Abstract
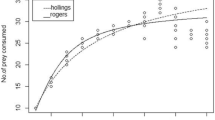
The two-spotted spider mite (TSSM), Tetranychus urticae Koch is a polyphagous widespread herbivore pest and one of the most economically important species which feeds more than 1,100 plant species. The functional response at five different constant temperatures (15, 20, 25, 30 and 35 °C), prey switching, and mutual interference behaviors of the predatory coccinellid, Stethorus gilvifrons Mulsant on TSSM nymphs were assessed in laboratory conditions (25 ± 1 °C, 60 ± 5% RH and L:D 16:8 h) to evaluate its potential for biological control of TSSM. The predator showed a type II functional response at all tested temperatures. The shortest handling time (Th) and highest searching efficiency (α) were obtained at 30 °C and 20 °C, respectively. The highest and lowest values of the maximum attack rate (T/Th) of the predator were obtained at temperatures of 30 °C and 15 °C, respectively. The switching behavior was not observed in S. gilvifrons between different density proportions of TSSM nymph and adult. The highest value of total predation and lowest value of per capita predation rate belonged to the predator density of 16. The results revealed a strong predation capacity of S. gilvifrons on TSSM, especially in higher temperatures which makes it an efficient biocontrol agent in integrated TSSM management programs.




Similar content being viewed by others
References
Afshari A, Mossadegh MS, Kamali K (2001) Feeding behaviours of the ladybird beetle, Stethorus gilvifrons (Mulsant), and effect of different factors on its feeding rate in laboratory condition. J Agric Sci 23(2):71–90
Aggelis G, Vayenas DV, Tsagou V, Pavlou S (2005) Prey–predator dynamics with predator switching regulated by a catabolic repression control mode. Ecol Model 183(4):451–462. https://doi.org/10.1016/j.ecolmodel.2004.07.034
Akist T, Cakmak I, Ozer G (2007) Effect of temperature and photoperiod on development and fecundity of an acarophagous ladybird beetle Stethorus glivifrons. Phytoparasitica 35(4):357–366
Beddington JR (1975) Mutual interference between parasites or predators and its effect on searching efficiency. J Anim Ecol 44(1):331–340. https://doi.org/10.2307/3866
Biddinger DJ, Weber DC, Hull LA (2009) Coccinellidae as predator of mites: Stethorini in biological control. Biol Control 51(2):268–283. https://doi.org/10.1016/j.biocontrol.2009.05.014
Bolland HR, Gutierrez J, Flechtmann CHW (1998) World catalogue of the spider mite family (Acari: Tetranychidae). Brill Academic Publishers, Leiden, The Netherlands
Chazeau J (1985) Predaceous insects. In: Helle W, Sabelis MW (eds) World Crop Pests, Spider Mites: Their Biology, Natural Enemies and Control. Elsevier Publication, Amsterdam, pp 211–246
Costa JF, Matos CHC, de Oliveira CRF, da Silva TGF, Lima Neto IFA (2017) Functional and numerical responses of Stethorus tridens Gordon (Coleoptera: Coccinellidae) preying on Tetranychus bastosi Tuttle, Baker & Sales (Acari: Tetranychidae) on physic nut (Jatropha curcas). Biol Control 111:1–5. https://doi.org/10.1016/j.biocontrol.2017.04.015
Dalir S, Hajiqanbar HR, Fathipour Y, Khanamani M (2021a) Age-dependent functional and numerical responses of Neoseiulus cucumeris (Acari: Phytoseiidae) on two-spotted spider mite (Acari: Tetranychidae). J Econ Entomol 114(1):50–61. https://doi.org/10.1093/jee/toaa266
Dalir S, Hajiqanbar HR, Fathipour Y, Khanamani M (2021b) A comprehensive picture of foraging strategies of Neoseiulus cucumeris and Amblyseius swirskii on western flower thrips. Pest Manag Sci 77(12):5418–5429. https://doi.org/10.1002/ps.6581
Farazmand A, Fathipour Y, Kamali K (2012) Functional response and mutual interference of Neoseiulus calfornicus and Typhlodromus bagdasarjani (Acari: Phytoseiidae) on Tetranychus urticae (Acari: Tetranychidae). Int J Acarol 38(5):369–376. https://doi.org/10.1080/01647954.2012.655310
Farazmand A, Fathipour Y, Kamali K (2013) Functional response and mutual interference of Scolothrips longicornis (Thysanoptera: Thripidae) on two spotted spider mite. IOBC/WPRS Bull 93:39–44
Fathipour Y, Hosseini A, Talebi AA, Moharramipour S (2006) Functional response and mutual interference of Diaeretiella rapae (Hymenoptera: Aphidiidae) on Brevicoryne brassicae (Homoptera: Aphididae). Entomol Fenn 17(2):90–97. https://doi.org/10.33338/ef.84293
Fathipour Y, Karimi M, Farazmand A, Talebi AA (2017) Age-specific functional response and predation rate of Amblyseius swirskii (Phytoseiidae) on two-spotted spider mite. Syst Appl Acarol 22(2):159–169. https://doi.org/10.11158/saa.22.2.1
Fathipour Y, Karimi M, Farazmand A, Talebi AA (2018) Age-specific functional response and predation capacity of Phytoseiulus persimilis (Phytoseiidae) on the two-spotted spider mite. Acarologia 58(1):31–40. https://doi.org/10.24349/acarologia/20184225
Fathipour Y, Maleknia B (2016) Mite predators. In: Omkar (ed) Ecofriendly pest management for food security. Elsevier, San Diego, pp 329–366
Fathipour Y, Maleknia B, Bagheri A, Soufbaf M, Reddy VP (2020) Functional and numerical responses, mutual interference, and resource switching of Amblyseius swirskii on two-spotted spider mite. Biol Control 146:104266. https://doi.org/10.1016/j.biocontrol.2020.104266
Free C, Beddington J, Lawton J (1977) On the inadequacy of simple models of mutual interference for parasitism and predation. J Anim Ecol 46(2):543–544. https://doi.org/10.2307/3829
Gitonga LM, Overholt WA, Lohr B, Magambo JK, Mueke JM (2002) Functional response of Orius albidipenis (Hemiptera: Anthocoridae) to Megalurothrips sjostedti (Thysanoptera: Thripidae). Biol Control 24:1–6. https://doi.org/10.1016/S1049-9644(02)00001-4
Godfray HC, Pacala SW (1992) Aggregation and the population dynamics of parasitoids and predators. Am Nat 140(1):30–40. https://doi.org/10.1086/285401
Haji-Zadeh J, Kamali K, Mossadegh MS (1996) Studies on the feeding behavior of Stethorus gilvifrons Mulsant (Col: Coccinellidae). Sci J Agri 18:71–88. https://doi.org/10.22055/ppr.1996.12801
Handoko H, Affandi A (2012) Life-history traits of Stethorus gilvifrons (Mulsant) (Coleoptera: Coccinellidae) on phytophagous mites Eutetranychus orientalis Klein (Acari: Tetranychidae). Agrivata, J Agric Sci 34(1):7–13. https://doi.org/10.17503/AGRIVITA-2012-34-1-P007-013
Hassell MP, Varley GC (1969) New inductive population model for insect parasites and its bearing on biological control. Nature 223(5211):1113–1137. https://doi.org/10.1038/2231133a0
Helle W, Sabelis MW (1985) Spider mites: their biology, natural enemies and control. Volume 1A. Elsevier, Amsterdam
Henne DC, Johnson SJ (2010) Laboratory evaluation of aggregation, direct mutual interference, and functional response characteristics of Pseudacteon tricuspis Borgmeier (Diptera: Phoridae). Biol Control 55(1):63–71. https://doi.org/10.1016/j.biocontrol.2010.07.001
Holling CS (1959) Some characteristics of simple types of predation and parasitism. Can Entomol 91(7):385–398. https://doi.org/10.4039/Ent91385-7
Hull LA (1997) The functional and numerical Response of Stethorus punctum (Coleoptera: Coccinellidae) to densities of the Panonychus ulmi (Acarina: Tetranychidae). Dissertation, Pennsylvania State University
Imani Z, Shishehbor P, Sohrabi F (2009) The effect of Tetranychus turkestani and Eutetranychus orientalis (Acari: Tetranychidae) on the development and reproduction of Stethorus gilvifrons (Coleoptera: Coccinellidae). J Asia-Pac Entomol 12(4):213–216. https://doi.org/10.1016/j.aspen.2009.05.004
Jafari S, Fathipour Y, Faraji F (2012) The influence of temperature on the functional response and prey consumption of Neoseiulus barkeri (Phytoseiidae) on two-spotted spider mite. J Entomol Soc Iran 31(2):39–52
Juliano SA (2001) Nonlinear curve fitting: predation and functional response curves. In: Scheiner SM, Gurevitch J (eds) Design and analysis of ecological experiments. Oxford University Press, New York, pp 178–196
Karami Jamour T, Shishehbor P (2012) Host plant effects on the functional response of Stethorus gilvifrons to strawberry spider mites. Biocontrol Sci Technol 22(1):101–110. https://doi.org/10.1080/09583157.2011.646240
Khanamani M, Fathipour Y, Hajiqanbar H, Sedaratian A (2014) Two-spotted spider mite reared on resistant eggplant affects consumption rate and life table parameters of its predator, Typhlodromus bagdasarjani (Acari: Phytoseiidae). Exp Appl Acarol 63(2):241–252. https://doi.org/10.1007/s10493-014-9785-z
Khanamani M, Fathipour Y, Talebi AA, Mehrabadi M (2017) Quantitative analysis of long-term mass rearing of Neoseiulus californicus (Acari: Phytoseiidae) on almond pollen. J Econ Entomol 110(4):1442–1450. https://doi.org/10.1093/jee/tox116
Kouhjani-Gorji M, Fathipour Y, Kamali K (2009) The effect of temperature on the functional response and prey consumption of Phytoseius plumifer (Acari: Phytoseiidae) on the two-spotted spider mite. Acarina 17(2):231–237
Latifian M (2012) Voracity and feeding preferences of larvae and adult stages of Stethorus gilvifrons Mulsant. (Coleoptera: Coccinellidae) on larvae and adult of Oligonychus afrasiaticus McGregor (Acarina: Tetranychidae). Intl J Agri Crop Sci 4(9):540–546
Madadi H, Enkegaard A, Brodsgaard HF, Kharrazi-Pakdel A, Mohaghegh J, Ashouri A (2007) Host plant effects on the functional response of Neoseiulus cucumeris to onion thrips larvae. J of Appl Entomol 131(10):728–733. https://doi.org/10.1111/j.1439-0418.2007.01206.x
Migeon A, Dorkeld F (2007) Spider Mites Web: a comprehensive database for the Tetranychidae. http://www.montpellier.inra.fr/CBGP/spmweb. Accessed 20 May 2021
Murdoch WW (1969) Switching in general predators: experiments on predator specificity and stability of prey populations. Ecol Monogr 39(4):335–354. https://doi.org/10.2307/1942352
Nauen R, Stumpf N, Elbert A, Zebitz CPW, Kraus W (2001) Acaricide toxicity and resistance in larvae of different strains of Tetranychus urticae and Panonychus ulmi (Acari: Tetranychidae). Pest Manag Sci 57(3):253–261. https://doi.org/10.1002/ps.280
Nicholson AJ (1933) The balance of animal populations. J Anim Ecol 2(1):132–178. https://doi.org/10.2307/954
Pakyari H, Fathipour Y (2009a) Mutual interference of Scolothrips longicornis Priesner (Thysanoptera: Thripidae) on Tetranychus urticae Koch. (Acari: Tetranychidae). IOBC/WPRS Bull 50:65–68
Pakyari H, Fathipour Y (2009b) No-choice prey stage preference and functional response of Scolothrips longicornis on Tetranychus urticae. IOBC/WPRS Bull 49:139–144
Pakyari H, Fathipour Y, Rezapanah M, Kamali K (2009) Temperature-dependent functional response of Scolothrips longicornis (Thysanoptera: Thripidae) preying on Tetranychus urticae. J Asia-Pac Entomol 12(1):23–26. https://doi.org/10.1016/j.aspen.2008.12.001
Perumalsamy K, Selvasundrama R, Roobakkumar A, Jasin Rahman V, Nair Muraeedharan N (2010) Life table and predatory efficiency of Stethorus gilvifrons (Coleoptera: Coccinellidae), an important predator of the red spider mite, Oligonychus coffeae (Acari: Tetranychidae), infesting tea. Exp Appl Acarol 50(2):141–150. https://doi.org/10.1007/s10493-009-9290-y
Rogers D (1972) Random search and insect population models. J Anim Ecol 41(2):369–383. https://doi.org/10.2307/3474
Royama T (1971) A comparative study of models for predation and parasitism. Res Popul Ecol 13(1):1–91. https://doi.org/10.1007/BF02511547
Sohrabi F, Shishehbor P (2007) Functional and numerical responses of Stethorus gilvifrons Mulsant feeding on strawberry spider mite, Tetranychus turkestani Ugarov and Nikolski. Pak J Biol Sci 10(24):4563–4566. https://doi.org/10.3923/pjbs.2007.4563.4566
SPSS (2011) IBM SPSS software statistics for Windows, version 20.0. IBM Crop, New York
Svendsen MS, Enkegaard A, Brodsgaard H (1999) Influence of humidity on the functional response of larvae of the gall midge (Feltiella acarisuga) feeding on spider mite eggs. IOBC/WPRS Bull 22:243–246
Taghizadeh M, Irani-Nejad KH, Iranipour S, Moghaddam Vahed M (2018) Daily consumption and functional response of Stethorus gilvifrons (Coleoptera: Coccinellidae) and Orius albidipennis (Hemiptera: Anthocoridae) to Tetranychus urticae (Acari: Tetranychidae). Persian J Acarol 7(4):363–380. https://doi.org/10.22073/pja.v7i4.38181
Tirello P, Pozzebon A, Cassanelli S, Van Leeuwen T, Duso C (2012) Resistance to acaricides in Italian strains of Tetranychus urticae: toxicological and enzymatic assays. Exp Appl Acarol 57:53–64. https://doi.org/10.1007/s10493-012-9536-y
van Baalen M, Krivan V, van Rijn PCJ, Sabelis MW (2001) Alternative food, switching predators, and persistence of predator–prey systems. Am Nat 157(5):1–13. https://doi.org/10.1086/319933
Veeravel R, Baskaran P (1997) Functional and numerical responses of Coccinella transversalis Fab and Cheilomenes sexmaculatus Fab feeding on the Melon Aphid, Aphis gossypii Glov. Int J Trop Insect Sci 17:335–339. https://doi.org/10.1017/S1742758400019159
Xiao Y, Osborne LS, Chen J, McKenzie CL (2013) Functional responses and prey stage preferences of a predatory gall midge and two predacious mites with two spotted spider mites, Tetranychus urticae, as host. J Insect Sci 13(1):8. https://doi.org/10.1673/031.013.0801
Yang X, Shen M, Xiong J, Guo Z (1996) Approaches to enhance the effectiveness of biocontrol of Panonychus citri (Acarina: Tetranychidae) with Stethorus punctillum (Coleoptera: Coccinellidae) in citrus orchards in Guizhou. Syst Appl Acarol 1:21–27
Acknowledgements
The financial and technical support for this research was provided by the Department of Entomology, Tarbiat Modares University (Tehran, Iran), which is greatly appreciated.
Funding
This study was supported by the Department of Entomology, Tarbiat Modares University, Tehran, Iran.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
There are no ethical concerns regarding the organisms used in this research.
Conflict interests
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fathipour, Y., Mehrkhou, F., Mirhosseini, M.A. et al. Comprehensive foraging behavior of acarophagous ladybird, Stethorus gilvifrons (Coleoptera: Coccinellidae) on Tetranychus urticae (Trombidiformes: Tetranychidae): implications for biological control. Int J Trop Insect Sci 44, 215–226 (2024). https://doi.org/10.1007/s42690-023-01148-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42690-023-01148-7