Abstract
Bacteria isolated from bioaerosols emitted by a clarifier in a wastewater treatment plant (Dobre Miasto, Kosyń, Poland) were analyzed. A total of 27 morphologically different bacterial colonies were isolated, and 14 strains antagonistic towards Fusarium culmorum in vitro were selected for analysis. Most of the analyzed bacteria did not increase the germination capacity or the height of wheat seedlings. The only exception was strain PSDM20 which was characterized by multiple plant growth–promoting properties, but also by the lowest metabolic activity and lowest substrate assimilation. Strain PSDM16 deteriorated the status of wheat seedlings. Bacterial strains PSDM3, PSDM6, PSDM10, PSDM13, PSDM14, PSDM17, and PSDM20 prevented the deterioration of the biometric parameters of wheat seedlings exposed to F. culmorum and F. graminearum. Strains PSDM3, PSDM6, PSDM10, and PSDM17 most effectively protected wheat seedlings against infections caused by the above pathogens. Strain PSDM6 produced indole acetic acid (IAA), and it significantly contributed to plant elongation. Correlations were not observed between the growth-promoting properties, metabolic activity, and fungistatic properties of the evaluated bacteria. Pseudomonas putida PSDM3, Proteus penneri PSDM6, Enterobacter hormaechei PSDM10, and Advenella sp. PSDM17 were most effective in limiting the spread of Fusarium spp. infections in spring wheat, and they can be used as biological fungicides. The results of this study indicate that bacteria isolated from non-agricultural ecosystems are capable of protecting and fertilizing crops. The growth-promoting properties of bacterial strains of the genus Proteus are comparable with those of the widely investigated Pseudomonas spp. strains.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Continuous population growth and decreasing availability of farmland necessitate intensive crop farming. The yield potential of crops can be increased by developing new varieties; applying effective fertilizer treatments; and, if possible, minimizing the adverse effects of abiotic (temperature, light availability, soil quality) and biotic factors, mostly phytopathogens (viral, bacterial, and fungal), pests, and weeds. Massive quantities of crop protection agents, mostly mineral fertilizers, fungicides, insecticides, and herbicides, are applied on the global scale. These products can adversely influence agricultural ecosystems, increase pathogen resistance to active ingredients, eliminate beneficial microorganisms, and decrease soil fertility. Biological fertilizers offer a safer alternative to chemical agents. Biofertilizers improve crop yields and quality; they suppress the influence of harmful microorganisms and, above all, exert less toxic effects on the environment than chemical products. Bacterial biopreparations are among the most popular biological crop protection products (Compant et al. 2005; Bonilla et al. 2012; Robačer et al. 2016; Liu et al. 2018).
Bacterial communities that enhance plant growth are known as plant growth–promoting bacteria (PGPB). They exert beneficial effects on plants by inhibiting the growth of pathogenic microorganisms, limiting the spread of pathogens in ecological niches, increasing the availability of nutrients, synthesizing growth-promoting substances, increasing plant resistance to abiotic stress, and inducing plant defense responses. Plant growth–promoting bacteria exert indirect effects by eliminating pathogens and enhancing plant growth, and their effectiveness is determined by the rate at which they colonize a given niche (soil or plant). Beneficial microorganisms exert direct effects by producing phytohormones (such as IAA), solubilizing mineral phosphorus with the involvement of organic acids which are the products of bacterial metabolism, fixing nitrogen, ammonifying organic compounds, producing chelating compounds (such as siderophores), enzymes which improve the biochemical properties of the soil solution (phosphatases, urease, dehydrogenase, lipase, and protease), and antimicrobial compounds (Compant et al. 2005; Gray and Smith 2005; Ahmed and Kibret 2014).
Plant growth–promoting rhizobacteria (PGPR) are the most widely researched group of PGPB. Rhizobacteria colonize plants, the rhizosphere, and rhizoplane and enhance plant growth. Selected PGPR enter plant roots and create new populations. Many of them penetrate root endodermal cells, cortex cells, and vascular tissues, and they form communities of endophytes in stems, leaves, tubers, and other plant organs. Some PGPR convert tryptophan to IAA and improve the health status of seedlings, increase root mass and the availability of soil nutrients for plants (Compant et al. 2005; Cummings 2009; Yang et al. 2009; Beneduzi et al. 2012).
Fungi of the genus Fusarium (in particular F. culmorum, F. oxysporum, F. graminearum, F. moniliforme, F. pseudograminearum, F. sambucinum, F. solani) are among the most toxic pathogens of germinating plants. In early stages of growth, seedlings are usually infected by fungal pathogens which colonize soil or seeds. The spread of soil-borne infections can be controlled by seed dressing with synthetic fungicides which improve plant health during the growing season. However, synthetic fungicides target specific fungi and are not effective against pathogens resistant to a given active ingredient (Saremi et al. 2011; Przemieniecki et al. 2014a, b; Jadon et al. 2015). The search for new methods of controlling soil-borne pathogen continues, and recent research has revealed that PGPB can effectively prevent crop infections without exerting a negative impact on the environment (Compant et al. 2005; Sallam et al. 2013, Przemieniecki et al. 2015, 2017). The following bacterial genera enhance plant growth and are suitable for biological crop protection: Azotobacter, Bacillus, Beijerinckia, Burkholderia, Enterobacter, Erwinia, Flavobacterium, Microbacterium, Pseudomonas, Rhizobium, and Serratia. The above list is likely to be expanded as new advancements are made in research (Mendes et al. 2012; Ahmed and Kibret 2014). There is growing evidence that bacteria which do not naturally colonize plants are highly effective in enhancing plant growth and inhibiting the growth of phytopathogens.
In this study, bacteria were isolated from an anthropogenic environment, and their ability to promote plant growth, act as a growth biostimulant and inhibit the development Fusarium sp. pathogens on spring wheat during germination, was evaluated. The effect of IAA-producing bacteria on winter oilseed rape seedlings (used for biometric measurements) was analyzed. The aim of the study was to determine whether non-rhizosphere bacteria can be effectively used as biological fertilizers and crop protection agents.
2 Materials and Methods
Bacteria were sampled from the wastewater treatment plant in Dobre Miasto (Region of Warmia and Mazury, Poland; 54° 00′ 05.5″ N, 20° 23′ 54.8″ E). Bioaerosol emissions from a wastewater clarifier were sampled with the use of a microbial air sampler kit (Merck, Germany) and plated on King’s B medium (without antibiotics). The plates were incubated at 37 °C for 48 h in the laboratory.
2.1 Bacterial Identification
Selected bacteria were identified by comparing a fragment of the 16S rDNA sequence (Lane 1991) with the reference sequences in GenBank (NCBI) with the use of the BLAST algorithm. The applied PCR and sequencing protocols were described in a previous study (Przemieniecki et al. 2016). A phylogenetic analysis of the evaluated strains was performed in the MEGA5.2 program (Kumar et al. 2008). All sequences were deposited in GenBank under accession numbers MG722771-MG722784.
2.2 Evaluation of the Properties of Plant Growth–Promoting Bacteria
Every isolated bacterial colony was characterized by different morphological properties. Bacterial isolates were analyzed to determine their antagonistic activity against pathogenic fungi (Fusarium culmorum, F. graminearum), their ability to solubilize Ca2(PO)4, degrade cellulose, ammonify organic compounds, produce lipases, proteases, siderophores, hydrogen cyanide, and indole acetic acid (IAA).
The biochemical properties of bacterial strains were analyzed with API® 20NE and API® ZYM kits (Biomerieux, France) according to the manufacturer’s recommendations. Both assays were carried out at a temperature of 28 °C. API® 20NE was incubated for 24 h, and API® ZYM for 4 h and 30 min.
2.2.1 Cellulose Degradation
The cellulose-degrading ability of the tested strains was determined by placing an overnight culture on a medium containing 0.7 g of KH2PO4 l−1, 0.3 g of K2HPO4 l−1, 0.25 g of MgSO4 l−1, 0.2 g of yeast extract, 2 g of cellulose powder l−1, 2 g of gelatin l−1, 0.2 g of yeast extract, and 15 g of agar l−1. After 48 h of incubation at 28 °C for 48 h, the cultures were rinsed for 10 min with 1% solution of Congo red dye which binds to bacterial lipopolysaccharides. The solution was removed, and the diameter of the clear zone around the colony (cellulose degradation) was measured (Lu et al. 2004).
2.2.2 Production of Indole Acetic Acid
An overnight bacterial culture was transferred to 100 ml of nutrient broth (Merck, Germany) enriched with l-tryptophan (50 mg L−1). After 48 h of incubation at 28 °C, the resulting suspension was transferred to a 15-ml Falcon tube and centrifuged (6000 rpm, 15 min), and 1 ml of clear liquid above the suspension was transferred to a fresh 15-ml tube. One drop of orthophosphoric acid and 2 ml of Salkovsky’s reagent were added (35% perchloric acid + 1 ml of 0.5 M FeCl3 in 50 ml). Red-stained samples (positive result) were incubated for 30 min and analyzed in a spectrophotometer at 535 nm against control (1 ml of nutrient broth with one drop of orthophosphoric acid and 2 ml of Salkovsky’s reagent). The results were compared against the standard curve for IAA (Mohite 2013).
2.2.3 Phosphate Solubilizing Bacteria
Phosphate-solubilizing bacteria (PSB) were identified on Pikovskaya’s medium containing 10 g of glucose, 2.5 g of Ca3(PO4)2, 0.5 g of (NH4)2SO4, 0.2 g of NaCl, 0.1 g of MgSO4 7H2O, 0.2 g of KCl, 0.5 g of yeast extract, 0.002 g of FeSO4 7H2O, 0.002 g of MnSO4 7H2O, and 15 g of agar (Nautiyal 1999). Bacteria from an overnight culture were spotted on a medium. After 7 days of incubation at 28 °C, PSB were identified based on the presence of clear zones around bacterial cultures.
2.2.4 Hydrogen Cyanide Production
The tested bacteria’s ability to produce HCN was determined by transferring 100 μl of an overnight culture to tryptic soy agar (TSA, Merck, Germany) containing 4.4% glycine. Filter paper rinsed in a mixture of 2% sodium carbonate and 0.5% picric acid was attached to the inner side of the lid covering a Petri plate, and the plate was wrapped in parafilm. After 4 days of incubation at 28 °C, the presence of HCN was confirmed in plates where filter paper changed color from yellow to brown (Bakker and Schipper 1987).
The results were expressed on a scale of 0 to 4, where 0 denoted the absence of HCN (yellow color) and 4 denoted the presence of strain DEPMD-PS1 which is capable of producing HCN (dark brown discoloration).
2.2.5 Ammonia Production
The tested bacteria’s ability to produce ammonia was determined by adding 10 ml of peptone water (10 g of peptone l−1, 5 g of NaCl l−1) to 100 ml of an overnight culture. The presence of ammonia was detected with the use of Nessler’s reagent (0.5 ml) (Przemieniecki et al. 2015). After 3 days of incubation at 28 °C, cultures whose color changed from yellow to dark yellow or brown were regarded as capable of producing ammonia. The intensity of discoloration was expressed on a scale of 0 to 4.
2.2.6 Lipase Production
An overnight culture was incubated on a selective medium containing 10 g of peptone, 0.1 g of CaCl2, 5 g of NaCl, 15 g of agar, and 10 ml of Tween 20. All bacteria were streaked on the medium and incubated at 28 °C for 48 h. Lipase activity was determined based on the presence of depositions around bacterial colonies. Bacterial ability to produce lipase was evaluated on a scale of 0 to 4 (0—no lipase production, 1—low production, 2—moderate production, 3—high production, 4—very high production) (Ghodsalavi et al. 2013).
2.2.7 Protease Production
An overnight bacterial culture was transferred to a selective medium containing 15 g of skim milk, 0.5 g of yeast extract, and 9.2 g of agar. The bacteria were spotted on plates of SMA medium and incubated at 27 °C for 48 h. Their ability to produce protease was determined by measuring the diameter of clear zones around bacterial colonies (Ghodsalavi et al. 2013).
2.3 Inhibition of Fusarium spp.
2.3.1 Dual-Culture Analysis
In order to determine the usefulness of isolated bacteria, the in vitro antagonism of the microbial culture was evaluated. Ten microliters of an overnight bacterial culture was plated on a potato dextrose agar (PDA) on the opposite edges of the dish (3 cm from the center). A mycelial disc with a diameter of 5 mm was placed in the center of the plate. In the control sample, the bacterial suspension was replaced with sterile distilled water. The plates were incubated for 5 days at 28 °C. The bacteria’s antagonistic activity against Fusarium culmorum was determined.
2.3.2 Seed Inoculation
Spring wheat (cv. Bombona) kernels were inoculated with selected bacterial strains. Fifty kernels were plated on 1% water agar:
-
a)
Treatment I—containing F. culmorum and F. graminearum spores at a concentration of one·105 ml−1,
-
b)
Treatment II—containing sterile carboxymethyl cellulose (CMC).
After 7 days, the germination capacity of seeds, the height of wheat seedlings, and the severity of fungal infection were evaluated on a scale of 0 to 5 (0—no infection, 5—necrotized plant).
2.4 The Influence of IAA-Producing Bacteria on the Height of Winter Oilseed Rape Seedlings
Bacterial strains PSDM6 and PSDM7 characterized by the highest IAA-producing (Indole-3-acetic acid) ability were cultured overnight, and cell suspensions with a concentration of 5 108 CFU were prepared in 1% sterile CMC. Before the application of the bacterial suspension, winter oilseed rape seeds (cv. Adriana) were disinfected with 70% ethyl alcohol for 1 min, 1% sodium hypochlorite for 1 min and rinsed three times in sterile deionized water. Sterile 1% CMC without bacteria was the control suspension. Seeds were placed in containers with 50 ml of 1% water agar and incubated for 14 days at 80% humidity with a 12-h light (25 °C) and 12-h dark (18 °C) cycle. Seedlings were placed on a millimeter paper, photographed, and measured from the root to cotyledons. Every treatment consisted of three separate containers filled with 10 seeds each.
2.5 Statistical Analysis
Data were processed with the use of Duncan’s test (ANOVA) at p = 0.05 in the Statistica 12 (Dell) program. The protocol for measuring yeast growth inhibition zones was described by Przemieniecki et al. (2014a, b).
3 Results
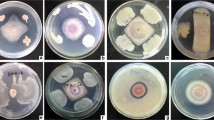
A total of 27 morphologically different (typical and not typical of Pseudomonadaceae) bacterial colonies were isolated from the cultures. In this group, 14 strains inhibited the growth of F. culmorum mycelia by more than 50%. Pseudomonas putida strain PSDM3 was most effective in inhibiting fungal growth (Table 1).
An analysis of bacterial properties revealed that Pseudomonas putida PSDM3, Enterobacter sp. PSDM16, Advenella sp. PSDM17, and Proteus sp. PSDM21 produced hydrogen cyanide, Staphylococcus sp. PSDM15 demonstrated both proteolytic and lipolytic activity, and Proteus sp. PSDM21 demonstrated lipolytic activity. Proteus sp. PSDM7 was the only strain incapable of solubilizing phosphorus. Staphylococcus pasteuri PSDM20 and Proteus sp. PSDM21 degraded cellulose (Table 2). Most bacterial strains were successfully cultured on both acidic and alkaline media. Proteus penneri PSDM6 and Staphylococcus sp. PSDM15 were the only strains that did not proliferate well at pH 5. With the exception of Enterobacter sp. PSDM16, all strains were highly tolerant of salinity stress up to 3%. Furthermore both strains Proteus spp. PSDM6 and PSDM7 produced auxin (IAA) that had a positive effect on plant growth (Table 2, Fig. 1).
PSDM strains 6 and 7 were characterized by the highest metabolic diversity (7 properties). PSDM strains 6, 7, 10, 12, 13, 15, 17, and 21 were capable of assimilating the highest number of substrates (minimum 10 properties). Two of the tested bacterial strains, Proteus penneri PSDM6 and Proteus sp. PSDM7, produced indole acetic acid (Table 3).
Spring wheat kernels inoculated with Proteus peneri PSDM21 were characterized by significantly lower germination capacity than control, and the average number of germinated kernels inoculated with Enterobacter sp. PSDM16 was significantly lowest in the group of the tested strains. The remaining bacterial strains did not significantly influence the number of germinated wheat kernels (Fig. 2).
The inoculation of spring wheat kernels with Staphylococcus pasteuri PSDM20 produced significantly tallest seedlings (around 6.5 cm). Seed inoculation with Enterobacter sp. PSDM16 produced the shortest seedlings. In the remaining cases, significant differences were not observed relative to control. Seed inoculation with strains PSDM3, PSDM9, PSDM10, PSDM12, PSDM14, PSDM17, and PSDM21 led to a minor and non-significant increase in plant height (around 6 cm) (Fig. 3).
Seed germination was estimated at 76–95%. In treatments infected with F. culmorum, germination capacity was significantly reduced by more than 10% relative to control. Strains PSDM3, PSDM6, PSDM10, PSDM13, PSDM14, PSDM17, and PSDM20 significantly inhibited the adverse effect of F. culmorum on germinating kernels, whereas the remaining bacterial strains did not significantly influence seed germination. The germination capacity of kernels inoculated with F. graminearum was estimated at 93% and did not differ from control. Strain PSDM16 significantly decreased the number of germinating kernels (Fig. 4).
Spring wheat seedlings differed in height. The height of control plants was determined at 3 cm, whereas the height of plants exposed to F. culmorum was significantly reduced (by around 1 cm, i.e., 30%). Strains PSDM3, PSDM6, PSDM8, PSDM9, PSDM10, PSDM13, PSDM14, PSDM17, PSDM20, and PSDM21 significantly minimized the pathogen’s negative influence on plant growth. Seed inoculation with F. graminearum had no negative effect on the height of wheat seedlings. In the above treatment, seed inoculation with strains PSDM3, PSDM9, and PSDM13 led to a significant increase in seedling height (by nearly 1 cm) relative to control (Fig. 4).
The severity of infection caused by each of the analyzed pathogenic species was highest in treatments with non-dressed kernels (F. culmorum—4 points, F. graminearum—2.7 points). Fusarium culmorum infections were more severe than F. culmorum infections, even when seeds were dressed with bacterial strains. Strains PSDM3, PSDM6, PSDM10, and PSDM17 most effectively suppressed the symptoms of F. culmorum infection (1.7–2.0). Strains PSDM12, PSDM13, PSDM14, and PSDM20 also significantly reduced (by around 1.5 points) the severity of infection in wheat plants. Twelve of the 14 tested bacterial suspensions were effective in reducing the symptoms of F. graminearum infection. The rate of infection after treatment with effective bacteria was estimated at 1.2 points (decrease by more than 50%; Fig. 5).
4 Discussion
Bacterial antagonists of fungal pathogens are generally isolated from plant habitats. However, some studies demonstrated that the microorganisms isolated from other ecological niches, such as water environments, also effectively inhibited the growth of pathogens and promoted plant growth (Goswami et al. 2013; Przemieniecki et al. 2015).
In our study, we tested a total of 27 morphologically varied bacterial colonies derived from the bioaerosol emissions of a wastewater clarifier. Various bacterial properties were investigated in those strains to determine the presence of correlations between tolerance to environmental stressors and the ability to inhibit the growth of Fusarium pathogens. Most of the tested bacteria did not increase germination capacity or the height of wheat seedlings; however, more than half of the analyzed strains exhibited antagonistic activity against Fusarium culmorum in the dual-culture method. In addition, nearly all strains were tolerant of environmental stressors. They were capable of growth on both acidic and alkaline media; they tolerated salt stress up to 5% NaCl and assimilated various substrates as sources of energy and carbon (Table 3). Pseudomonas putida PSDM3, Enterobacter sp. PSDM16, Advenella sp. PSDM17, and Proteus sp. PSDM21 produced hydrogen cyanide and were characterized by the highest proteolytic activity, whereas Staphylococcus sp. PSDM15 demonstrated both proteolytic and lipolytic activity. Most bacterial strains were capable of growth on both acidic and alkaline media. Staphylococcus sp. PSDM15 was the only strain whose growth was inhibited at pH 5, whereas Lactococcus raffinolactis PSDM12 was successfully cultured on all growth media. An analysis of bacterial properties did not reveal any correlations between tolerance to environmental stressors and the ability to inhibit the growth of Fusarium pathogens.
All bacterial strains were capable of ammonification and, excluding PSDM7, of solubilizing phosphates. However, only some strains displayed the properties enabling the elimination of phytopathogens (activity of protease, lipase, β-glucosidase, and β-galactosidase). In those strains, enzyme production was not closely linked with the ability to suppress symptoms of infection in wheat seedlings. Strains PSDM3, PSDM6, PSDM10, and PSDM17 were most effective in inhibiting the growth of F. culmorum mycelia and minimizing the symptoms of infection on seedlings. Strain PSDM6 significantly increased the height of winter oilseed rape plants on a medium enriched with tryptophan by secreting indole acetic acid which promotes plant growth. Strain PSDM20 was less effective in inhibiting the growth of Fusarium spp. than the remaining antagonistic strains, which could be attributed to its lowest metabolic activity and lowest ability to assimilate substrates. The above strain was characterized by multiple plant growth–promoting properties in contrast to strain PSDM16 which had an adverse effect on plant health. Pseudomonas putida PSDM3, Proteus penneri PSDM6, Enterobacter cloacea PSDM10, Lactococcus raffinolactis PSDM12, Proteus penneri PSDM13, Staphylococcus hominis PSDM14, Advenella incernata PSDM17, Staphylococcus pasteuri PSDM20, and Proteus penneri PSDM21 improved the biometric parameters of wheat seedlings infected with F. culmorum by preventing fungal colonization, whereas Proteus penneri PSDM6, Proteus sp. PSDM7, Enterobacter cloacea PSDM10, Lactococcus raffinolactis PSDM12, Proteus penneri PSDM13, Staphylococcus hominis PSDM14, and Staphylococcus epidermis PSDM15 promoted the growth of seedlings infected with F. graminearum. Strains PSDM3, PSDM6, PSDM10, and PSDM17 were most effective in protecting seedlings against the analyzed pathogens. An analysis of winter oilseed rape seedlings treated with IAA-producing strains revealed a significant increase in plant height under the influence of strain PSDM6. Pseudomonas putida PSDM3, Proteus penneri PSDM6, Enterobacter hormaechei PSDM10, and Advenella sp. PSDM17 were most effective in inhibiting the colonization of spring wheat seedlings by Fusarium spp., and they can be used as biological fungicides. However, the example of Pseudomonas putida PSDM3, which was not characterized by high levels of metabolic or proteolytic activity, indicates that plant infections caused by Fusarium spp. were most effectively inhibited by other mechanisms of action than those analyzed in the present study.
The results of this study demonstrated that non-rhizosphere bacteria can significantly improve the properties of crop plants, which is in congruence with other studies. Pseudomonas OG isolated from sea water (Goswami et al. 2013) was capable of producing siderophores, HCN, IAA, and catalase; solubilizing phosphates; and producing ammonia. Inoculation of chickpea (Cicer arietinum L.) and green gram (Vigna radiata (L.) seeds with the above strain significantly improved the biometric parameters of germinating seedlings. Bacterial inoculation had the most stimulatory effect on the accumulation of dry matter which increased by more than 26% in both plants.
In another study, the plant growth–promoting properties of Pseudomonas luteola SP0113, isolated from a water environment (defunct water well), were confirmed (Przemieniecki et al. (2015). The above strain also strongly inhibited the growth of phytopathogens and was resistant to high glyphosate concentrations (Przemieniecki et al. 2017). The analyzed strain produced catalase and peroxidase and was capable of ammonifying organic compounds and solubilizing phosphates. It was highly resistant to environmental stressors and demonstrated antagonistic activity against Fusarium spp. and Monographella nivalis. The combination of Pseudomonas luteola SP0113 with a high glyphosate dose (recommended by the manufacturer for weed control) led to nearly complete inhibition of Fusarium fungi.
Examples of bacterial antagonists of fungal pathogens isolated from ecological niches other than plant habitats also include Pseudomonas fluorescens, P. luteola, and Bacillus brevis isolated from the rhizosphere of Physalis peruviana. Those strains effectively counteracted the spread of Fusarium oxysporum. Strain P. fluorescens B-3,4 significantly delayed the appearance of disease symptoms on P. peruviana plants (Urrea et al. 2011).
Przemieniecki et al. (2016) demonstrated that bacteria can be transferred from the rhizosphere of one plant species to that of another plant species without the loss of their plant growth-promoting properties. Bacteria isolated from the rhizosphere of rye (Serratia fonticola ART-8 and Pseudomonas putida ART-9) were characterized by multiple PGP traits, but Pseudomonas putida ART-9 possessed more PGP traits, it was capable of growth in a temperature range of 4 °C to 28 °C, it demonstrated cellulolytic, proteolytic and lipolytic activity, produced siderophores (pioverdin) and solubilized phosphates. Despite a wide range of PGP traits, neither strain was effective in inhibiting the growth of Fusarium mycelia in vitro. In a greenhouse experiment, the above strains increased the size of spring wheat spikes and 1000 kernel weight, but seed inoculation with both bacterial strains decreased plant height. Przemieniecki et al. (2018) demonstrated that Bacillus subtilis SP-A9 isolated from the rhizosphere of rye was highly effective in eliminating plant pathogens and promoting the growth of spring wheat plants. The analyzed strain was characterized by moderate metabolic activity and a moderate range of assimilated substrates, but it was capable of synthesizing several enzymes, including protease, esterase, lipase, β-glucosidase and β-galactosidase, which can potentially counteract the growth of phytopathogens. Bacillus subtilis SP-A9 was also highly tolerant of environmental stressors, including high salinity. In a greenhouse experiment, seed inoculation with Bacillus subtilis SP-A9 improved selected biometric parameters of wheat plants exposed to Fusarium culmorum and F. oxysporum. Wheat plants grown from seeds inoculated with Bacillus subtilis SP-A9 were characterized by a significantly higher number of spikes and higher grain yield, although no significant changes were observed in 1000 kernel weight. Plant height was also significantly decreased in the above treatment. The cited experiment demonstrated that beneficial bacteria do not have to be isolated from the rhizosphere of a specific plant species and can establish a symbiotic relationship with a variety of host plants.
Bacteria of the genus Bacillus produce antifungal antibiotics which inhibit the growth of Fusarium spp. pathogens. In a study by Zhao et al. (2014), the growth of Fusarium fungi was effectively inhibited by various bacteria. The most effective bacterial strain was Bacillus subitilis which suppressed the growth of F. culmorum. The analyzed bacterial strain produced antifungal antibiotics bacillomycin, fengycin, iturin A, surfactin, and bacilysin which had been previously described by Mora et al. (2011). Zhao et al. (2014) also demonstrated that Bacillus sp. significantly decreased the production of deoxynivalenol (DON) and was more effective in reducing the symptoms of Fusarium head blight under field conditions than carbendazim.
Bacteria producing IAA were isolated mainly from the rhizosphere in agricultural ecosystems. Kumar et al. (2010) analyzed the antagonistic activity of 80 rhizosphere bacteria (mostly Bacillus spp.) against Sclerotium rolfsii and Colletotrichum capsici. More than 21% of isolates demonstrating antagonistic activity against phytopathogenic fungi were capable of producing IAA (> 20 μg ml−1). The cited authors also observed that PGP traits such as siderophore production and phosphate solubilization were correlated with inhibition of mycelial growth. Karnwal (2009) reported that Pseudomonas fluorescens and P. aeruginosa isolated from the rhizosphere were capable of promoting plant growth. The above strains converted l-tryptophan to IAA whose concentration in inoculated rice seeds increased from 1.6 to around 2.2 pmol ml−1. The growth-promoting properties of Pseudomonas putida have been confirmed in other studies. The bacterial strain isolated by Hernández-Montiel et al. (2017) produced a high concentration of IAA (23 μg mL−1), which, in combination with other factors, contributed to an improvement in the biometric parameters of tomatoes as microcapsule fertilizer. In the current study, bacterial strains PSDM6 and PSDM7 produced IAA on a growth medium enriched with l-tryptophan in concentrations higher than 20 μl ml−1. The inoculation of winter oilseed rape seeds enhanced seedling growth relative to control; however, PSDM6 was the only bacterial strain capable of inducing a significant improvement. These results suggest that IAA production is not the only trait which promotes plant growth.
5 Conclusions
The results of our study indicate that PGPR isolated from agricultural ecosystems are not the only microorganisms capable of promoting plant growth and protecting crops against pathogens. Bacteria of the genera Pseudomonas, Proteus, Staphulococcus, and Advenella isolated from an environment subjected to high anthropogenic pressure (wastewater treatment plant) strongly inhibited the growth of Fusarium spp., but were characterized by fewer PGP traits. The above suggests that rhizosphere bacteria possess other antifungal mechanisms, such as the production of antibiotics. In contrast to rhizosphere bacteria, bacteria of the genera Proteus and Pseudomonas were most effective in the analyzed group of microbial communities. They were characterized by multiple PGP traits, and they effectively inhibited the growth of Fusarium pathogens. However, only bacteria of the genus Proteus produced IAA in amounts that were sufficient for the promotion of plant growth. Our findings suggest that non-rhizosphere PGPB can be effectively used as biological agents to stimulate plant growth and protect plants against pathogens.
References
Ahmed M, Kibret M (2014) Mechanisms and applications of plant growth promoting rhizobacteria: current perspective. J King Saud Univ - Sci 26:1–20
Bakker AW, Schipper B (1987) Microbial cyanide production in the rhizosphere in relation to potato yield reduction and Pseudomonas spp.-mediated plant growth-stimulation. Soil Biol Biochem 19:451–457
Beneduzi A, Ambrosini A, Passaglia LMP (2012) Plant growth-promoting rhizobacteria (PGPR): their potential as antagonists and biocontrol agents. Genet Mol Biol 34:1044–1051
Bonilla N, Gutiérrez-Barranquero JA, Vicente A, Cazorla FM (2012) Enhancing soil quality and plant health through suppressive organic amendments. Diversity 4:475–491
Compant S, Duffy B, Nowak J, Clément C, Ait Barka E (2005) Use of plant growth-promoting bacteria for biocontrol of plant diseases: principles, mechanisms of action, and future prospects. Appl Environ Microbiol 71:4951–4959
Cummings SP (2009) The application of plant growth promoting rhizobacteria (PGPR) in low input and organic cultivation of graminaceous crops; potential and problems. Environ. Biotechnol 5:43–50
Ghodsalavi B, Ahmadzadeh M, Soleimani M, Madloo PB, Taghizad-Farid R (2013) Isolation and characterization of rhizobacteria and their effects on root extracts of Valeriana officinalis. Aust J Crop Sci 7:338–344
Goswami D, Vaghela H, Parmar S, Dhandhukia P, Thakker JN (2013) Plant growth promoting potentials of Pseudomonas spp. strain OG isolated from marine water. J Plant Interact 8:281–290
Gray EJ, Smith DL (2005) Intracellular and extracellular PGPR: commonalities and distinctions in the plant-bacterium signaling processes. Soil Biol Biochem 37:395–412
Hernández-Montiel LG, Chiquito-Contreras CJ, Murillo-Amador B, Vidal-Hernández L, Quiñones-Aguilar EE, Chiquito-Contreras RG (2017) Efficiency of two inoculation methods of Pseudomonas putida on growth and yield of tomato plants. J Soil Sci Plant Nutr 17:1003–1012
Jadon KS, Thirumalaisamy PP, Kumar V, Koradia VG, Padavi RD (2015) Management of soil borne diseases of groundnut through seed dressing fungicides. Crop Prot 78:198–203
Karnwal A (2009) Production of indol acetic acid by fluorescent Pseudomonas in the presence of l-tryptophan and rice root exudates. J Plant Pathol 91:61–63
Kumar S, Dudley J, Nei M, Tamura K (2008) MEGA: a biologist-centric software for evolutionary analysis of DNA and protein sequences. Brief Bioinform 9:299–306
Kumar K, Amaresan N, Bhagat S, Madhuri K, Srivastava RC (2010) Isolation and characterization of rhizobacteria associated with coastal agricultural ecosystem of rhizosphere soils of cultivated vegetable crops. World J Microbiol Biotechnol 27:1625–1632
Lane DJ (1991) Nucleic acid techniques in bacterial systematics: 16S/23S rRNA sequencing. John Wiley and Sons, New York
Liu H-A, Comino JR, Wu H-S, Yang G-Y, Ma X-L, Wang X-J, Chen K-K, Liu Y-D, Brevik EC (2018) Assessment of a new bio-organic remediation as a biofungicide in fusarium-infested soils of watermelon monoculture areas from China. J Soil Sci Plant Nut 18:735–751
Lu WJ, Wang HT, Nie YF, Wang ZC, Huang DY, Qiu XY, Chen JC (2004) Effect of inoculating flower stalks and vegetable waste with ligno-cellulolytic microorganisms on the composting process. J Environ Sci Health B 39:871–887
Mendes R, Kruijt M, de Bruijn I, Dekkers E, van der Voort M, Schneider JHM, Piceno YM, DeSantis TZ, Andersen GL, Bakker PAHM, Raaijmakers JM (2012) Deciphering the rhizosphere microbiome for disease-suppressive bacteria. Science 332:1097–1100
Mohite B (2013) Isolation and characterization of indole acetic acid (IAA) producing bacteria from rhizospheric soil and its effect on plant growth. J Soil Sci Plant Nut 13:638–649
Mora I, Cabrefiga J, Montesinos E (2011) Antimicrobial peptide genes in Bacillus strains from plant environments. Int Microbiol 14:213–223
Nautiyal SC (1999) An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol Lett 170:265–270
Przemieniecki SW, Kurowski TP, Korzekwa K (2014a) Chemotypes and geographic distribution of the Fusarium graminearum species complex. Environ Biotechnol 10:45–59
Przemieniecki SW, Kurowski TP, Korzekwa K, Karwowska A (2014b) The effect of psychrotrophic bacteria isolated from the root zone of winter wheat on selected biotic and abiotic factors. J Plant Prot Res 54:407–413
Przemieniecki SW, Kurowski TP, Karwowska A (2015) Plant growth promoting potential of Pseudomonas sp. SP0113 isolated from potable water from a closed water well. Arch Biol Sci 67:663–673
Przemieniecki SW, Kurowski PT, Kotlarz K, Krawczyk K, Damszel M, Karwowska A (2016) Plant growth promoting properties of Serratia fonticola ART-8 and Pseudomonas putida ART-9 and their effect on the growth of spring wheat (Triticum aestivum L.). Environ Biotechnol 12:35–39
Przemieniecki SW, Kurowski TP, Damszel MM, Karwowska A, Adamiak E (2017) Effect of roundup 360 SL on survival of Pseudomonas sp. SP0113 strain and effective control of phytopathogens. J Agric Sci Technol 19:1417–1427
Przemieniecki S, Kurowski T, Damszel M, Krawczyk K, Karwowska A (2018) The effectiveness of the Bacillus sp. SP-A9 strain as a biological control agent for spring wheat (Triticum aestivum L.). J Agric Sci Technol 20:609–619
Robačer M, Canali S, Lakkenborg Kristensen H, Bavec F, Grobelnik Mlakar S, Jakop M, Bavec M (2016) Cover crops in organic field vegetable production. Sci Hortic 208:104–110
Sallam NA, Raid SN, Mohamed MS, El-eslam AS (2013) Formulations of Bacillus spp. and Pseudomonas fluorescens for biocontrol of cantaloupe root rot caused by Fusarium solani. J Plant Prot Res. 53:295–300
Saremi H, Okhovvat SM, Ashrafi SJ (2011) Fusarium diseases as the main soil borne fungal pathogen on plants and their control management with soil solarization in Iran. Afr J Biotechnol 10:18391–18398
Urrea R, Cabezas L, Sierra R, Cárdenas M, Restrepo S, Jiménez P (2011) Selection of antagonistic bacteria isolated from the Physalis peruviana rhizosphere against Fusarium oxysporum. J Appl Microbiol 111:707–716
Yang J, Kloepper JW, Ryu CM (2009) Rhizosphere bacteria help plants tolerate abiotic stress. Trends Plant Sci 14:1–4
Zhao Y, Selvaraj JN, Xing F, Zhou L, Wang Y, Song H, Tan S, Sun L, Sangare L, Folly YME, Lin Y (2014) Antagonistic action of Bacillus subtilis strain SG6 on Fusarium graminearum. PLoS One 9(3):e92486
Acknowledgements
The authors would like to thank Karol Korzekwa and Beata Duch for collecting samples from the wastewater treatment plant in Dobre Miasto.
Funding
This study was financed under research projects from the University of Warmia and Mazury in Olsztyn (No. 20.620.019-300) and the Ministry of Science and Higher Education (No. 20.610.16-300).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Przemieniecki, S.W., Kurowski, T.P., Kotlarz, K. et al. Bacteria Isolated from Treated Wastewater for Biofertilization and Crop Protection Against Fusarium spp. Pathogens. J Soil Sci Plant Nutr 19, 1–11 (2019). https://doi.org/10.1007/s42729-018-0001-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-018-0001-9