.100128
Farhad M, Rana MAK, Ahmad R, Virk ZA, Iqbal M, Ilyas MF, Gill S, Khan Sh A, Ramzani PMA, Afzal H, Tauqeer HM (2023) Roles of Organic Acids in Plant Stress Tolerance, Food Security, and Soil Remediation. Climate-Resili Agr 1:713–729. Cham: Springer International Publishing. https://doi.org/10.1007/978-3-031-37424-1
Fasusi OA, Cruz C, Babalola OO (2021) Agricultural sustainability: microbial biofertilizers in rhizosphere management. Agriculture 11:163. https://doi.org/10.3390/agriculture11020163
Fu J, Xiao Y, Wang YF, Liu ZH, Yang K (2021) Saline–alkaline stress in growing maize seedlings is alleviated by Trichoderma asperellum through regulation of the soil environment. Sci Rep 11:11152. https://doi.org/10.1038/s41598-021-90675-9
Glick BR, Gamalero E (2021) Recent developments in the study of plant microbiomes. Microorganisms 9:1533. https://doi.org/10.3390/microorganisms9071533
Gomez KA, Gomez AA (1984) Statistical procedures for agricultural research. Wiley
Grote U, Fasse A, Nguyen TT, Erenstein O (2021) Food security and the dynamics of wheat and maize value chains in Africa and Asia. Front Sustain Food Syst 4:617009. https://doi.org/10.3389/fsufs.2020.617009
Gul S, Javed S, Azeem M, Aftab A, Anwaar N, Mehmood T, Zeshan B (2023) Application of Bacillus subtilis for the alleviation of salinity stress in different cultivars of Wheat (Tritium Aestivum L). Agronomy 13:437. https://doi.org/10.3390/agronomy13020437
Hemathilake DMKS, Gunathilake DMCC (2022) Agricultural productivity and food supply to meet increased demands. Future Foods (pp. 539–553). Academic Press. https://doi.org/10.1016/C2020-0-02752-0
Huang J, Hartemink AE (2020) Soil and environmental issues in sandy soils. Earth-Sci Rev 208:103295. https://doi.org/10.1016/j.earscirev.2020.103295
Jackson ML (1973) Soil chemical analysis Prentice Hall. Inc. Englewood Cliffs NJ 498:183–204
Javed Z, Tripathi GD, Mishra M, Dashora K (2021) Actinomycetes–the microbial machinery for the organic-cycling, plant growth, and sustainable soil health. Biocatal Agr Biotechnol 31:101893. https://doi.org/10.1016/j.bcab.2020.101893
Ji BY, Zhao CP, Yue WU, Wei HAN, Song JQ, Bai WB (2022) Effects of different concentrations of super-absorbent polymers on soil structure and hydro-physical properties following continuous wetting and drying cycles. J Integ Agr 21:3368–3381. https://doi.org/10.1016/j.jia.2022.08.065
Junaid MD, Gokce AF (2024) Global agricultural losses and their causes. Bull Biol all Sci Res 9:66. https://doi.org/10.54112/bbasr.v2024i1.66
Kamal RK, Athisayam V, Gusain YS, Kumar V (2018) Trichoderma: a most common biofertilizer with multiple roles in agriculture. Biomed J Sci Tech Res 4:4136–4137. 10.26717/ BJSTR.2018.04.001107
Khan A, Panthari D, Sharma RS, Punetha A, Singh AV, Upadhayay VK (2023) Biofertilizers: a microbial-assisted strategy to improve plant growth and soil health. Advanced microbial techniques in agriculture, environment, and health management. Academic, pp 97–118
Khodadadi Dehkordi D (2018) Evaluation of two types of superabsorbent polymer on soil water and some soil microbial properties. Paddy Water Environ 16:143–152. https://doi.org/10.1007/s10333-017-0623-x
Kontro MH, Yaradoddi JS, Ganachari SV, Banapurmath NR, Umesh MK (2022) Actinomycetes in Agriculture and Forestry. Actinobacteria: Ecology, Diversity, classification and extensive applications. Springer Nature Singapore, Singapore, pp 213–232. http://www.springer.com/series/15861
Kumar S, Sharma PK, Yadav MR, Sexena R, Gupta KC, Kumar R, Garg NK, Yadav HL (2019) Effect of irrigation levels and moisture conserving polymers on growth, productivity and profitability of wheat. Indian J Agr Sci 89:509–514
Kumar A, Meena RS, Nirmal DE, Gurjar DS, Singh A, Yadav GS, Pradhan G (2020) Response of polymers and biofertilizers on soybean (Glycine max) yield under rainfed conditions. Indian J Agr Sci 90:767–770
Kumar S, Singh A, Naresh RK, Kumar M, Shahi UP (2022) Effect of irrigation regime, Pusa hydrogel and vermicompost on growth, productivity and soil-nutrient status in late-sown wheat (Triticum aestivum). Indian J Agro 67:20–25. https://doi.org/10.59797/ija.v67i1.79
Kumar C, Tomar A, Pandey S, Prasad MNV (2023) Plant Growth promoting Rhizobacteria sustaining saline and Metal Contaminated Soils. Agroecological approaches for sustainable Soil Management. Chapter 9:437–456. https://doi.org/10.1002/9781119911999.ch19
Langridge P, Alaux M, Almeida NF, Ammar K, Baum M, Bekkaoui F, Zhang X (2022) Meeting the challenges facing wheat production: the strategic research agenda of the Global Wheat Initiative. Agronomy 12:2767. https://doi.org/10.3390/agronomy12112767
Lee KM, Min JH, Oh S, Lee H, Koh WG (2020) Preparation and characterization of superabsorbent polymers (SAPs) surface-crosslinked with polycations. React Function Polym 157:104774. https://doi.org/10.1016/j.reactfunctpolym.2020.104774
Li Y, Shi H, Zhang H, Chen S (2019) Amelioration of drought effects in wheat and cucumber by the combined application of super absorbent polymer and potential biofertilizer. PeerJ Plant Biol 7:e6073. https://doi.org/10.7717/peerj.6073
Lindsay WL, Norvell WA (1978) Development of DTPA soil test for zinc, iron, manganese and copper. Soil Sci Soc Am J 42:421–428
Mahgoub NA (2020) Effectiveness of hydrogel application on tomato (Solanum lycopersicum L.) growth and some sandy soil chemical properties under drip irrigation system. J Soil Water Sci 5:49–54
Malik S, Chaudhary K, Malik A, Punia H, Sewhag M, Berkesia N, Nagora M, Kalia S, Malik K, Kumar A, Boora K (2023) Superabsorbent polymers as a Soil Amendment for Increasing Agriculture Production with reducing Water losses under water stress Condition. Polymers 15:161. https://doi.org/10.3390/polym15010161
Mamedov AI, Huang CH, Aliev FA, Levy GJ (2017) Aggregate stability and water retention near saturation characteristics as affected by soil texture, aggregate size and polyacrylamide application. Land Degrad Develop 28:543–552. https://doi.org/10.1002/ldr.2509
McLean EO (1982) Soil pH and lime requirement. In Page, A. L., R. H. Miller and D. R. Keeney (eds.) Methods of soil analysis. Part 2 - Chemical and microbiological properties. (2nd Ed.). Agronomy 9:199–223
Metwally RA (2020) Arbuscular mycorrhizal fungi and Trichoderma viride cooperative effect on biochemical, mineral content, and protein pattern of onion plants. J Basic Microbiol 60:712–721. https://doi.org/10.1002/jobm.202000087
Nosheen S, Ajmal I, Song Y (2021) Microbes as biofertilizers, a potential approach for sustainable crop production. Sustainability 13:1868. https://doi.org/10.3390/su13041868
Oladosu Y, Rafii MY, Arolu F, Chukwu SC, Salisu MA, Fagbohun IK, Muftaudeen TK, Swaray S, Haliru BS (2022) Superabsorbent Polymer hydrogels for sustainable agriculture: a review. Horticulturae 8:605. https://doi.org/10.3390/horticulturae8070605
Oljira AM, Hussain T, Waghmode TR, Zhao H, Sun H, Liu X, Wang X, Liu B (2020) Trichoderma enhances net photosynthesis, water use efficiency, and growth of wheat (Triticum aestivum L.) under salt stress. Microorganisms 8:1565. https://doi.org/10.3390/microorganisms8101565
Olsen SR, Cole CV, Watanabe F, Dean LA (1954) Estimation of available phosphorus in soil by extraction with sodium bicarbonate. Cric. 939, USDA, Washington, D. C
Omran AA (2008) Integration of remote sensing, geophysics and GIS to evaluate groundwater potentiality-a case study in Sohag region, Egypt. The 3rd International Conference on Water Resources and Arid Environments and the 1st Arab Water Forum
Ostrand MS, DeSutter TM, Daigh AL, Limb RF, Steele DD (2020) Superabsorbent Polymer characteristics, properties, and applications. Agrosys Geosci Environ 3:e20074. https://doi.org/10.1002/agg2.20074
Palanivelu SD, Armir NAZ, Zulkifli A, Hair AHA, Salleh KM, Lindsey K, Che-Othman MH, Zakaria S (2022) Hydrogel application in urban farming: potentials and limitations- A review. Polymers 14:2590. https://doi.org/10.3390/polym14132590
Piper CS (1950) Soil analysis. Inter science. PUBLISHER Inc., New York
Prakash J, Arora NK (2019) Phosphate-solubilizing Bacillus sp. enhances growth, phosphorus uptake and oil yield of Mentha arvensis L. 3Biotech 9: 126. https://doi.org/10.1007/s13205-019-1660-5
Radhakrishnan R, Hashem A, AbdAllah EF (2017) Bacillus: a biological tool for crop improvement through bio-molecular changes in adverse environments. Front Physiol 8:667. https://doi.org/10.3389/fphys.2017.00667
Ren C, Liu K, Dou P, Li J, Wang K (2022) The changes in soil microorganisms and soil chemical properties affect the heterogeneity and stability of soil aggregates before and after grassland conversion. Agriculture 12:307. https://doi.org/10.3390/agriculture12020307
Roy T, Kumar S, Chand L, Kadam DM, Bihari B, Shrimali SS, Bishnoi R, Maurya UK, Singh M, Muruganandam M, Singh L, Sharma SK, Kumar R, Mallik A (2019) Impact of Pusa hydrogel application on yield and productivity of rainfed wheat in North West Himalayan region. Curr Sci 116:1246–1251. https://www.jstor.org/stable/27138018
Saeed Q, Xiukang W, Haider FU, Kučerik J, Mumtaz MZ, Holatko J, Naseem M, Kintl A, Ejaz M, Naveed M, Brtnicky M, Mustafa A (2021) Rhizosphere bacteria in plant growth promotion, biocontrol, and bioremediation of contaminated sites: a comprehensive review of effects and mechanisms. Inter J Molec Sci 22:10529. https://doi.org/10.3390/ijms221910529
Santoyo G, Guzmán-Guzmán P, Parra-Cota FI, Santos-Villalobos SDL, Orozco-Mosqueda MDC, Glick BR (2021) Plant growth stimulation by microbial consortia. Agronomy 11:219. https://doi.org/10.3390/agronomy11020219
Sattar A, Naveed M, Ali M, Zahir ZA, Nadeem SM, Yaseen M, Meena SV, Farooq M, Singh R, Rahman M, Meena HN (2019) Perspectives of potassium solubilizing microbes in sustainable food production system: a review. Appl Soil Ecol 133:146–159. https://doi.org/10.1016/j.apsoil.2018.09.012
Saxena AK, Kumar M, Chakdar H, Anuroopa N, Bagyaraj DJ (2020) Bacillus species in soil as a natural resource for plant health and nutrition. J Appl Microbiol 128:1583–1594. https://doi.org/10.1111/jam.14506
Senbeta AF, Worku W (2023) Ethiopia’s wheat production pathways to self-sufficiency through land area expansion, irrigation advance, and yield gap closure. Heliyon 9:e20720. https://doi.org/10.1016/j.heliyon.2023.e20720
Shankar A, Prasad V (2023) Potential of desiccation-tolerant plant growth-promoting rhizobacteria in growth augmentation of wheat (Triticum aestivum L.) under drought stress. Front Microbiol 14:1017167. https://doi.org/10.3389/fmicb.2023.1017167
Sharma A, Kumar P, Pahal V, Kumar J, Pandey SS (2023) Endophytic phytohormone production and utilization of functional traits in Plant Growth Promotion. Plant Microbiome for Plant Productivity and sustainable agriculture. Springer Nature Singapore, Singapore, pp 365–385
Shukla SK, Jaiswal VP, Sharma L, Tiwari R, Pathak AD, Gaur A, Awasthi SK, Srivastava A (2022) Trash management and Trichoderma harzianum influencing photosynthesis, soil carbon sequestration, and growth and yield of sugarcane ratoon in subtropical India. Eur J Agro 141:126631. https://doi.org/10.1016/j.eja.2022.126631
Singh SP, Singh RK, Kumar S (2017) Response of irrigation schedule, mulching and hydrogel on various growth analysis attributes and nutrient uptake of wheat (Triticum aestivum L). J Pharma Phytochem 6:2569–2573
Singh N, Agarwal S, Jain A, Khan S (2021) 3-Dimensional cross linked hydrophilic polymeric network hydrogels: an agriculture boom. Agri Water Manage 253:106939. https://doi.org/10.1016/j.agwat.2021.106939
Singh K, Tripathi S, Chandra R (2023) Bacterial assisted phytoremediation of heavy metals and organic pollutants by Cannabis sativa as accumulator plants growing on distillery sludge for ecorestoration of polluted site. J Environ Manag 332:117294. https://doi.org/10.1016/j.jenvman.2023.117294
Sri L, Chanu PH, Rani P, Rai S, Prasad SK, Singh RK (2019) Effect of hydrogel on soil moisture stress. J Pharma Phytochem 8:316–320
Sroka K, Sroka P (2024) Superabsorbent Hydrogels in the Agriculture and Reclamation of degraded areas. Sustainability 16:2945. https://doi.org/10.3390/su16072945
Subbiah BV, Asija GL (1956) A rapid procedure for the estimation of available nitrogen in soils. Curr Sci 25:259–260
Sun B, Bai Z, Bao L, Xue L, Zhang S, Wei Y, Zhang Z, Zhuang G, Zhuang X (2020) Bacillus subtilis biofertilizer mitigating agricultural ammonia emission and shifting soil nitrogen cycling microbiomes. Environ Inter 144:105989. https://doi.org/10.1016/j.envint.2020.105989
Tahir M, Khalid U, Ijaz M, Shah GM, Naeem MA, Shahid M, Kareem F (2018) Combined application of bio-organic phosphate and phosphorus solubilizing bacteria (Bacillus strain MWT 14) improve the performance of bread wheat with low fertilizer input under an arid climate. Brazelian J Microbiol 49(1):15–24. https://doi.org/10.1016/j.bjm.2017.11.005
Takahashi M, Kosaka I, Ohta S (2023) Water Retention characteristics of Superabsorbent polymers (SAPs) used as soil amendments. Soil Syst 7:58. https://doi.org/10.3390/soilsystems7020058
Warrad M, Hassan YM, Mohamed MSM, Hagagy N, Al-Maghrabi OA, Selim S, Saleh AM, AbdElgawad HA (2020) Bioactive fraction from Streptomyces sp. Enhances Maize Tolerance against Drought stress. J Microbiol Biotechnol 30:1156–1168. https://doi.org/10.4014/jmb.2003.03034
Wu Y, Li S, Chen G (2023) Hydrogels as water and nutrient reservoirs in agricultural soil: a comprehensive review of classification, performance, and economic advantages. Environ Develop Sustain 1–33. https://doi.org/10.1007/s10668-023-03706-y
Yang F, Cen R, Feng W, Liu J, Qu Z, Miao Q (2020) Effects of superabsorbent polymer on soil remediation and crop growth in arid and semi-arid areas. Sustainability 12:7825. https://doi.org/10.3390/su12187825
Yang Y, Zhang S, Wu J, Gao C, Lu D, Tang DW (2022) Effect of long term application of super absorbent polymer on soil structure, soil enzyme activity, photosynthetic characteristics, water and nitrogen use of winter wheat. Front Plant Sci 13:998494. https://doi.org/10.3389/fpls.2022.998494
Yaseen R, Hegab R, Kenawey M, Eissa D (2020) Effect of super absorbent polymer and bio fertilization on Maize productivity and soil fertility under drought stress conditions. Egytian J Soil Sci 60:377–395. https://doi.org/10.21608/ejss.2020.35386.1372
Zambrano-Mendoza JL, Sangoquiza-Caiza CA, Campaña-Cruz DF, Yánez-Guzmán CF (2021) Use of biofertilizers in agricultural production. Technol Agri 193. https://doi.org/10.5772/intechopen.98264
Zangana DD, Aljburi JM (2023) Impact of hydrogel and its relationship to yield, some of its components and grain quality of bread wheat genotypes (Triticum aestivum L.). IOP Conference Series: Earth Environ Sci 1214: 012042. IOP Publishing. https://doi.org/10.1088/1755-1315/1214/1/012042
Zhang JF, Zhao TN, Sun BP, Song SS, Guo HB, Shen HJ, Wu Y (2018) Effects of biofertilizers and super absorbent polymers on plant growth and soil fertility in the arid mining area of Inner Mongolia, China. J Mater Sci 15:1920–1935. https://doi.org/10.1007/s11629-017-4801-5
Zhang W, Wang P, Liu S, Chen J, Chen R, He X, Ma G, Lei Z (2021) Factors affecting the properties of superabsorbent polymer hydrogels and methods to improve their performance: a review. J Mater Sci 56:16223–16242. https://doi.org/10.1007/s10853-021-06306-1
Zheng H, Mei P, Wang W, Yin Y, Li H, Zheng M, Ou X, Cui Z (2023) Effects of super absorbent polymer on crop yield, water productivity and soil properties: a global meta-analysis. Agr Water Manage 282:108290. https://doi.org/10.1016/j.agwat.2023.108290
Funding
The authors did not receive any external funds.
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors state that they have no competing interests relevant to this article content.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Negim, O.I.A., Moharam, M.H.A., Elsayed, E.F. et al. The Combination Between Super Absorbent Polymers (SAPs) and Biofertilizers Could be an Ecofriendly Approach for Soil Chemical Properties Improving and Sustainable Wheat (Triticum Sativum) Production in Sandy Loam Soil. J Soil Sci Plant Nutr (2024). https://doi.org/10.1007/s42729-024-01839-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42729-024-01839-1