Abstract
Salinity stress is a major abiotic factor that affects medicinal plant growth, performance, and secondary compounds. Malva parviflora L. and Rumex dentatus L. plants were collected from three habitats in the northeastern Nile Delta governorates of Damietta and El-Dakahlia. Conductivity (salinity) classified the habitats as mesophytic, moderately saline, and saline. Chemical and physical soil characteristics varied by habitat. Results show that M. parviflora L. and R. dentatus L. had high soluble sugars, total carbohydrates, electrolyte leakage, and proline in the saline habitat. In contrast, mesophytic habitats showed low content. In addition, R. dentatus L. had more antioxidant enzymes and elements in saline habitats than in mesophytic habitats. In saline habitats, M. parviflora L. and R. dentatus L. were characterized by more calcium and sodium increase than mesophytic habitats. Moreover, R. dentatus L. had more phenols, alkaloids, flavonoids, anthocyanin, and tannins under saline conditions than M. parviflora. Meanwhile, in the saline habitat, plant hormones, i.e., indole acetic acid and gibberellic acid, decreased significantly in both M. parviflora and R. dentatus than in the mesophytic habitat. Scanning Electron Microscopy (SEM) of the tested plants showed the highest stomatal frequency and area on the lower surface of mesophytic plant leaves compared to either its upper surface or both leaf surfaces in saline habitat. Hence, it can be concluded that R. dentatus plant can mitigate the negative effects of salinity by improving the qualitative and quantitative performance under salinity stress more than M. parviflora plant.
Similar content being viewed by others
1 Introduction
Globally, the problem of soil salinity has a detrimental effect on agricultural development and production (Yan et al. 2020). Salinity is defined as the presence of too many soluble salts, such as magnesium (Mg2+), sodium (Na+), chloride (Cl-), calcium (Ca2+), and trace levels of nitrates and carbonates, in the top layer of soil that exceeds the threshold (0.15 g salt/100 g soil) (Dawood et al. 2022; Rady et al. 2011; Sadak 2023). The spread of salt in soil has grown to be a significant worldwide issue, affecting agricultural yield and growth. According to Agha et al. (2021), the present impacted area is above 900 million ha, and the trend is predicted to continue at a pace of 2 Mha per year (Singh 2018). For the next 30 years, this condition is predicted to worsen, possibly resulting in a 50% rise in damaged acreage and a decrease in agricultural productivity (Wang et al. 2021).
Agriculture in arid and semi-arid environments is more significantly impacted by salinity. Arid and semi-arid agricultural zones are significantly affected negatively by soil salinity. When low-quality irrigation water is used, salts build up in the soil (Mushtaq et al. 2020), which hinders a plant’s capacity to absorb nutrients and water. Among other detrimental impacts on plant growth and development, this diminished resource availability causes enzyme failure, hormone abnormalities, disturbance of the photosynthetic machinery, and ion toxicity (Hanafy and Sadak 2023; Shahid et al. 2020).
Rumex dentatus is a member of the Polygonaceae family, also known as the toothed dock or Aegean dock. It has many culinary uses and is useful in ethnomedicine (Khalil et al. 2022). It responded to unfavorable environmental conditions by inducing ROS and malondialdehyde (Radic and Marijana 2006). According to ALHaithloul et al. (2022), the whole plant provides medicinal benefits and cures several diseases. Besides acting as an aphrodisiac, the plant is a stimulant and tonic. Also, Mostafa et al. (2011) reported that it is used in the treatment of tumors, hepatic diseases, constipation, impaired digestion, pains, heart troubles, calculi, diseases of the hiccoughs, asthma, piles, dyspepsia, leukoderma, nausea, and scabies. The plant may also control cholesterol contents and reduce biliary disorders. R. dentatus is a traditional medicinal plant used to treat antitumor, antidermatitis, diarrhea, anti-inflammatory, constipation, eczema, and locally known Toothed dock (Rehan et al. 2020).
The leaves of M. parviflora L. contain mucilaginous cells and are edible and therapeutic (Munir et al. 2021). The mucilage in its fruits and leaves is a natural source of antitussive, gastro-protective, and anti-inflammatory chemicals (Altyar et al. 2022). In Egypt, it is an annual medicinal plant. Because M. parviflora contains natural antioxidants, it may be used as a medicinal agent to halt aging and treat oxidative stress-related degenerative disorders (Sun and Shahrajabian 2023). Furthermore, its phenolic components have a variety of biological effects and work as antioxidants by inhibiting the oxidation of platelets, low-density lipoproteins, red blood cell destruction, and aggregation (Khatana et al. 2020).
How to guarantee the safe use of plants with varying concentrations in salty soils is uncertain, given the lack of understanding of the mechanisms by which plants tolerate salinity in diverse environments and the reactions of their active antioxidant components to salinity. There is a wide variation in plant mineral element uptake and tolerance for salinity among species and soil salinity concentrations; therefore, a systematic study of multiple plant populations under different salinity concentrations is necessary. This study assessed (1) the physiological stress responses of M. parviflora and R dentatus under salinity stress and their interactions with non-enzymatic and enzymatic antioxidant systems. (2) The effects of salinity on M. parviflora and R dentatus uptake of mineral elements and polysaccharide content have been studied, and (3) exploring the possibility of safely using M. parviflora and R dentatus which have grown in salty soils.
2 Materials and Methods
2.1 Study Area
The research region is located at the northeastern section of the Nile Delta, namely, El-Dakahlia and Damietta governorates. The aerial portions of two plant species, M. parviflora (Fig. 1a) and R. dentatus, were collected during the field study in March and April 2015 (Fig. 1b).
Three different habitats (Fig. 1c) were sampled in plastic bags and transported directly to the laboratory for analysis. As a result of the measured conductivity (salinity) for each habitat, three categories were determined: mesophytic, moderately saline, and saline.
2.2 Soil Analyses
The soil samples (0–20 cm depth) were collected as three replicates from the rhizosphere surrounding each plant in each habitat. After collection, all samples were air-dried and sieved through a 2 mm to remove trash and coarse grit. These air-dried samples’ chemical and physical properties were determined according to Page et al. (1982) (Tables 1 and 2).
2.3 Plant Analysis
2.3.1 Photosynthetic Pigments
One gram of fresh leaves was extracted with 100 mL of 80% aqueous acetone (v/v) to remove the colors. After the complete extraction of photosynthetic pigments, the optical density of the extract was computed to measure the chlorophyll a, chlorophyll b, and carotenoid amount as determined by the Wellburn (1994) technique at 649, 665, and 470 nm.
2.3.2 Total Soluble Sugars and Polysaccharides Contents
Total soluble sugars were determined according to Haroun (1985) method. In brief, 200 mg of dried leaves were mixed with 96% v/v ethanol (5 mL) and centrifuged at 3500 ×g for 10 min. The samples were kept at 4 °C for some time. After that, 0.1 mL of the extract was heated for 10 min in a water bath with 3 mL of newly made anthrone reagent (100 mL sulfuric acid (72%) + 150 mg anthrone). Following cooling, a spectrophotometer was used to measure each sample’s absorbance at 625 nm.
Polysaccharide contents were determined according to Sadasivam and Manickam (1996). In brief, 80% ethanol-treated 0.2 g dry tissues were centrifuged, and the collected residue was dried. The residue was collected and increased to 100 ml with distilled water. Analysis used 0.5 and 1 ml. Next, 4 mL of anthrone reagent was mixed with 1 mL supernatant and cooked for 8 min in a boiling water bath. After cooling, a spectrophotometer measured the color at 630 nm. The total carbohydrates were determined as the sum of the sample’s total soluble sugars and polysaccharides.
2.3.3 Determination of Electrolyte Leakage, Phenolics and Proline, and Total Lipids
Calculations of electrolyte leakage (EL) were made from the leaves of plants using the Lutts et al. (1996) approach. First, 20 discs (1 cm2) were randomly chosen after 20 h of room-temperature shaking, and they were placed in a boiling tube with 10 mL of deionized water. An Acromet AR20 electrical conductivity meter (Fisher Scientific, Chicago, IL, USA) was used to test the electrical conductivity (EC1). The EC2 was measured after the mixture had been heated to 45–55 °C for 30 min in a water bath. Finally, the sample was boiled at 100 °C for 10 min, and the EC3 value was noted.
The proline content of dried leaves was estimated using the Bates et al. (1973) method by ninhydrin solution, glacial acetic acid, and sulfosalicylic acid. The sample was heated to 100 °C and cooled, and then 5 mL of toluene was added. The toluene layer’s absorbance was measured at 528 nm using a spectrometer.
Phenols content was determined using Folin-Ciocalteu reagent (FCR) and Na2CO3 solution according to Ziouti et al. (1992). A total of 100 mg of dried leaves were combined with 6.5 mL of 50% methanol. After a vortex, the samples were darkened for 95 min and centrifuged for 5 min at 15,000 ×g. Tubes were incubated at 42 °C for 10 min. Then, undergo a spectrophotometer measurement at 7765 nm.
According to AoOA and Horwitz (1975), the technique was used to estimate total lipids in dried leaves. First, 10 g of oven-dried leaves were extracted in a soxhlet device for 18 h using a 1:2 mixture of petroleum ether and diethyl ether. After complete extraction of oil, the remaining solvent must be completely removed from the samples. Then, samples were weighed.
2.3.4 Antioxidant Enzyme Activities
Tests were conducted on antioxidant enzyme activity using homogenized leaf tissues extracted in pre-child extraction buffer of Triton X-100 at 0.05%, phenylmethylsulfonyl fluoride at 1 mM, polyvinylpyrrolidone at 1% (w/v), and 50 mM K-phosphate buffer (pH = 7.0). The catalase (CAT) activity was determined, as depicted previously (Starlin and Gopalakrishnan 2013). The peroxidase (POX) and polyphenol oxidase (PPO) activity tests were performed by the method reported in Bergmeyer and Bernt (1974), using benzidine and a spectrophotometer to record the absorbance at 470 nm.
2.3.5 Element’s Content
Total nitrogen was quantified by Haroun (1981). When phosphate and molybdic acid combine to create a blue complex in the presence of a reducing agent, the amount of phosphorus may be determined, according to Margesin and Schinner (2005). The atomic absorption spectrophotometry was used to determine Mg and Ca ions, followed by Chapman and Pratt (1978). Finally, the flame emission technique determines K and Na (Allen et al. 1974).
2.3.6 Quantitative Determination of Some Secondary Metabolites
According to Siji and Nandini (2017), the approach was used to determine total tannin. First, 50 mL of methanol was used to extract 1 g of plant material. Swirling occasionally to combine. Next, 1 ml of the supernatant was mixed with 5 ml of freshly prepared vanillin hydrochloride reagent (4% vanillin and 8% HCl in methanol 1:1 v/v). After 20 min, the color was evaluated at 500 nm.
The total flavonoids in the dried leaves were extracted and filtered using 80% cold methanol. In the filtrate, the total flavonoids were measured using a spectrophotometric technique that has been previously described by Boham and Kocipai-Abyazan (1974). A total of 250 mL of the filtrate from the methanolic extract was combined with 1.25 mL of distilled water and 75 uL of a 5% NaNO2 solution. A total of 150 uL of a 10% AlCl3H2O solution was added after 5 min. The combination included 275 mL of distilled water and 500 L of 1 M NaOH and was well mixed before the intensity of the pink hue was determined at 510 nm.
Alkaloid levels were determined using the Harborne (1973) method. First, a 250 ml beaker containing 5 g of the sample and 200 ml acetic acid (10%) in ethanol (v/v) were combined, sealed, and allowed to stand for 4 h. After filtration, the extract was concentrated to a fourth of its initial volume in a water bath. After the precipitation was complete, the extract was gradually treated with concentrated ammonium hydroxide. Finally, the precipitate was collected, washed with diluted ammonium hydroxide, and filtered once the whole solution had settled.
Anthocyanin content was initially measured using ammonia HCl (Egbuna et al. 2018). A total of 2 mL each of extract, 2N HCl, and ammonia was added. As a result, anthocyanin turns pink-red to blue-violet. Next, the pH differential approach was used to calculate total anthocyanin concentration using pH 1.0 and 4.5 absorbance data and structural changes in chemical forms. Finally, crude extracts were diluted separately with 0.025 M hydrochloric acid-potassium chloride buffer (pH 1) and 0.4 M sodium acetate buffer (pH 4.5). The buffers diluted each sample, resulting in absorbance readings from 0.2 to 1.4 V UV-Vis spectrophotometer at 700 nm (UV1601; Shimadzu, Kyoto, Japan).
2.3.7 Phytohormones Estimation
Phytohormones estimation: the methods for extraction and separation of the different phytohormones from the plant’s leaves were according to Shindy and Smith (1975). Gas-liquid chromatography (GLC) is used for the determination of acidic hormones like auxin (IAA), gibberellins (GAs), and abscisic acid (ABA).
2.3.8 Scanning Electron Microscopy (SEM)
Electron Microscopy (SEM) of the plant’s leaves was operated at 30 KV at the Electron Microscopy Unit, Mansoura University, in a Jeol JSM-6510 L.V SEM using a modified Karnovsky (1965).
2.4 Statistical Analysis
An analysis of variance was conducted for a completely randomized factorial experiment. After performing Levene’s test to determine if the error variances were homogeneous and a normality test (Levene 1960), the differences between means were compared using Fisher’s test at p < 0.05. The SPSS 27 Edition was used to perform the statistical analysis. Principal component analysis (PCA) identified substantial differences in physiological parameters under different habitats. Cell structure was analyzed using the public domain software package Image J, which is commonly used for measuring the size of cells and other organisms.
3 Results
3.1 Physical and Chemical Analysis of the Soil Samples
Analyses of the soil from various environments revealed that mesophytic soil had lower soil TDS, pH, CaCO3, SO4, Cl-, EC, Na+, HCO3, Ca++, Mg++, and K+ values than saline soils. In contrast, the mesophytic environment was distinguished by its clayey texture, maximum porosity values, and ability to store water, TP, OC, and TN. This might be explained by the Nile Delta’s farmed land being disturbed by irrigation canal networks dispersed across it and the excessive use of organic and synthetic fertilizers (Tables 1 and 2).
3.2 Photosynthetic Pigments
Total chlorophyll, carotenoids, and total photosynthetic pigments of M. parviflora and R. dentatus plants under different salinity concentrations are illustrated in Fig. 2. Generally, all chlorophyll pigment levels diminished in M. parviflora and R. dentatus plants were grown under high salt concentrations. In addition, compared to the other habitats, total chlorophyll content and total pigments increased significantly in M. parviflora and R. dentatus plants in the mesophytic habitat. The highest rise was observed with R. dentatus, which showed more efficiency than M. parviflora. For example, total pigment content varied from 16.83 ± 0.09 to 14.35 ± 0.16 when R. dentatus plants grew under mesophytic and saline habitats, respectively (Fig 2a–c).
Variation in a total chlorophyll, b carotenoids, c total pigments, d total soluble sugars, e polysaccharides, and f total carbohydrates of Malva parviflora and Rumex dentatus plants at the three different habitats. Statistically, different letters indicate significant differences according to the Fisher test at p-value <0.05
According to the present findings, M. parviflora and R. dentatus in the saline habitat were characterized by the highest concentrations of total soluble sugars, polysaccharides, and total carbohydrates. In contrast, the mesophytic ecosystem detected minimal content (Fig. 2d–f). For example, total carbohydrate content varied from 146.0 ± 0.4 to 278.2 ± 6.25 when R. dentatus plants grew under mesophytic and saline habitats, respectively (Fig. 2d–f).
3.3 Electrolyte Leakage, Proline, Lipid percentage, and Antioxidant Enzyme Activity
Under saline habitats, electrolyte leakage in M. parviflora leaves is significantly higher than R. dentatus plants (Fig. 3a). However, M. parviflora and R. dentatus plants grew under saline habitats, causing a significantly higher increase in proline and lipid content compared to mesophytic habitats. Results demonstrate that R. dentatus alleviates osmotic stress derived from elevated salt concentrations more efficiently than M. parviflora (Fig. 3b–c).
Variation in a electrolyte leakage (%), b proline content, c total lipids (%), d peroxidase, e polyphenol oxidase, and f catalase of Malva parviflora and Rumex dentatus plants at the three different habitats. Statistically, different letters indicate significant differences according to the Fisher test at p-value <0.05
Figure 3d–f shows the activity of POX, PPO, and CAT enzymes in the leaves of M. parviflora and R. dentatus plants to mitigate the stress caused by elevated salt concentrations. POX, PPO, and CAT activities responded highly to salt levels. Results depicted in Fig. 3d–f show a significant increase in peroxidase, polyphenol oxidase, and catalase content in M. parviflora leaves and R. dentatus plants that grew under saline habitats. However, under saline habitats, peroxidase, polyphenol oxidase, and catalase content in the leaves of M. parviflora is lower than that of R. dentatus plants.
3.4 Elements Content
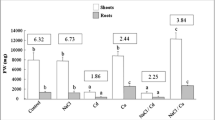
A comparison of the effects of various habitats on a few elements in M. parviflora and R. dentatus revealed that nitrogen (N), phosphorus (P), potassium (K), and magnesium (Mg) considerably rose in mesophytic environments compared to saline habitats (Fig. 4). However, saline habitats notice a higher calcium and sodium increase than mesophytic habitats. The highest contents of P, N, K, and Mg increase were observed with R. dentatus, which showed more efficiency than M. parviflora.
3.5 Secondary Metabolites
The data in this study showed that alkaloids, phenols, flavonoids, tannins, and anthocyanin content for M. parviflora and R. dentatus increased significantly in saline habitats compared to mesophytic habitats (Fig. 5a–e). For example, alkaloids (varied from 8.0 ± 0.16 to 13.22 ± 0.12), phenolics (varied from 4.79 ± 0.09 to 9.57 ± 0.10), flavonoids (varied from 6.47 ± 0.10 to 11.97 ± 0.12), tannins ( varied from 1.73 ± 0.03 to 3.65 ± 0.06), and anthocyanin (varied from 0.36 ± 0.01 to 0.82 ± 0.02) content when R. dentatus plants grew under mesophytic and saline habitat, respectively (Fig. 5a–e).
3.6 Phytohormones
In the current study, changes in indole acetic acid (IAA) and gibberellic acid (GA3) for M. parviflora and R. dentatus showed a significant reduction in its content in saline habitat compared with mesophytic habitat (Fig. 6). In contrast, the current study showed a marked significant decrease in abscisic acid (ABA) biosynthesis in M. parviflora and R. dentatus plants that grew under saline habitats compared to the mesophytic habitat.
3.7 Scanning Electron Microscope
The present study noticed that, for the two studied plants, the stomatal frequency was significantly lower in the saline habitat than in the mesophytic habitat, with the maximum number of stomata recorded on the lower surface of leaves in the mesophytic habitat as compared to the upper surface (Fig. 7).
Additionally, in comparison to the lower surface, the stomatal frequency is generally lower on the upper surfaces of M. parviflora (Fig. 8a–d) and R. dentatus (Fig. 9a–d) leaves, while the stomatal area and stomatal opening area are higher on the lower surfaces of leaves in mesophytic habitat when compared to the upper surfaces in the same habitat or with those in saline habitat.
3.8 Principal Component Analysis
PCA identified substantial differences in physiological parameters under different habitats (Fig. 10). The PCA identified two primary components of the measured variables, and PC1 and PC2 accounted for 52.52% and 39.32% of the total variance in M. parviflora and R. dentatus plants (Fig. 10).
4 Discussion
Salinity is one of the greatest abiotic stresses, which slows down plant development and causes many metabolic changes (Noor et al. 2023; Sadak 2022). According to plants, salt tolerance is a hereditary trait that varies across species. Therefore, salt buildup in plant cells may deleteriously affect normal physiological processes. As a result, plants have developed a variety of fast defense mechanisms to lessen the effects of salt (Agha et al. 2023). Additionally, the availability of phytohormones and signaling molecules enhances a plant’s capacity to adjust to detrimental environmental conditions (Rady et al. 2019). This investigation revealed that M. parviflora and R. dentatus plants could tolerate salinity to a certain extent by modifying their free radical scavenging capacity and cell osmoprotection. This allows them to maintain proper water balance even when exposed to high salt levels. Plants may also modify their growth and development in response to salinity stress.
In plants, chlorophyll is essential for photosynthetic capacity, while soil salinity degrades chlorophyll significantly (Yasir et al. 2021). In R. dentatus rather than M. parviflora, the total chlorophyll and total photosynthetic pigment contents decreased with rising NaCl concentrations, whereas carotenoids increased by increasing salinity (Fig. 2). Under higher NaCl concentrations, the plant’s root system might be disrupted, choking water absorption (Li et al. 2012). The findings could suggest that M. parviflora and R. dentatus use independently developed and modified mechanisms to function in soil salinity. The antennae pigments may need to be more efficient in order to give enough energy to cope with the energy-intensive responses to soil salinity. By boosting chlorophyll concentrations to have greater light harvesting ability and thus higher photosynthesis, protecting chloroplast enzymes, and enhancing the manufacture of photosynthetic pigments, R. dentatus exhibits remarkable resistance to soil salt (Ragaey et al. 2022). Our research supports previous research that found species-dependent responses to salt stress were seen in the concentrations of chlorophylls a and b (Agha et al. 2021). Additionally, we noticed that M. parviflora’s photosynthetic pigments dramatically reduced under salt stress. It is possible that salinity caused the creation of reactive oxygen species (ROS) like H2O2 and O2, which caused lipid peroxidation and chlorophyll oxidation, reducing chlorophyll concentration. Furthermore, it has been found that in saline conditions, chlorophyll concentration increased in salt-tolerant plants such as mustard while reduced in salt-sensitive plants like soybean (Agha et al. 2023; El-Bassiouny and Sadak 2015).
Secondary metabolites are organic chemicals produced by plants that take the role of primary metabolism but do not directly impact growth and development, according to Tiago et al. (2017). Our research indicates that as environments changed, so did interspecific differences in plant proline and soluble sugar content. Salt stress had different impacts on the amount of soluble sugar, lipids, alkaloids, phenolics, flavonoids, tannins, and anthocyanins in plants. In previous research, salinity stress dramatically increased sugars, flavonoids, tannins, anthocyanin, and proline (Mogazy and Hanafy 2022). Proline is an amino acid that serves as an osmolyte compound and an antioxidant when plants are exposed to various stresses (El-Sheshtawy et al. 2022). Proline is an osmoprotectant that helps plants to cope with water or osmotic stress. According to studies, salt alters the metabolism of proline, flavonoids, tannins, and anthocyanins, reducing a plant’s ability to withstand stress. Our results are consistent with research demonstrating that R. dentatus plants had considerably higher proline, flavonoids, tannins, and anthocyanin contents under salt stress than M. parviflora plants (Khan et al. 2021) .
The proline result agrees with many authors (Karimi and Yusef-Zadeh 2013; Mahboobeh and Akbar 2013). This rise in proline levels under salinity stress may be due to the activation of the glutamate ligase enzyme that converts glutamine to proline (Szepesi and Szőllősi 2018). In stressed plants, proline is adaptive in facilitating osmotic adjustment and safeguarding subcellular structures. Proline is found in higher plants in greater abundance than other amino acids and regulates the accumulation of usable N (Abrahám et al. 2003). In addition, proline buildup occurs regularly in the cytosol, contributing significantly to cytoplasmic osmotic correction (Pecherina et al. 2023).
This supports the findings of Misra and Gupta (2006), who noted that seedlings of Catharanthus spp. responded to salt by increasing the concentration of alkaloids in the leaves and roots. Plants are unavoidably subjected to various forms of stress during normal growth and development, which may increase ROS formation. Under severely suppressive circumstances, these alkaloids operate as ROS scavengers (Smirnoff 1993). In addition, phenolics have critical functions in plant growth, notably in pigment and lignin biosynthesis (Elsayed et al. 2020).
Alkaloids and flavonoids may be produced synthetically, which is a powerful anti-ROS strategy (Posmyk et al. 2009). Furthermore, Dardanelli et al. (2008) also found that Azospirillum brasilense raised the synthesis of nod gene-inducing alkaloids and flavonoid compounds as well as accelerated root branching in bean seedling roots when exposed to salt stress. One of the most prevalent groups of plant phenolics, flavonoid compounds, is essential for plant defense.
Various plant types produce different chemicals called tannins. Through their capacity to defend against abiotic stress and control plant development, they have a significant impact on plants. Furthermore, Paolocci et al. (2005) claimed that condensed tannins influence mutualistic or pathogenic interactions between microbes and plants and plant responses to abiotic stressors.
Additionally, anthocyanins are phytopigments classified within the phenylpropanoid flavonoid subclass (Grotewold 2006). These findings support Zahedi et al. (2020) observation that salt stress enhanced the antioxidant capacity of the antioxidant pools in strawberry fruit (anthocyanins and superoxide dismutase). Photoprotection and stress signaling are two abiotic stress-related functions of anthocyanins (Tattini et al. 2014). Therefore, high levels of polyphenols, particularly anthocyanins, may demonstrate their effective involvement in limiting oxidative damage by H2O2 production. High levels of polyphenols, particularly anthocyanins, may demonstrate their effective involvement in limiting oxidative damage by H2O2 production (Hichem and Mounir 2009).
Therefore, electrolyte leakage (EL), lipid percentage, and proline of M. parviflora and R. dentatus were raised in the saline habitat compared with the other two habitats. The EL findings are comparable to those obtained by Mahmoudi et al. (2011). Furthermore, plasma membranes are the principal location of ion-specific salt damage. As a result, one of the most significant selection criteria for finding salt-tolerant plants is electrolyte leakage from plasma membranes (Ashraf and Ali 2008).
Plants utilize a variety of non-enzymatic and enzymatic antioxidants to protect themselves from oxidative stress and to maintain ROS concentrations within a small functioning range (Ozgur et al. 2013). The current research shows a significant rise in the estimated enzymatic antioxidant enzymes (PPO, CAT, and POX) in saline habitats compared with mesophytic habitats for M. parviflora and R. dentatus. In this regard, Mittova et al. (2003) found that plants fight against ROS by inducing the activity of antioxidative enzymes that scavenge ROS, like POX, SOD, CAT, and GR. Additionally, high salt concentrations enhanced the antioxidant enzyme activity of cotton, including ascorbate peroxidase, glutathione reductase, and catalase (Hamani et al. 2020). Catalase is the primary H2O2 scavenging enzyme in all-aerobic organisms that is involved in the breakdown of H2O2 into oxygen and water (Yang and Poovaiah 2002). It is essential for maintaining redox equilibrium under oxidative stress and serves as a cellular H2O2 sink (Shu et al. 2023).
The influence of different habitats on specific elements of M. parviflora and R. dentatus was studied. It was shown that nitrogen, phosphorus, potassium, and magnesium considerably increased in mesophytic environments compared to salty habitats. However, compared to mesophytic ecosystems, saline habitats see greater increases in salt and calcium. In this regard, Kader and Lindberg (2005) established a correlation between rising cytosolic Na+ concentrations and salt. In contrast, Gomes et al. (2011) found that increasing Na+ decreased cytosolic K+ levels. Na+ also causes plants to increase cytosolic Ca2+ in response (Munns and Tester 2008; Rahman et al. 2021). Furthermore, salinity has been found to impact plant development through Mg2+ deficit (Khan et al. 2000), which is consistent with our magnesium findings for M. parviflora and R. dentatus.
High EL levels may be attributable to naturally high potassium levels rather than damaged plasma membranes caused by stress, as Mansour and Salama (2004) suggested. According to Demidchik et al. (2014), EL is mainly caused by the efflux of K+, which is plentiful in plant cells. Furthermore, proline is an osmotically active amino acid that helps membrane stability and reduces the influence of NaCl on cell membrane rupture.
A summary of the available data revealed that when compared to the mesophytic environment, the saline habitat had the most significant value for M. parviflora and R. dentatus’ Ca2+ concentration. These results are in accordance with those of Hessini et al. (2009), who found that Spartina alterniflora significantly increased its Ca2+ levels in saline environments. The fact that Ca2+ is a non-toxic inorganic nutrient with detoxifying properties in the saline medium may influence how M. parviflora and R. dentatus respond to salinity stress due to increased Ca2+ levels in salty surroundings (Izzo et al. 2008).
In this regard, researchers emphasized that because Na+ ions prevent the absorption of K+ ions, the reduction in K+ concentration during stress is caused by the buildup of Na+ ions. In addition, it is claimed that the K+ ion is crucial for osmotic adjustment, especially in aged leaves (Haroun 2002; Khan et al. 2022). These findings support those of Grewal (2010), who found that the decline in soil salinity levels negatively impacted the K+/Na+ ratio of wheat leaves and chickpeas.
Furthermore, calcium mitigates the adverse effects of salt on plants. Cramer et al. (1987) found that salinity reduces plant Ca2+ absorption, potentially by displacing it from the cell membrane or changing membrane function. A direct influence of calcium on cell wall extensibility has also been seen in a salinized halophyte (Rygol and Zimmermann 1990). Furthermore, it has been shown that salt inhibits plant development through Mg2+ deficiency, which is similar to our results of M. parviflora and R. dentatus magnesium.
Indole acetic acid (IAA) and gibberellic acid (GA3) content in M. parviflora and R. dentatus showed a significant reduction in its content in saline habitat compared with mesophytic habitat. The important function of GA3 in controlling plant development under abiotic stress conditions is attributed to DELLA protein-mediated growth restraint during abiotic stress exposure (Achard et al. 2008; Zuluaga et al. 2023).
Furthermore, increased auxin concentrations promoted hypersensitivity to salt stress via overexpression of auxin biosynthesis (Jung and Park 2011). In fact, salt stress reduced IAA levels in tomatoes by around 75%. As a result, decreased plant growth and development under stress circumstances might result from altered auxin accumulation and redistribution (Abdelhamid et al. 2020; Akbari et al. 2007).
In contrast to the mesophytic habitats, the two analyzed saline habitats exhibited a markedly significant rise in ABA production, according to the present research. In this context, the plant stress hormone ABA has long been hypothesized to be a crucial signaling mediator for regulating various plant developmental processes and adaptive responses to a broad range of abiotic stimuli (Hadiarto and Tran 2011; Sofy et al. 2022). Furthermore, ABA is widely recognized as an endogenous signal molecule that allows plants to tolerate harsh environmental circumstances like drought and salt stress (Raghavendra et al. 2010). According to Jaschke et al. (1997), ABA buildup may ameliorate the inhibitory impact of salinity on photosynthesis, growth, and assimilate translocation in Hordeum vulgare L. These results are consistent with those of the current investigation.
Furthermore, the ABA hormone aids plants in adjusting to decreasing water supply by shutting stomata and collecting a variety of proteins and osmoprotectants for osmotic adjustment. Higher levels of endogenous ABA were shown to be highly connected with growth problems in plants such as Phaseolus vulgaris (Cabot et al. 2009) and Brassica (He and Cramer 1996).
All plants are exposed to tremendous stressors during their life cycle. Plants react to stress in various ways depending on their species and the source of the stress. Two key environmental factors reduce plant production: drought and salt (Sharaf et al. 2009; Sabagh et al. 2019). The lower surface of M. parviflora and R. dentatus leaves in the mesophytic habitat has a greater number of stomata and a larger opening area than the upper surface in the same habitat or in the saline habitat. Halophytes may partly close their stomata as salinity rises, reducing transpiration and salt transfer to the leaves. Fricke et al. (2006) found that stomatal closure is one of the most rapid reactions to salinity. In addition, reduced guard cell turgor pressure decreased stomatal aperture and allowed plants to quickly minimize water loss in response to external cues. This response is thought to be critical for minimizing plant water loss under hyperosmotic conditions in their rhizosphere (Kong et al. 2005; Selvakumar et al. 2014).
The present research demonstrates that the observed decrease in stomatal area in salt-stressed plants may be caused by abscisic acid buildup (Fricke et al. 2006).
At both the cellular and whole plant levels, salinity is a key limiting factor for crop development (Yan et al. 2020). Therefore, they investigated the effects of salinity on stomatal physiology, morphology, water relations, and seedling growth in peas and quinoa to compare the salt tolerance mechanisms of these two species. They found that salt stress significantly reduced stomatal conductance, density, and length in both plants, but quinoa was less affected by salinity.
According to Rasouli et al. (2021), analyzing how salinity stress affects both lycophyte’s and halophytes’ stomatal operation could improve salt stress tolerance in traditional crops. Fast stomatal responses to internal signals and environmental factors may be critical to synchronize stomatal conductance and photosynthetic responses. As a result, optimizing water use efficiency under changing conditions is essential. Some plants, as Rosmarinus officinalis, acquire tolerance and avoidance strategies when exposed to salt. These processes rely on stomatal closure and decreased leaf area to limit water loss via transpiration (Alarcón et al. 2006; Badawy et al. 2022). However, Acosta-Motos et al. (2017) discovered that changes in stomatal opening alter transpiration, a method by which leaves cool themselves.
Furthermore, similar to our findings, Maricle et al. (2009) discovered that freshwater species (S. cynosuroides, Spartina gracilis, and S. pectinata) contain stomata on both the adaxial and abaxial sides of the leaves (amphistomatous leaves). On the other hand, salt marsh plants have stomata nearly entirely on the adaxial leaf surfaces (epistomatous leaves). As a result, organisms that flourish in high marshes are likely to have adaptations to high salinity. As a result, these species have few stomata on the abaxial side of their leaves, which might limit water loss during periods of extreme water stress. They also discovered that stomata were preferentially found on adaxial leaf surfaces and that leaves curled to hide adaxial surfaces in response to a water shortage.
5 Conclusion
Through chemical, physical, and biological processes in soil, which provide a favorable air-moisture regime for plant growth, soil plays an important role in plant life as the major growth medium for plants. This study determined various parameters of M. parviflora and R. dentatus in three different habitats: mesophytic, moderately saline, and saline. So, it became evident from this study that multiple strategies help plants tolerate salinity stress. But the two used plants, M. parviflora and R. dentatus, appeared to differ concerning the physiological approaches required for best growth. Thus, under the saline habitat’s conditions, the two plants vary in the induction of several defense components, such as soluble sugars, proline, antioxidant enzymes and compounds, IAA, ABA, electrolyte leakage, and stomatal frequency.
Data Availability
Not applicable
References
Abdelhamid MT, Sekara A, Pessarakli M, Alarcón J, Brestic M, El-Ramady H, Gad N, Mohamed HI, Fares WM, Heba SS, Sofy MR, El-Kafafi ES (2020) New approaches for improving salt stress tolerance in rice. Rice research for quality improvement: genomics and genetic engineering. Breed Tech Abiot Stress Tolerance 1:247–268. https://doi.org/10.1007/978-981-15-4120-9_10
Abrahám E, Rigó G, Székely G, Nagy R, Koncz C, Szabados L (2003) Light-dependent induction of proline biosynthesis by abscisic acid and salt stress is inhibited by brassinosteroid in Arabidopsis. Plant Mol Biol 51(3):363–372. https://doi.org/10.1023/A:1022043000516
Achard P, Renou J-P, Berthomé R, Harberd NP, Genschik P (2008) Plant DELLAs restrain growth and promote survival of adversity by reducing the levels of reactive oxygen species. Curr Biol 18(9):656–660. https://doi.org/10.1016/j.cub.2008.04.034
Acosta-Motos JR, Ortuño MF, Bernal-Vicente A, Diaz-Vivancos P, Sanchez-Blanco MJ, Hernandez JA (2017) Plant responses to salt stress: adaptive mechanisms. Agronomy 7(1):18. https://doi.org/10.3390/agronomy7010018
Agha MS, Abbas MA, Sofy MR, Haroun SA, Mowafy AM (2021) Dual inoculation of Bradyrhizobium and Enterobacter alleviates the adverse effect of salinity on Glycine max seedling. Not Bot Horti Agro Cluj-Nap 49(3):12461. https://doi.org/10.15835/nbha49312461
Agha MS, Haroun SA, Abbas MA, Sofy MR, Mowafy AM (2023) Growth and metabolic response of Glycine max to the plant growth-promoting Enterobacter Delta PSK and Bradyrhizobium japonicum under salinity stress. J Plant Growth Regul:1–15. https://doi.org/10.15835/nbha49312461
Akbari G, Sanavy S, Yousefzadeh S (2007) Effect of auxin and salt stress (NaCl) on seed germination of wheat cultivars (Triticum aestivum L.). Pak J Biol Sci: PJBS 10(15):2557–2561. https://doi.org/10.3923/pjbs.2007.2557.2561
Alarcón J, Morales M, Ferrández T, Sánchez-Blanco M (2006) Effects of water and salt stresses on growth, water relations and gas exchange in Rosmarinus officinalis. J Hortic Sci Biotechnol 81(5):845–853. https://doi.org/10.1080/14620316.2006.11512148
Alhaithloul HAS, Khan MI, Musa A, Ghoneim MM, ALrashidi AA, Khan I, Azab E, Gobouri AA, Sofy MR, El-Sherbiny M (2022) Phytotoxic effects of Acacia saligna dry leachates on germination, seedling growth, photosynthetic performance, and gene expression of economically important crops. PeerJ 10:e13623. https://doi.org/10.7717/peerj.13623
Allen SE, Grimshaw HM, Parkinson JA, Quarmby C (1974) Chemical analysis of ecological materials. Blackwell Scientific Publications
Altyar AE, Munir A, Ishtiaq S, Rizwan M, Abbas K, Kensara O, Elhady SS, Rizg WY, Youssef FS, Ashour ML (2022) Malva parviflora leaves and fruits mucilage as natural sources of anti-inflammatory, antitussive and gastro-protective agents: a comparative study using rat models and gas chromatography. Pharmaceuticals 15(4):427. https://doi.org/10.3390/ph15040427
Ashraf M, Ali Q (2008) Relative membrane permeability and activities of some antioxidant enzymes as the key determinants of salt tolerance in canola (Brassica napus L.). Env Expl Bot 63(1-3):266–273. https://doi.org/10.1016/j.envexpbot.2007.11.008
Badawy IH, Hmed AA, Sofy MR, Al-Mokadem AZ (2022) Alleviation of cadmium and nickel toxicity and phyto-stimulation of tomato plant l. by endophytic Micrococcus luteus and Enterobacter cloacae. Plants 11(15):2018. https://doi.org/10.3390/plants11152018
Bates LS, Waldren RP, Teare I (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39(1):205–207. https://doi.org/10.1007/BF00018060
Bergmeyer HU, Bernt E (1974) UV-assay with pyruvate and NADH Methods of enzymatic analysis. Elsevier, pp 574–579. https://doi.org/10.1016/B978-0-12-091302-2.50010-4
Boham B, Kocipai-Abyazan R (1974) Flavonoids and condensed tannins from leaves of Hawaiian vaccinium vaticulatum and V calycinium. Pac Sci 48(4):458–463
Cabot C, Sibole JV, Barceló J, Poschenrieder C (2009) Abscisic acid decreases leaf Na+ exclusion in salt-treated Phaseolus vulgaris L. J Plant Growth Reg 28(2):187–192. https://doi.org/10.1007/s00344-009-9088-5
Chapman H, Pratt P (1978) Methods of analysis for soils, plant and water. California Univ. Division Agric Sciences Priced Publication, p 50
AoOA C, Horwitz W (1975) Official methods of analysis, vol 222. Association of Official Analytical Chemists Washington, DC
Cramer GR, Lynch J, Läuchli A, Epstein E (1987) Influx of Na+, K+, and Ca2+ into roots of salt-stressed cotton seedlings: effects of supplemental Ca2+. Plant Physiol 83(3):510–516. https://doi.org/10.1104/pp.83.3.510
Dardanelli M, Córdoba F, Espuny M, Carvajal M (2008) AR; Díaz, MES; Serrano, AMG; Okon, Y.; Megías, M. Effect of Azospirillum brasilense coinoculated with Rhizobium on Phaseolus vulgaris flavonoids and nod factor production under salt stress. Soil Biol Biochem 40:2713–2721. https://doi.org/10.1016/j.soilbio.2008.06.016
Dawood MF, Sofy MR, Mohamed HI, Sofy AR, Abdel-kader HA (2022) Hydrogen sulfide modulates salinity stress in common bean plants by maintaining osmolytes and regulating nitric oxide levels and antioxidant enzyme expression. J Soil Sci Plant Nutr 22(3):3708–3726. https://doi.org/10.1007/s42729-022-00921-w
Demidchik V, Straltsova D, Medvedev SS, Pozhvanov GA, Sokolik A, Yurin V (2014) Stress-induced electrolyte leakage: the role of K+-permeable channels and involvement in programmed cell death and metabolic adjustment. J Exp Bot 65(5):1259–1270. https://doi.org/10.1093/jxb/eru004
Egbuna C, Ifemeje JC, Maduako MC, Tijjani H, Udedi SC, Nwaka AC, Ifemeje MO (2018) Phytochemical test methods: qualitative, quantitative and proximate analysis Phytochemistry. Apple Academic Press, pp 381–426. https://doi.org/10.1201/9780429426223-15
El-Bassiouny H, Sadak MS (2015) Impact of foliar application of ascorbic acid and α-tocopherol on antioxidant activity and some biochemical aspects of flax cultivars under salinity stress. Acta Biol Colomb 20(2):209–222. https://doi.org/10.15446/abc.v20n2.43868
El-Sheshtawy HS, Mahdy HM, Sofy AR, Sofy MR (2022) Production of biosurfactant by Bacillus megaterium and its correlation with lipid peroxidation of Lactuca sativa. Egypt J Pet 31(2):1–6. https://doi.org/10.1016/j.ejpe.2022.03.001
Elsayed RH, Kamel EM, Mahmoud AM, El-Bassuony AA, Bin-Jumah M, Lamsabhi AM, Ahmed SA (2020) Rumex dentatus L. phenolics ameliorate hyperglycemia by modulating hepatic key enzymes of carbohydrate metabolism, oxidative stress and PPARγ in diabetic rats. Food Chem Toxicol 138:111202. https://doi.org/10.1016/j.fct.2020.111202
Fricke W, Akhiyarova G, Wei W, Alexandersson E, Miller A, Kjellbom PO, Richardson A, Wojciechowski T, Schreiber L, Veselov D (2006) The short-term growth response to salt of the developing barley leaf. J Exp Bot 57(5):1079–1095. https://doi.org/10.1093/jxb/erj095
Gomes MAC, Suzuki MS, Cunha M, Tullii CF (2011) Effect of salt stress on nutrient concentration, photosynthetic pigments, proline and foliar morphology of Salvinia auriculata Aubl. Acta Limnol Bras 23:164–176. https://doi.org/10.1590/S2179-975X2011000200007
Grewal HS (2010) Water uptake, water use efficiency, plant growth and ionic balance of wheat, barley, canola and chickpea plants on a sodic vertosol with variable subsoil NaCl salinity. Agric Water Manag 97(1):148–156. https://doi.org/10.1016/j.agwat.2009.09.002
Grotewold E (2006) The genetics and biochemistry of floral pigments. Annu Rev Plant Biol 57(1):761–780. https://doi.org/10.1146/annurev.arplant.57.032905.105248
Hadiarto T, Tran L-SP (2011) Progress studies of drought-responsive genes in rice. Plant Cell Rep 30(3):297–310. https://doi.org/10.1007/s00299-010-0956-z
Hamani AKM, Wang G, Soothar MK, Shen X, Gao Y, Qiu R, Mehmood F (2020) Responses of leaf gas exchange attributes, photosynthetic pigments and antioxidant enzymes in NaCl-stressed cotton (Gossypium hirsutum L.) seedlings to exogenous glycine betaine and salicylic acid. BMC Plant Biol 20(1):1–14. https://doi.org/10.1186/s12870-020-02624-9
Hanafy RS, Sadak MS (2023) Foliar spray of stigmasterol regulates physiological processes and antioxidant mechanisms to improve yield and quality of sunflower under drought stress. J Soil Sci Plant Nutr 23(2):2433–2450. https://doi.org/10.1007/s42729-023-01197-4
Harborne J (1973) Phytochemical methods chapman and Hall. Ltd London 4:49–188
Haroun S (2002) Fenugreek growth and metabolism in response to gibberellic acid and seawater. Bulletin of The Faculty of Science, Assiut Univ(Egypt)
Haroun SA (1981) Studies on pigmentation and metabolism in leaves. . MS Thesis,. Botany Department, Faculty of Science - Mansoura University, Egypt
Haroun SA (1985) Studies on adaptation of plants to water stress. PhD Thesis,. Botany Department, Faculty of Science - Mansoura University, Egypt
He T, Cramer GR (1996) Abscisic acid concentrations are correlated with leaf area reductions in two salt-stressed rapid-cycling Brassica species. Plant Soil 179(1):25–33. https://doi.org/10.1007/BF00011639
Hessini K, Martínez JP, Gandour M, Albouchi A, Soltani A, Abdelly C (2009) Effect of water stress on growth, osmotic adjustment, cell wall elasticity and water-use efficiency in Spartina alterniflora. Environ Exp Bot 67(2):312–319. https://doi.org/10.1016/j.envexpbot.2009.06.010
Hichem H, Mounir D (2009) Differential responses of two maize (Zea mays L.) varieties to salt stress: changes on polyphenols composition of foliage and oxidative damages. Ind Crops Prod 30(1):144–151. https://doi.org/10.1016/j.indcrop.2009.03.003
Izzo R, Incerti A, Bertolla C (2008) Seawater irrigation: Effects on growth and nutrient uptake of sunflower plants Biosaline agriculture and high salinity tolerance. Springer, pp 61–69. https://doi.org/10.1007/978-3-7643-8554-5_6
Jaschke WD, Peuke AD, Pate JS, Hartung W (1997) Transport, synthesis and catabolism of abscisic acid (ABA) in intact plants of castor bean (Ricinus communis L.) under phosphate deficiency and moderate salinity. J Exp Bot 48(9):1737–1747. https://doi.org/10.1093/jxb/48.9.1737
Jung J-H, Park C-M (2011) Auxin modulation of salt stress signaling in Arabidopsis seed germination. Plant Signal Behav 6(8):1198–1200. https://doi.org/10.4161/psb.6.8.15792
Kader MA, Lindberg S (2005) Uptake of sodium in protoplasts of salt-sensitive and salt-tolerant cultivars of rice, Oryza sativa L. determined by the fluorescent dye SBFI. J Exp Bot 56(422):3149–3158. https://doi.org/10.1093/jxb/eri312
Karimi H, Yusef-Zadeh H (2013) The effect of salinity level on the morphological and physiological traits of two grape (Vitis vinifera L.) cultivars. Inter J Agro Plant Prod 4(5):1108–1117
Karnovsky M (1965) A formaldehyde-glutaradehyde fixation of high osmolality for use in electron microscopy. J Cell Biol 27:61–65
Khalil AAK, Zeb F, Khan R, Shah SA, Küpeli Akkol E, Khan IN, Khan J, Babar Jamal S, Khuda F, Haider A (2022) An overview on Rumex dentatus L.: Its functions as a source of nutrient and health-promoting plant. Evidence-Based Compl Alternative Med 2022. https://doi.org/10.1155/2022/8649119
Khan A, Ali S, Murad W, Hayat K, Siraj S, Jawad M, Khan RA, Uddin J, Al-Harrasi A, Khan A (2021) Phytochemical and pharmacological uses of medicinal plants to treat cancer: a case study from Khyber Pakhtunkhwa. North Pakistan J Ethnopharmacol 281:114437. https://doi.org/10.1016/j.jep.2021.114437
Khan I, Muhammad A, Chattha MU, Skalicky M, Bilal Chattha M, Ahsin Ayub M, Rizwan Anwar M, Soufan W, Hassan MU, Rahman MA (2022) Mitigation of salinity-induced oxidative damage, growth, and yield reduction in fine rice by sugarcane press mud application. Front Plant Sci 13:840900. https://doi.org/10.3389/fpls.2022.840900
Khan MA, Ungar IA, Showalter AM (2000) Effects of salinity on growth, water relations and ion accumulation of the subtropical perennial halophyte. Atriplex griffithii var stocksii Ann Bot 85(2):225–232. https://doi.org/10.1006/anbo.1999.1022
Khatana C, Saini NK, Chakrabarti S, Saini V, Sharma A, Saini RV, Saini AK (2020) Mechanistic insights into the oxidized low-density lipoprotein-induced atherosclerosis. Oxidative Med Cell Longev 2020. https://doi.org/10.1155/2020/5245308
Kong L, Wang M, Bi D (2005) Selenium modulates the activities of antioxidant enzymes, osmotic homeostasis and promotes the growth of sorrel seedlings under salt stress. Plant Growth Regul 45:155–163. https://doi.org/10.1007/s10725-005-1893-7
Levene H (1960) Robust tests for equality of variances. Contrib Prob Stat:278–292
Li J, Liao J, Guan M, Wang E, Zhang J (2012) Salt tolerance of Hibiscus hamabo seedlings: a candidate halophyte for reclamation areas. Acta Physiol Plant 34:1747–1755. https://doi.org/10.1007/s11738-012-0971-5
Lutts S, Kinet J, Bouharmont J (1996) NaCl-induced senescence in leaves of rice (Oryza sativaL.) cultivars differing in salinity resistance. Ann Bot 78(3):389–398. https://doi.org/10.1006/anbo.1996.0134
Mahboobeh R, Akbar EA (2013) Effect of salinity on growth, chlorophyll, carbohydrate and protein contents of transgenic Nicotiana Plumbaginifolia over expressing P5CS gene. J Env Res Manag 4:0163–0170. https://doi.org/10.1007/s11738-010-0696-2
Mahmoudi H, Kaddour R, Huang J, Nasri N, Olfa B, M’Rah S, Hannoufa A, Lachaâl M, Ouerghi Z (2011) Varied tolerance to NaCl salinity is related to biochemical changes in two contrasting lettuce genotypes. Acta Physiol Plant 33(5):1613–1622. https://doi.org/10.1007/s11738-010-0696-2
Mansour MMF, Salama KH (2004) Cellular basis of salinity tolerance in plants. Environ Exp Bot 52(2):113–122. https://doi.org/10.1016/j.envexpbot.2004.01.009
Margesin R, Schinner F (2005) Manual for soil analysis-monitoring and assessing soil bioremediation, vol 5. Springer Sci Business Media. https://doi.org/10.1007/3-540-28904-6
Maricle BR, Koteyeva NK, Voznesenskaya EV, Thomasson JR, Edwards GE (2009) Diversity in leaf anatomy, and stomatal distribution and conductance, between salt marsh and freshwater species in the C4 genus Spartina (Poaceae). New Phytol 184(1):216–233. https://doi.org/10.1111/j.1469-8137.2009.02903.x
Misra N, Gupta AK (2006) Effect of salinity and different nitrogen sources on the activity of antioxidant enzymes and indole alkaloid content in Catharanthus roseus seedlings. J Plant Physiol 163(1):11–18. https://doi.org/10.1016/j.jplph.2005.02.011
Mittova V, Tal M, Volokita M, Guy M (2003) Up-regulation of the leaf mitochondrial and peroxisomal antioxidative systems in response to salt-induced oxidative stress in the wild salt-tolerant tomato species Lycopersicon pennellii. Plant Cell Environ 26(6):845–856. https://doi.org/10.1046/j.1365-3040.2003.01016.x
Mogazy AM, Hanafy RS (2022) Foliar spray of biosynthesized zinc oxide nanoparticles alleviate salinity stress effect on Vicia faba plants. J Soil Sci Plant Nutr 22(2):2647–2662. https://doi.org/10.1007/s42729-022-00833-9
Mostafa H, Elbakry A, Eman AA (2011) Evaluation of antibacterial and antioxidant activities of different plant parts of Rumex vesicarius L.(Polygonaceae). Int. J Pharm Pharm Sci 3(2):109–118
Munir A, Youssef FS, Ishtiaq S, Kamran SH, Sirwi A, Ahmed SA, Ashour ML, Elhady SS (2021) Malva parviflora leaves mucilage: an eco-friendly and sustainable biopolymer with antioxidant properties. Polymers 13(23):4251. https://doi.org/10.3390/polym13234251
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651. https://doi.org/10.1146/annurev.arplant.59.032607.092911
Mushtaq Z, Faizan S, Gulzar B (2020) Salt stress, its impacts on plants and the strategies plants are employing against it: a review. J Appl Biol Biotechnol 8(3):8–1. https://doi.org/10.7324/JABB.2020.80315
Noor M, Fan J-B, Zhang J-X, Zhang C-J, Sun S-N, Gan L, Yan X-B (2023) Bermudagrass responses and tolerance to salt stress by the physiological, molecular mechanisms and proteomic perspectives of salinity adaptation. Agronomy 13(1):174. https://doi.org/10.3390/agronomy13010174
Ozgur R, Uzilday B, Sekmen AH, Turkan I (2013) Reactive oxygen species regulation and antioxidant defence in halophytes. Funct Plant Biol 40(9):832–847. https://doi.org/10.1071/FP12389
Page A, Miller R, Keeney D (1982) Methods of soil analysis. Part 2. Chemical and microbiological properties 2nd ed. American Soc of Agronomy, Inc Soil Sci Soc America, Inc Madison, Wisconsin, USA:1159
Paolocci F, Bovone T, Tosti N, Arcioni S, Damiani F (2005) Light and an exogenous transcription factor qualitatively and quantitatively affect the biosynthetic pathway of condensed tannins in Lotus corniculatus leaves. J Exp Bot 56(414):1093–1103. https://doi.org/10.1093/jxb/eri101
Pecherina A, Grinberg M, Ageyeva M, Zanegina D, Akinchits E, Brilkina A, Vodeneev V (2023) Salt-induced changes in cytosolic pH and photosynthesis in tobacco and potato leaves. Int J Mol Sci 24(1):491. https://doi.org/10.3390/ijms24010491
Posmyk M, Kontek R, Janas K (2009) Antioxidant enzymes activity and phenolic compounds content in red cabbage seedlings exposed to copper stress. Ecotoxicol Envir Safety 72(2):596–602. https://doi.org/10.1016/j.ecoenv.2008.04.024
Radic SR-S, Marijana P-KB (2006) Influence of NaCl and mannitol on peroxidase activity and lipid peroxidation in Centaurea ragusina L. roots and shoots. J Plant Physiol 163(12):1284–1292. https://doi.org/10.1016/j.jplph.2005.08.019
Rady M, Sadak MS, El-Bassiouny H, El-Monem A (2011) Alleviation the adverse effects of salinity stress in sunflower cultivars using nicotinamide and α-tocopherol. Aust J Basic Appl Sci 5(10):342–355
Rady MM, Talaat NB, Abdelhamid MT, Shawky BT, Desoky E-SM (2019) Maize (Zea mays L.) grains extract mitigates the deleterious effects of salt stress on common bean (Phaseolus vulgaris L.) growth and physiology. J HortSci Biotechnol 94(6):777–789. https://doi.org/10.1080/14620316.2019.1626773
Ragaey MM, Sadak MS, Dawood MF, Mousa NH, Hanafy RS, Latef AAHA (2022) Role of signaling molecules sodium nitroprusside and arginine in alleviating salt-Induced oxidative stress in wheat. Plants 11(14):1786. https://doi.org/10.3390/plants11141786
Raghavendra AS, Gonugunta VK, Christmann A, Grill E (2010) ABA perception and signalling. Trends Plant Sci 15(7):395–401. https://doi.org/10.1016/j.tplants.2010.04.006
Rahman K, Ahmed N, Raihan MRH, Nowroz F, Jannat F, Rahman M, Hasanuzzaman M (2021) Jute responses and tolerance to abiotic stress: mechanisms and approaches. Plants 10(8):1595. https://doi.org/10.3390/plants10081595
Rasouli F, Kiani-Pouya A, Tahir A, Shabala L, Chen Z, Shabala S (2021) A comparative analysis of stomatal traits and photosynthetic responses in closely related halophytic and glycophytic species under saline conditions. Environ Exp Bot 181:104300. https://doi.org/10.1016/j.envexpbot.2020.104300
Rehan M, Shafiullah AFA, Singh O (2020) Isolation, identification, antibacterial activity and docking of fatty acid and fatty alcohol from Rumex dentatus leaf extract. Int J Pharm Sci Rev Res 64(1):7–11. https://doi.org/10.47583/ijpsrr.2020.v64i01.002
Rygol J, Zimmermann U (1990) Radial and axial turgor pressure measurements in individual root cells of Mesembryanthemum crystallinum grown under various saline conditions. Plant Cell Environ 13(1):15–26. https://doi.org/10.1111/j.1365-3040.1990.tb01295.x
Sabagh AE, Hossain A, Barutçular C, Islam MS, Ratnasekera D, Kumar N, Meena RS, Gharib HS, Saneoka H, da Silva JAT (2019) Drought and salinity stress management for higher and sustainable canola ('Brassica napus' L.) production: a critical review. Australian. J Crop Sci 13(1):88–96
Sadak M (2022) Biochemical responses of white termis to pyridoxine and Mycorrhizae treatment under salinity stress. Egypt J Chem 65(10):1–2. https://doi.org/10.21608/ejchem.2022.118032.5319
Sadak MS (2023) Physiological role of Arbuscular Mycorrhizae and vitamin B1 on productivity and physio-biochemical traits of white lupine (Lupinus termis L.) under salt stress. Gesu Pfl 1-12. https://doi.org/10.1007/s10343-023-00855-y
Sadasivam S, Manickam A (1996) Biochemical method, 2nd edn. New Age Int Pvt Ltd. . Pub and TN Agricultural Univ, Coimbagore
Selvakumar G, Kim K, Hu S, Sa T (2014) Effect of salinity on plants and the role of arbuscular mycorrhizal fungi and plant growth-promoting rhizobacteria in alleviation of salt stress Physiological mechanisms and adaptation strategies in plants under changing environment. Springer, pp 115–144. https://doi.org/10.1007/978-1-4614-8591-9_6
Shahid MA, Sarkhosh A, Khan N, Balal RM, Ali S, Rossi L, Gómez C, Mattson N, Nasim W, Garcia-Sanchez F (2020) Insights into the physiological and biochemical impacts of salt stress on plant growth and development. Agronomy 10(7):938. https://doi.org/10.3390/agronomy10070938
Sharaf AEM, Farghal II, Sofy MR (2009) Response of broad bean and lupin plants to foliar treatment with boron and zinc. Aust J Basic Applied Sci 3(3):2226–2231
Shindy WW, Smith OE (1975) Identification of plant hormones from cotton ovules. Plant Physiol 55(3):550–554. https://doi.org/10.1104/pp.55.3.550
Shu DY, Chaudhary S, Cho K-S, Lennikov A, Miller WP, Thorn DC, Yang M, McKay TB (2023) Role of oxidative stress in ocular diseases: a balancing act. Metabolites 13(2):187. https://doi.org/10.3390/metabo13020187
Siji S, Nandini P (2017) Chemical and nutrient composition of selected banana varieties of Kerala. Int J Adv Eng Manag Sci 3(4):239829. https://doi.org/10.24001/ijaems.3.4.21
Singh A (2018) Alternative management options for irrigation-induced salinization and waterlogging under different climatic conditions. Ecol Indic 90:184–192. https://doi.org/10.1016/j.ecolind.2018.03.014
Smirnoff N (1993) Tansley Review No. 52. The role of active oxygen in the response of plants to water deficit and desiccation. New Phytolo:27–58. https://doi.org/10.1111/j.1469-8137.1993.tb03863.x
Sofy MR, Mancy AG, Alnaggar AEM, Refaey EE, Mohamed HI, Elnosary ME, Sofy AR (2022) A polishing the harmful effects of Broad Bean Mottle Virus infecting broad bean plants by enhancing the immunity using different potassium concentrations. Not Bot Horti Agro Cluj-Nap 50(1):12654–12654. https://doi.org/10.15835/nbha50112654
Starlin T, Gopalakrishnan VK (2013) Enzymatic and non-enzymatic antioxidant properties of Tylophora pauciflora Wight and Arn.–an in vitro study. Asian J Pharm Clin Res 6(4):68–71
Sun W, Shahrajabian MH (2023) Therapeutic potential of phenolic compounds in medicinal plants—natural health products for human health. Molecules 28(4):1845. https://doi.org/10.3390/molecules28041845
Szepesi Á, Szőllősi R (2018) Mechanism of proline biosynthesis and role of proline metabolism enzymes under environmental stress in plants Plant metabolites and regulation under environmental stress. Elsevier, pp 337–353. https://doi.org/10.1016/B978-0-12-812689-9.00017-0
Tattini M, Landi M, Brunetti C, Giordano C, Remorini D, Gould KS, Guidi L (2014) Epidermal coumaroyl anthocyanins protect sweet basil against excess light stress: multiple consequences of light attenuation. Physiol Plant 152(3):585–598. https://doi.org/10.1111/ppl.12201
Tiago O, Maicon N, Ivan RC, Diego NF, Vincius JS, Mauricio F, Alan JP, Velci QS (2017) Plant secondary metabolites and its dynamical systems of induction in response to environmental factors: a review. Afr J Agric Res 12(2):71–84. https://doi.org/10.5897/AJAR2016.11677
Wang F, Yang S, Wei Y, Shi Q, Ding J (2021) Characterizing soil salinity at multiple depth using electromagnetic induction and remote sensing data with random forests: a case study in Tarim River Basin of southern Xinjiang. China Sci Total Environ 754:142030. https://doi.org/10.1016/j.scitotenv.2020.142030
Wellburn AR (1994) The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Physiol 144(3):307–313. https://doi.org/10.1016/S0176-1617(11)81192-2
Yan H, Shah SS, Zhao W, Liu F (2020) Variations in water relations, stomatal characteristics, and plant growth between quinoa and pea under salt-stress conditions. Pak J Bot 52(1):1–7. https://doi.org/10.30848/PJB2020-1(9)
Yang T, Poovaiah B (2002) Hydrogen peroxide homeostasis: activation of plant catalase by calcium/calmodulin. Proc Nat Acad Sci 99(6):4097–4102. https://doi.org/10.1073/pnas.052564899
Yasir TA, Khan A, Skalicky M, Wasaya A, Rehmani MIA, Sarwar N, Mubeen K, Aziz M, Hassan MM, Hassan FA (2021) Exogenous sodium nitroprusside mitigates salt stress in lentil (Lens culinaris medik.) by affecting the growth, yield, and biochemical properties. Molecules 26(9):2576. https://doi.org/10.3390/molecules26092576
Zahedi SM, Hosseini MS, Abadía J, Marjani M (2020) Melatonin foliar sprays elicit salinity stress tolerance and enhance fruit yield and quality in strawberry (Fragaria× ananassa Duch.). Plant Physiol Biochem 149:313–323. https://doi.org/10.1016/j.plaphy.2020.02.021
Ziouti A, El Modafar C, Fleuriet A, El Boustani E (1992) Les polyphenols, marqueurs potentiels de la resistance du palmier dattier (Phoenix dactylifera L.) au Fausarium oxysporum f. sp. albedinis. Bulletin De Liaison-Groupe Polyphenols 16:346–346
Zuluaga MYA, Monterisi S, Rouphael Y, Colla G, Lucini L, Cesco S, Pii Y (2023) Different vegetal protein hydrolysates distinctively alleviate salinity stress in vegetable crops: a case study on tomato and lettuce. Front Plant Sci 14:1077140. https://doi.org/10.3389/fpls.2023.1077140
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. AMK, SAH, AAN, GAE, OAE, AFA, and RMEG performed material preparation, data collection, and analysis. The first draft of the manuscript was written by AMK, SAH, AAN, GAE, OAE, MRS, AFA, and RMEG; all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethical Approval
Not applicable
Consent to Participate
Not applicable
Consent for Publication
Not applicable
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kazamel, A.M., Haroun, S.A., Noureldin, A.A. et al. Ultrastructural, Secondary Metabolite, and Antioxidant Modulation in Response to Salt-Affected Habitats Induced Oxidative Stress and Their Accumulation in Malva parviflora L. and Rumex dentatus L.. J Soil Sci Plant Nutr 24, 389–407 (2024). https://doi.org/10.1007/s42729-023-01550-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-023-01550-7