Abstract
This research aimed to evaluate the influence of alccofine 1203 on a mineral admixture and sodium nitrite as a corrosion inhibitor on the properties of concrete. To achieve these aims, an experimental investigation was carried out on a set of composite samples comprising five distinct concrete formulations. Five different mixes for the concrete were used as the overlay materials such as NC as a reference concrete, alccofine concrete (i.e. 25% cement replaced with alccofine), and alccofine concrete with varying dosages of sodium nitrite (i.e. 1%, 1.25%, and 1.5%). Alccofine reduced the workability and water absorption and increased the compressive strength of the concrete (5%) at curing age of 28 days. Adding sodium nitrite further reduced water absorption of the concrete and workability but compressive strength of 29.15% and 26.93% for the curing period of 3 and 7 days, respectively. The pH of the concrete powdered solution became more alkaline with the replacement of alccofine and addition of sodium nitrite. Free chloride content dropped by 48 and 66%, respectively, with the introduction of GGBS and sodium nitrite. Corrosion properties of the concrete samples were examined using open circuit potentials and linear polarization resistance of various concrete mixes after being immersed in 1 M H2SO4 and 3% NaCl environment. The findings indicated a notable improvement in the corrosion resistance, water absorption test, thermal conductivity, strength properties, and microstructural properties of concrete with the incorporation of SN in combination with alccofine. The application of the response surface method allowed for the prediction, validation, and optimization of experimental data using a regression equation.
Article highlights
-
Alccofine 1203 enhances the performance of concrete and minimizes the utilization of cement.
-
The corrosion rate and durability of concrete are considerably improved by the utilization of alccofine and corrosion-inhibiting admixtures.
-
The response surface method is best suited to predict the experimental data.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The reinforced cement concrete has witnessed diverse applications in civil infrastructures, including but not limited to, highways, tunnels, bridges, dams, sewer system and marine structures etc. In developing countries like India, a major share of the economy is invested in the development of infrastructure and its repair and maintenance expenditure. Although, attributed to superior mechanical strength and durability characteristics, reinforced concrete structures are supposed to be durable even under severe conditions. However, a variety of deterioration mechanisms may limit the lifespan of these structures. Premature degradation of structures not only affects the quality of life as well as poses threat to safety of human life. The corrosion of reinforcement embedded in concrete stands out as a significant factor contributing to the deterioration, impacting the lifespan and durability of RCC structures. The deterioration of steel reinforcement in concrete is a global problem, which influences the service life and durability of concrete structural elements. The expansion of steel bars caused by rusting induces tensile stresses on the concrete within RCC structures. Concrete which is poor in resisting tensile stresses cannot resist tension and cracks occur which leads to brittle failures. In addition, corrosion of RCC structures imposes a significant economic burden on the society. The global cost of corrosion is estimated to be approximately $2.5 trillion, equivalent to 3.4 percent of the worldwide Gross Domestic Product (GDP) and the savings of between $375 and $875 billion per annum is possible on the global basis. Similarly, in India, corrosion is impacting nearly 4.2% of the country’s GDP [1, 2]. So, a huge amount is invested in the measures adopted in the repair and maintenance of structures which got affected by corrosion along with requirement of skilled labour and material costs, which otherwise can be utilized in the development of a country.
Corrosion is termed as the “electrochemical or chemical reaction between a material and its environment that leads to deterioration of the material and/or its properties” [3]. When there is an electrical potential difference get developed on the surface of steel embedded in concrete then it behaves as an electrochemical cell composed of cathodic and anodic regions while the pore water within the concrete acts as an electrolyte. In the electrochemical system so developed the portion of steel which has [4] more potential act as negative electrode, i.e. anode, starts corroding, whereas the cathode remains undamaged which will further increase the potential difference and accelerates the corrosion rate of rebars. Nevertheless, during the initial phase of the corrosion process, [5] when the hydration of concrete starts, a protective layer develops on the immediate surface of the rebars which consists of γ-Fe2O3 with a thickness of 10–3 to 10−1 µm [6]. This passive layer serves as a barrier, inhibiting the flow of ions between the rebar and the surrounding concrete, consequently diminishing the corrosion rate. This oxide layer which prevents rebar degradation remains stable at high pH value which is more than 12 [7]. So, for corrosion process to continue this passive layer must be damaged which happens the presence of chloride ions, carbonation the presence of moisture and oxygen is required. Hence, to mitigate corrosion, appropriate measures can be implemented [8, 9].
A variety of techniques have been in practice to safeguard the reinforcement in RCC structures against corrosion. There are various corrosion preventive methods such as epoxy coating of steel bars/impregnation of concrete, cathodic protection, use of mineral admixtures or superplasticizers and corrosion inhibiting admixtures (CIA). The first three strategies mentioned above would require significantly higher operational costs [10,11,12]. Inhibitors are substances that, when present in specific concentrations, reduce the corrosion rate without significantly impacting the concentration of any other corrosion-inducing agent. Such inhibitors include nitrites, phosphates, ferrous salts, benzoates and chromates. They are easy to handle and comparatively of low cost as compared to other corrosion preventive methods. Corrosion inhibiting admixtures are typically show resistant to corrosion by cathodic, anodic or mixed mechanisms. Cathodic inhibitors such as sodium hydroxide, suppress the reaction of cathodic corrosion, and decrease the rate of corrosion. Anodic inhibitors such as calcium or sodium nitrites, Suppress the anodic corrosion reaction, thereby reducing the corrosion rate by elevating the corrosion potential of steel.
Supplementary cementitious materials (SCMs) like ground granulated blast furnace slag (GGBS), silica fume (SF), and fly ash are also utilized to enhance the compactness of concrete matrix which may be helpful in the resist the process of carbonation up to some extent. Permeability reduction in concrete is a major factor in improving various aspects of durability performance such as alkali-silica reaction, mitigating sulphate attack and corrosion resistance. When permeability decreased the movement of deleterious compounds within the concrete and distribution of deterioration mechanisms are commensurately minimized [13,14,15,16]. Many researchers are of the opinion that the use of slag in concrete found to play a good role in controlling the rate of corrosion of steel in concrete [17,18,19,20,21]. It is well known that the use of the inclusion of slag in concrete can contribute to major decreases in permeability. It is decreased primarily by the high quantity of calcium silicate hydrate (CSH) produced in Portland/slag cement system. The microstructure is modified in concrete because the pores are partially filled with CSH in substitute of calcium hydroxide (CH). The total pore size is decreased and the interconnectivity ofpores becomes more winding. In comparison, decrease in permeability is strongly associated with the level of slag inclusion by 70% and more.
Andrade and Bujak [22] reported the effect of addition of slag and fly ash in the ordinary Portland cement on the corrosion of reinforcement. Slag based cement was found to be more chloride resistant but lesser resistant against carbonation in comparison to OPC. Mon temor et al. [23] investigated the utilization of fly ash in concrete and its effects on steel reinforcement in a NaCl environment through the application of electrochemical impedance spectroscopy (EIS). They reported that with addition of fly ash not only declines the corrosion rate but also slowdown the Corrosion initiation is influenced by the heightened resistivity of concrete. The use of ultra-fine GGBS on strength properties, (EC) electrical resistivity and chloride migration coefficient of hardened concrete was studied by Teng et al. [24]. The utilization of ultra-fine materials enhances the hydration rate as well as pozzolanic reaction because of their elevated specific surface area. The impact of GGBS and pre-manufactured ultrafine slag on the durability and strength characteristics of high-strength concrete were examined by Sharmila and Dhinakaran [25, 26]. It was found that GGBS showed better compaction by micro voids filling affect in the concrete than the ready-made ultra-fine slag. Haruehansapong et al. [27] studied the influence of nano sized silica particles on the compressive strength of cement mortar. It has been found that the addition of silica particles of size lesser than 40 nm don't significantly enhances the strength properties of concrete. Nevertheless, nano-silica not only decreases the porosity of the matrix but also enhances the microstructural characteristics of concrete. Saraswathy et al. [28] proposed enhancing corrosion resistance by incorporating various corrosion inhibitors, including sodium benzoate, stannous chloride, potassium chromate, and sodium nitrite. They observed that admixtures including stannate, citrate, and hydroxide enhance the compressive strength and delay the corrosion of reinforcement, thereby increasing the service life of reinforced cement concrete structures. The utilization of “Corrosion Inhibiting Admixtures (CIA)” in reinforced concrete such as primarily nitrites, amino alcohols, and mono-fluorophosphates was studied by Soylev and Richardson [29].
SCMs such as SF, GGBS, UFS, and FA are generally used in concrete to enhance the microstructure as well as to improve the mechanical properties. Some studies have shown that the incorporation of SCMs reduces just not reduces the permeability of concrete but also the rate of corrosion in the embedded rebar [30, 31]. The inclusion of GGBS in concrete can effectively be utilized to reduce the cumulate pore volume and to further improve the cement hydrate system which results in decreasing the chloride ions penetration [32]. The corrosion rate of steel is influenced by various factors, including the type of cement, concrete cover depth to reinforcement, and GGBS content. Concrete with Type I cement and GGBS showed a better improvement in the corrosion resistance of rebars more than twice that of concrete without GGBS [33]. Slag due to its low electrical conductivity may result in the finer microstructure which results in a reduction in corrosion [34].
Another important factor that controls corrosion is the alkalinity of the concrete surrounding the steel. If the alkalinity of the concrete is maintained then the rate of corrosion can be significantly reduced. Concrete with chemically activated fly ash (CFA) showed a lesser rate of corrosion as compared to other finely ground fly ashes (Thermally activated fly ash, physically activated fly ash, and As-received fly ash) because of higher CSH which maintains the alkalinity of concrete near the steel rebar [35]. Geopolymer concrete (GC) with fly ash (FA) showed a reduction in corrosion potential due to the initiation of the passive layer on the surface of steel rebars. Metakaolin when used as SCM in the concrete showed a significant improvement in the microstructure thereby enhancing the resistance to chloride ions penetration [36]. The higher content of GGBFS showed a decrease in the chloride ion diffusion coefficient of concrete. Due to hydraulic activity and the high specific area of GGBFS the chloride ion diffusion coefficient of concrete compared to concrete without GGBFS thereby increasing the corrosion resistance of concrete [37]. Corrosion resistance has been enhanced with the addition of nano-additives into the concrete. The inclusion of nano-additives and fly ash can develop strong bonded hydration products, it can speed up the pozzolanic activity and improve the pore microstructure of the concrete as a result decrease the corrosion rate and chloride ion ingress into the concrete [38]. Enhanced antibacterial activity, durability, and corrosion resistance were also reported in nanophase-modified fly ash concrete (i.e. Fly ash + Nano-CaCO3 and/or Nano-TiO2) to different environmental conditions. Nano-CaCO3-modified fly ash concrete showed not only strength enhancement and durability but also improved corrosion resistance as compared to Nano-TiO2-modified fly ash concrete and control concrete [39]. The combination of metakaolin, silica fume, and GGBS showed an increase in current and corrosion resistance compared to normal concrete [40]. The utilization of metakaolin showed better corrosion resistance than the silica fume for concrete cured in either seawater or portable water medium [41]. The inclusion of sugarcane bagasse ash sand (SBAS) leads to an increase the corrosion resistance, owing to the slowdown of the de-passivation of the rebars. This impact is mainly due to the enhancement of the alkalinity of concrete and reduced pores promoted by SBAS [42].
The present paper reports the influences of the addition of Sodium Nitrite (SN) as a corrosion inhibitor and ultrafine slag (UFS) in the prevention of the corrosion of reinforcement in concrete. The corrosion behavior is studied through metrics such as linear polarization resistance and opens circuit potential studies, thermal and microstructural properties of the concrete. This article also explores the effects of the inclusion of UFS and SN on workability in terms of the compaction factor test, the ultimate load-carrying capacity, and the water absorption values.
2 Research significance
This study focuses on producing early-strength concrete and examines the impact of various parameters on workability, strength properties, corrosion behavior, thermal characteristics, and microstructure. This investigation specifically considers the inclusion of UFS (Ultra-Fine Slag) and SN (additive or material denoted by SN). The research data presented in this paper are useful for understanding the behavior of early-strength concrete.
3 Materials
In the current study, OPC 53 grade according to Indian requirements is employed. The cement is evaluated for its different qualities as shown in Tables 1 and 2 and determined to meet the standards of IS: 8122-1989 [43]. River sand sourced from Chittoor district is utilized as the fine aggregate, possessing a specific gravity of 2.65 and a bulk density of 1650 kg/m3 and water absorption of 0.55%, respectively, according to IS 383-2016 [44]. Throughout the study, Alccofine (AL-1203), an ultrafine slag with a specific gravity of 2.9 that complies with ASTM C989 [45], sourced from firm Ambuja Cement Ltd, was utilized. Tables 3 and 4 furnish the physical and chemical attributes of AL-1203, respectively. Alccofine underwent EDX and SEM analysis at 20 keV. In Fig. 1a, ultrafine slag particles exhibit angular or irregular shapes, with sizes less than 30 µm. The properties of sodium nitrite are detailed in Table 5.
4 Methodology adopted
4.1 Mix design methodology
A concrete mix of M30 was formulated in accordance with. the mix design methodology described in IS: 10,262–2009 [46]. Table 6 presents the specifics of concrete mix proportions, maintaining a consistent water-binder ratio (W/B) of 0.45 across all mixes.
Cubes and cylindrical specimens were crafted to assess the compressive strength and corrosion resistance of the modified concrete, respectively. Cubes with dimensions of 150 mm and cylinders measuring 100 mm × 200 mm (D × H) were employed. Within the cylindrical specimens, a TMT (Thermo-Mechanically Treated) rebar with a diameter of 10 mm was inserted at a depth of 150 mm from the top surface.
4.2 Test methodology
Various tests performed in the current study and the testing methodology adopted is explained here afterward.
4.2.1 Mechanical properties
The effort needed to work with concrete is termed as workability of concrete, i.e., if concrete is handled without loss of homogeneity and segregation; it is known to be workable. This pertains to the characteristic of freshly mixed concrete. In this study, the workability of the concrete is evaluated before the casting process. The compaction factor test was performed as per BIS: 1199-1959 [47]. The compression strength assessment (CS) was conducted using a compression testing machine (CTM) of 2000 kN capacity in compliance with BIS 516-1959 [48], for normal concrete and UFS concrete cubes introducing sodium nitrite in different proportions with a curing period of 3, 7, 28 and 90 days. The water absorption test was conducted on cube specimens measuring 150 mm at 28 days, following ASTM C642-2013 [49] standards. Initially, the cube specimens were subjected to 24 h of drying in an oven at a temperature of 105 °C. Subsequently, the cubes were removed from the oven, and allowed to cool, and their weights were recorded. After immersing the cube samples in water for two days, their weights were measured again.
4.2.2 Measurement of free chloride content and pH level
Measurement of Free Chloride Content and pH Level (alkalinity) of the concrete next to the rebar play a crucial role in the initiation and corrosion rate. The process of chloride penetration through concrete is intricate and remains not fully comprehended [50,51,52].
When the concrete surface comes into contact with deicing salt or seawater, the aqueous solution is rapidly absorbed through capillary action initially. When the capillary absorption rate is negligible, chloride ions can be transferred further into the concrete through a slow diffusion process. Some quantity of the chloride ions will be chemically bound very nearer to the rebar, and the remaining will be trapped on the inner surface of the hardened cement paste. If the pH of the concrete samples reaches 9 indicates steel reinforcement has started to corrode. Due to these reasons, the pH content and free chloride concentration of concrete powder solution have to be examined with the addition of either mineral or chemical admixtures into the concrete.
After the compressive strength test, 100 g of powder samples from crushed concrete cubes were extracted and mixed with double distilled water to assess the free chloride and alkalinity of the concrete. The paste was taken from the solution and kept in jar test equipment for 60 min. The liquid powder sample of 20 ml was taken and titrated against the AgNO3 solution potassium chromate served as the indicator. The concentration of free chloride in the solution is expressed in parts per million (ppm). A 50 ml sample was taken for pH analysis using a digital meter.
4.2.3 Electrochemical analysis
Concrete specimens have been kept in three electrochemical cells for corrosion evaluation. The electrochemical cell consists of rebar-embedded cylindrical concrete samples that act as a working electrode, mesh made from stainless steel is used as a counter electrode placed exact center to the cylindrical specimen, and silver chloride is used as a reference electrode as shown in Fig. 2. Measurements of corrosion were performed in a 1 M H2SO4 and 3% NaCl environment. The open circuit potential (OCP) was examined for more than 600 s according to ASTM C-876 [53], until the potential stabilized and afterward over-potential range between − 10 mV to + 10 mV was slowly recorded at the rate of 1 mV/s. Corrosion resistance was obtained from these polarization studies using Ohm’s law.
4.2.4 Thermogravimetric analysis (TGA)
For TGA analysis, various concrete mix samples were ground and sieved (63 micron). Using acetone for solvent exchange, 30 g sieved samples were mixed with 100 ml acetone for 3 min. After drying for 12 h at 40 °C, oven-dried samples were stored until testing.
In a thermal analyzer, ten samples were examined, and small quantities were heated ranging from 24 °C to 800 °C at a rate of 10 °C/min, revealing weight decrease with increasing temperature and hydrate decomposition.
4.2.5 Microstructure morphology and chemical bonding of the deposited material at 28 days
The deposited material has been collected and mineralogical composition and morphological structures have been analyzed using XRD and SEM respectively. Further Fourier transform infrared spectroscopy (FTIR) test was conducted to know the chemical bonds of the deposited material.
5 Results and discussion
5.1 Compaction factor test
The assessment of the fresh properties of concrete was conducted through the compaction factor test. The obtained results using compaction factor testing on concrete are shown in Fig. 3. It can be analyzed from Fig. 3 that the workability of concrete is greatly influenced by the addition of alccofine and sodium nitrite. The value of the compacting factor for normal concrete mix (NC) is the highest in all the mixes under investigation. The compaction factor value of more than 0.90 indicates that the workability of normal concrete is of medium degree. Mix AC which is composed of 25% ultrafine slag shows a decrease in compaction factor value (0.88) which shows that the addition of UFS results in the concrete of decreased workability which may be due to the higher specific surface area of UFS demanding more water content to have for workable mix [54] which is very much similar to the study reported by Bharat Bhushan Jindal.
However, it is reported that the addition/replacement of up to 10% of alccofine (UFS) may result in an improvement in workability in the case of geopolymer-based concrete. In the current study higher percentage of UFS was taken to improve the micropore structure of concrete to enhance the compactness of the concrete matrix which may improve the corrosion-resisting performance of concrete.
The incorporation of Ultra-Fine Slag (UFS) in concrete is slightly affecting the workability of concrete; however, the outcomes show that with the inclusion of sodium nitrite, a noticeable effect on workability is observed. As the percentages of SN increase, there is a decreasing trend observed in the compaction factor value. From Fig. 3, it can be observed that with the addition of SN into the normal concrete mix, the compaction factor value decreased from 0.91 to 0.83 which indicates that with 25% UFS and 1.5% SN, the concrete workability reduces to a low workability state. However, with the addition of 1, 1.25, and 1.5% sodium nitrite, workability decreased by 1.02, 3.86, and 5.45%, respectively, in comparison to the mix containing alccofine (AC) at 0.45 W/B ratio. Introduction of sodium nitrite into UFS concrete (AC), sodium nitrite doesn’t have a significant improvement on the workability of concrete because of that it speeds up the setting time and thus curing time starts earlier in the concrete [55].
5.2 Compressive strength
In the present research, the compressive strength of concrete (CS) was assessed by a compression test. In general, the compressive strength of concrete is most important at not only early ages but also at later ages in the construction industry. It is noticed that a minor improvement in compressive strength is achieved with UFS (i.e. 25% cement replacement) at all ages are shown in Fig. 4. It has been realized that sodium nitrite enhances the concrete compressive strength at earlier ages than the normal concrete mix (NC) and alccofine concrete (AC). At early ages (3 and 7 days), the CS of mixtures AC + 1%SN, AC + 1.25%SN, and AC + 1.5%SN was enhanced by 9.56%, 29.15%, 17.23%, and 22.59%, 26.93%, 20.59%, respectively, in comparison to the mix containing UFS concrete (AC). The compressive strength is further enhanced significantly with SN, which is attributed to the reason that the concrete matrix gets increased the heat of hydration of cement phases like tricalcium silicate (C3S) and tricalcium aluminate (C3A), accordingly giving not only CS development but also heat evolution at early ages. Similar results patterns were examined at the age of 28 and 90 days. At 28- and 90-day curing period, the CS of mixes AC + 1%SN, AC + 1.25%SN, and AC + 1.5%SN showed enhancement by 3.29%, 10.89%, 6.78%, and 3.11%, 9.53%, 8.20%, respectively, in comparison to the mix containing UFS (AC). At the later ages (28 and 90 days), CS showed a minor change compared to 3 and 7 days because accelerators have no effect on the long-term properties [56].
5.3 Water absorption, free-chloride content, and pH value
WAT is one of the most important parameters for assessing the durability index property of concrete. The penetration of ions, water, and gases depends on the porosity and microstructure of concrete. The mixes with constant alccofine quantity and varying dosage of SN i.e. AC, AC + 1%SN, AC + 1.25%SN, and AC + 1.5%SN showed a decrease in WA percentage values of 2.64, 1.925, 1.68, and 1.912%, respectively, concerning the normal concrete mix at 28th day as shown in Fig. 5. WA significantly decreased with the incorporating of UFS in constant dosage due to small quantities of hydroxide phases produced, as well as the high surface area of UFS particles which settled in micro spaces in concrete [57].
Whereas chloride is available in water, it is transported with water to the steel reinforcement, that induces corrosion. Thus, the percentage of WA decreased is favorable for greater resistance to corrosion of the concrete. This reduction of WA was well associated with a major reduction in free chloride quantity in the powder concrete solution due to the addition of UFS and SN. While UFS caused a reduction in free chloride by 48%, adding 1, 1.25 and 1.5% SN further reduced it to 61, 64 and 66%, respectively as shown in Fig. 6.
Furthermore, pH tests revealed that the concrete solution became more alkaline with the addition of admixtures. Additionally, this indicates that introducing UFS and SN to the concrete, with better compressive strength than the normal concrete mix, is likely to improve corrosion resistance as well. The pH measurements for different concrete mixes are illustrated in Fig. 7.
5.4 Corrosion behaviour
Five different samples were tested for corrosion behaviour of early strength concrete. Evaluations of corrosion resistance (linear polarization resistance (LPR)) and open circuit potentials (OCP) of the specimens were carried out for fifty days once every 10 days. These samples were submerged in the medium of 1 M H2SO4 and 3% NaCl for 50 days. Figures 8 and 9 shows the OCP expressed in function of time and linear polarization observations, respectively.
The OCP of the normal concrete samples decreased, signifying a more negative value. This change is attributed to the ongoing hydration reactions in the samples over time.. Such reactions are the cause of changing the OCP. The decreased potentials indicate that the cathodic reaction may be retarded, resulting in a reduction of corrosion rate. The same trend was obtained over time with addition of alccofine in to the concrete as shown in Fig. 8. However, sodium nitrite shifts the OCP to more positive values compared to the normal concrete. Therefore, it can be observed that alccofine retards the cathodic reaction when sodium nitrite is a good inhibitor of the anodic reaction [58]. The process includes the reduction, oxidation, and Fe2+, as follows:
The deposition of nitrite ions on the rebar surface facilitates the release of O2−. Such O2− ions oxidize the Fe2+ formed on the rebar surface, to produce a stable barrier layer (γ-Fe2O3) above the rebar surface.
Linear polarization resistance (LPR) assessments give predictions of the rate of corrosion in the reinforcement for various concrete mixes that are being immersed in the medium of 1 M H2SO4 and 3%NaCl. From the LPR measurements, it can be observed that alccofine concrete specimens had approximately identical corrosion resistance as normal concrete specimens at initial ages and after that, the resistance significantly increased as shown in Fig. 9a–c. High corrosion resistance was observed with the addition of sodium nitrite and alccofine into the concrete. LPR is typically analyzed by applying small overpotentials around the gradually changing OCP. For a slowly corroding system such as concrete, these findings reflect the corrosion resistance observed at the time of measurement.
5.5 Thermogravimetric analysis
Figures 10 and 11 show the results of heat flow (i.e. decomposition of hydration compounds within the temperatures between 24 and 800 °C) and mass loss of various mixes such as NC, AC, AC + 1%SN, AC + 1.25%SN, and AC + 1.5%SN at 28 days, respectively. These results reveal a distinction between concrete containing alccofine and SN compared to normal and alccofine concrete without SN. The former exhibited increased heat absorption at 50 °C and 100 °C, indicating that alccofine concrete with SN contains more free water, possibly due to delayed hydration, and has a higher content of calcium silicate hydrate (CSH).
The approach involved assessing the weight loss of normal concrete, alccofine concrete with and without SN, during heating at 105 °C and 580 °C. Water removed at 105 °C and at 580 °C is considered as free water and bound water, respectively. Equations 4–5 are utilized to determine free water and bound water [59]. The calcium hydroxide content at 580 °C is obtained as a percentage weight, using Eq. 6 [60, 61]. The results indicate higher levels of free and bound water in AC, AC + 1%SN, AC + 1.25%SN, and AC + 1.5%SN compared to normal concrete, as depicted in Figs. 12 and 13, respectively.
Figure 14 displays the calcium hydroxide percentage in normal concrete and alccofine-added concrete with different doses of SN admixture, maintaining a constant water-to-binder ratio of 0.45, corresponding to the temperature. This is attributed to the reaction between alccofine and calcium hydroxide in the presence of water, leading to the formation of additional CSH in the concrete.
5.6 X-ray diffraction (XRD)
Samples were extracted from failed specimens and pulverized to pass through a 200 µm sieve. Figure 15 illustrates the peak intensities of NC, AC, and AC + 1.25%SN at 28 days for a water-to-binder (W/B) ratio of 0.45. In Fig. 8, the intensities of CSH, ettringite, Ca(OH)2, and CASH were identified at Bough’s angles (2Theta (2Ɵ)) of 20.64, 22.87, 26.46, and 28.29, respectively.
The analysis affirms that the intensities of these crystalline components are higher in alccofine concrete, both with and without sodium nitrite, compared to normal concrete samples. This suggests that the production ofCSH is more pronounced in alccofine concrete, with and without sodium nitrite, as Ca(OH)2 transforms into additional CSH. This transformation contributes to the overall enhancement of concrete strength.
5.7 FTIR
The FTIR spectrum results for the concrete mixes NC, AC, AC + 1%SN, AC + 1.25%SN, and AC + 1.5%SN are presented in Fig. 16, offering insights into the mineral composition. This figure illustrates the FTIR spectra curves of all specimens at 28 days within the range of 500 to 4000 cm⁻1. In normal concrete samples, the Si–O stretching vibration of CSH is observable at 962 cm⁻1. With the introduction of alccofine and sodium nitrite, the absorption peaks of Si–O of CSH shift to higher values, ranging between 964 and 975 cm⁻1. This shift signifies changes in the n(Ca)/n(Si) ratio attributed to the presence of silicon and aluminum phases in alccofine, leading to the generation of extra calcium silicate hydrate (CSH) gels in concrete. As a result, this phenomenon plays a role in the noted enhancements in compressive strength observed with the inclusion of sodium nitrite and alccofine.
The absorption peak of Ca(OH)2, induced by the stretching vibration, is identified in the range of 3691–3694 cm⁻1. Notably, the Ca(OH)2 absorption peak diminishes with the addition of sodium nitrite and alccofine, signifying a reduction in intensity due to the consumption of Ca(OH)2. This trend aligns with findings from XRD analysis. Bands at 3507–3536 and 1652–1664 cm⁻1are attributed to the stretching vibration of H2O, while the asymmetric stretching range of 1410–1420 cm⁻1 induces the CO32− band.
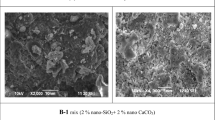
5.8 SEM
In Fig. 17, SEM analysis of normal and alccofine-added concrete with varying percentages (0, 1, 1.25, and 1.5%) of sodium nitrite (SN) is presented. Image (a) reveals that normal concrete without alccofine and SN exhibits high porosity and is less compact. In contrast, image (b) clearly indicates that alccofine-added concrete possesses a denser microstructure, possibly attributed to the formation of additional gel (white precipitates), as alccofine is a rich source of calcium and silica. Likewise, in images (c–e), the impact of SN becomes apparent. Image (d) illustrates a more compact microstructure with 1.25% SN, while higher percentages of SN show needle-like structures, potentially contributing to a reduction in concrete strength.
Comparing normal and alccofine-added concrete samples after a 28-day curing period, it is evident that alccofine-added concrete with 1.25% SN exhibits a compact and dense structure, likely due to the formation of more hydrated products such as CASH, CAH &CSH thereby enhancing compressive strength.
6 Response surface method
A set of statistical methods that are that are utilized to create, enhance and optimize products is termed as response surface methodology (RSM) and is typically used where several components impact at least one performance characteristics. It is commonly used to enhance an individual or set of reactions to meet a given arrangement of details. In this work, RSM has been used to develop a regression expression that predicts the concrete strength by obtaining the best values of the considered variables. In this study, the considered variables are the curing age, the amount of sodium nitrite added, the compressive strength of the concrete. The compressive strength results of concrete are utilized to develop the regression models. A Multivariate analysis with 95% confidence levels (α = 0.05) is carried out using MINITAB program [62, 63]. The parameters considered are x, y, z which represents the curing age, the amount of sodium nitrite added and compressive respectively.
The application of RSM bought about an exact relation among x, y, and z with the regression Eq. (7) respectively.
where 95% confidence bound coefficients are a = 12.53 (11.79, 13.27), b = 1.953 (0.1628, 3.744), c = 1.062 (1.022, 1.103), d = − 0.7526 (− 1.959, 0.4536), e = − 0.0004608 (− 0.013, 0.01208), f = − 0.008775 (− 0.00917, − 0.00838).
The values of the Coefficient of Determination (COD) obtained from Table 7 and residual error obtained in Table 8. These values confirm the appropriate fit of the models as the error of total variation is observed to be within 95% confidence level which is in the acceptable range. Figure 18 depicts the response surface model for the early strength concrete. From these figure it observed that the optimum amount of sodium nitrite concentration 1.25% have maximum compressive strength. Table 7 and Fig. 18 validate the regression models obtained in Eq. (7) which is a good fit.
7 Conclusion
The influences of introducing alccofine ultra-fine particles and a sodium nitrite as a corrosion inhibitor, on the composition, corrosion behaviour and mechanical properties of concrete were examined. Composition of variousmixes were studied by XRD analysis, while the compaction factor test, compressive strength,water absorption test of various concrete mixes were evaluated. Further, free chloride and pH content of concretepowder samples were calculated. Opencircuit potentials and linear polarization resistances were achieved in a 1 M H2SO4 and 3%NaCl environment. The main findings ofthe study are as follows:
-
With addition of constant alccofine quantity showed moderate enhancement in compressive strength of concrete at all ages as the addition of alccofine results in the enhanced hydrated products. The additional hydrated gel leads to denser microstructure and improved mechanical strength and durability.
-
Water absorption testing revealed a notable decrease in water uptake for concrete containing alccofine and SNF compared to the reference mix. This enhancement can be ascribed to the pore-filling mechanism of alccofine particles, resulting in a denser and less permeable concrete matrix.The decrease in water absorption may indicate the improvement in the durability of concrete.
-
Corrosion potentials declined over time for all samples.
-
The deposition of nitrite ions on the rebar surface facilitates the release of O2−. Such O2− ions oxidize the Fe2+ formed on the rebar surface, to produce a stable barrier layer above the rebar surface.
-
In thermogravimetric analysis, concrete incorporating alccofine exhibited reduced mass loss and an increased occurrence of hydrate decomposition when subjected to temperatures ranging from 24 to 800 °C. The TGA results indicated that the combination of alccofine with varying dosages of SN resulted in lower calcium hydroxide content and higher bound water content compared to normal concrete.
-
XRD analysis revealed that the presence of alccofine, with or without sodium nitrite, contributed to an augmentation in the formation of CSH and calcium aluminosilicate hydrate (CASH) in the concrete.
-
Through SEM analysis, it was observed that the CASH formation, CSH and microstructure in the concrete were enhanced by alccofine and the combination of alccofine with SN.
-
Moreover, the experimental data is subjected to validation through RSM, and the observed error falls within an acceptable range, affirming the reliability and accuracy of the experimental results.
Data availability
All data is provided within the manuscript.
References
https://www.pcimag.com/blogs/14-pci-blog/post/103869-the-cost-of-corrosion
Popov BN. Corrosion engineering: principles and solved problems. Amsterdam: Elsevier; 2015.
Razaqpur A, Isgor O. Prediction of reinforcement corrosion in concrete structures. Front Technol Infrastruct Eng Struct Infrastruct. 2009;4:45–69.
Cicek V. Corrosion Engineering and Cathodic Protection Handbook: With Extensive Question and Answer Section. New York: Wiley; 2017.
Kurdowski W. Chloride corrosion in cementitious system, Structure and Performance of Cements. (2002): 295–309.
Berrocal CG, Lundgren K, Löfgren I. Corrosion of steel bars embedded in fibre reinforced concrete under chloride attack: state of the art. Cement Concrete Res. 2016;80:69–85.
Ebell G, Burkert A, Fischer J, Lehmann J, Müller T, Meinel D, Paetsch O. Investigation of chloride‐induced pitting corrosion of steel in concrete with innovative methods. Mater Corros. 2016;67(6):583–90.
Neville A. Chloride attack of reinforced concrete: an overview. Mater Struct. 1995;28(2):63.
Bertolini L, Bolzoni F, Pedeferri P, Lazzari L, Pastore T. Cathodic protection and cathodic prevention in concrete: Principles and applications. J Appl Electrochem. 1998;28:1321–31.
McHattie JS, Perez IL, Kehr JA. Factors affecting cathodic disbondment of epoxy coatings for steel reinforcing bars. Cement Concr Compos. 1996;18:93–103.
Yeih W, Chang JJ, Chang CC, Chen KL, Chi MC. Electrochemical chloride removal for reinforced concrete with steel rebar cage using auxiliary electrodes. Cem Concr Compos. 2016;74:136–46.
Philip N, Ganga VR, Syriac T. Effectiveness of bacteria-based self-healing concrete under corrosive environment. Iran J Sci Technol Trans Civil Eng. 2023;48:1–14.
Philip N, Varughese AE, James J. Correlation of mechanical properties of concrete with partial metakaolin replacement with various depended factors. Iran J Sci Technol Trans Civil Eng. 2023;47(1):1–19.
Philip N, Jędrzejewska A, Varughese AE, James J. Influence of pore structure on corrosion resistance of high performance concrete containing Metakaolin. Cement – WapnoBeton. Cem Lime Concr. 2023;27(5):302–19.
Roy DM. Hydration, microstructure and chloride diffusion of slag-cement plasters and mortars, fly ash, silica fume, and natural pozzolans in concrete, SP-114. Am Concr Inst Farmington Hills MI. 1989;2:1265–81.
Fulton FS. The properties of portland cement containing milled granulated blast-furnace slag. Monograph: Portland Cement Institute, Johannesburg; 1974. p. 4–46.
Hogan FJ, Muesel JW. Evaluation for durability and strength development of a ground granulated blast-furnace slag. Cem Concr Aggr. 1981;3(1):40–52.
Mehta PK. “Durability of Concrete in Marine Environment-A Review”, Performance of Concrete in Marine Environment, SP-65. American Concrete Institute: Farmington Hills, MI; 1980. p. 1–20.
Bakker RFM. On the Cause of Increased Resistance of Concrete Made from Blast-Furnace Cement to Alkali Reaction and to Sulfate Corrosion. Thesis RWTH-Aachen, 1980.
Hope BB, Ip AKC. Corrosion of steel in concrete made with slag cement. ACI Mater J. 1987;84:525–31.
Andrade C, Buják R. Effects of some mineral additions to Portland cement on reinforcement corrosion. Cem Concr Res. 2013;53:59–67.
Montemor MF, Simões AMP, Salta MM. Effect of fly ash on concrete reinforcement corrosion studied by EIS. Cem Concr Compos. 2000;22:175–85.
Teng S, Lim TYD, SabetDivsholi B. Durability and mechanical properties of high strength concrete incorporating ultra fine ground granulated blast-furnace slag. Constr Build Mater. 2013;40:875–81.
Sharmila P, Dhinakaran G. Compressive strength, porosity and sorptivity of ultra fine slag based high strength concrete. Constr Build Mater. 2016;120:48–53.
Sharmila P, Dhinakaran G. Strength and durability of ultra fine slag based high strength concrete. Struct Eng Mech. 2015;55(3):675–86.
Haruehansapong S, Pulngern T, Chucheepsakul S. Effect of the particle size of nanosilica on the compressive strength and the optimum replacement content of cement mortar containing nano-SiO2. Constr Build Mater. 2014;50:471–7.
Saraswathy V, Muralidharan S, Kalyanasundaram RM, Thangavel K, Srinivasan S. Evaluation of a composite corrosion-inhibiting admixture and its performance in concrete under macrocell corrosion conditions. Cem Concr Res. 2001;31:789–94.
Soylev TA, Richardson MG. Corrosion inhibitors for steel in concrete: State-of-the-art report. Constr Build Mater. 2008;22:609–22.
Sarma VVS, Alisha SS, Vijay K, Kumar PG, Sai Kumar KS. Mechanical performance enhancement of recycled aggregate concrete using GGBS and fly ash for sustainable construction. Multiscale Multidiscip Model Exp Des. 2023. https://doi.org/10.1007/s41939-023-00271-9.
Vijay K, Paluri Y, Reddy MS, Rao IV, John K, Dayanand N. Performance evaluation of reclaimed asphalt pavement (RAP) aggregate in concrete pavements: a state-of-the-art review. J Build Pathol Rehabil. 2023. https://doi.org/10.1007/s41024-023-00335-w.
Li S, Roy DM. Investigation of relations between porosity, pore structure and chloride diffusion of fly ash blended cement pastes. Cem Concr Res. 1986;16:749–59.
Yeau KY, Kim EY. An experimental study on corrosion resistance of concrete with ground granulated blast furnace slag. Cem Concr Res. 2005;35:1391–9.
Gu P, Beaudoin JJ, Zhang MH, Malhotra VM. Performance of reinforcing steel in concrete containing silica fume and blast furnace slag ponded with sodium chloride solution. ACI Mater J. 2000;97(3):254–62.
Saraswathy V, Muralidharan S, Thangavel K, Srinivasan S. Influence of activated fly ash on corrosion-resistance and strength of concrete. Cem Concr Compos. 2003;25(7):673–80.
Asmara YP, Siregar JP, Tezara C, Nurlisa W, Jamiluddin J. Long term corrosion experiment of steel rebar in fly ash-based geopolymer concrete in NaCl solution. Int J Corros. 2016;2016:1–5. https://doi.org/10.1155/2016/3853045.
Park JH, Park C, Joh SH, Lee HS. Effect of curing condition on resistance to chloride ingress in concrete using ground granulated blast furnace slag. Materials. 2019;12(19):3233.
Supit SWM, Shaikh FUA. Durability properties of high volume fly ash concrete containing nano-silica. Mater Struct. 2015;48(8):2431–45.
Sudha Uthaman RP, George VV, Harilal M, Philip J. Enhanced seawater corrosion resistance of reinforcement in nanophase modified fly ash concrete. Constr Build Mater. 2019;221:232–43.
Ghanooni-Bagha M, Shayanfar MA, Shirzadi-Javid AA, Ziaadiny H. Corrosion-induced reduction in compressive strength of self-compacting concretes containing mineral admixtures. Constr Build Mater. 2016;113:221–8.
Diab AM, Elyamany HE, AbdElmoty AM. Effect of mix proportions, seawater curing medium and applied voltages on corrosion resistance of concrete incorporating mineral admixtures. Alex Eng J. 2011;50(1):65–78.
Almeida FCR, Sales A, Moretti JP, Mendes PCD. Use of sugarcane bagasse ash sand (SBAS) as corrosion retardant for reinforced Portland slag cement concrete. Constr Build Mater. 2019;226:72–82.
BIS 8112. Ordinary Portland Cement 43 Grade Specification. India: New Delhi; 2013.
BIS. Specification for Coarse and Fine Aggregate from Natural Sources for Concrete, Second Revision (IS 383: 2016). India: New Delhi; 2016.
ASTM C989. Standard Specification for Ground Granulated Blast-furnace Slag for Use in Concrete and Mortars. USA: West Conshohocken; 1999.
BIS 10262. Guidelines for Concrete Mix Design Proportioning. New Delhi, India: Bureau of Indian Standards; 2009.
BIS 1199. Methods of Sampling and Analysis of Concrete. India: New Delhi; 1959.
BIS 516. Methods of Tests for Strength of Concrete. India: New Delhi; 1959.
ASTM C642. Standard Test Method for Density, Absorption, and Voids in Hardened Concrete. USA: West Conshohocken; 2013.
Conciatori D, Laferrière F, Brühwiler E. Comprehensive modeling of chloride ion and water ingress into concrete considering thermal and carbonation state for real climate. Cem Concr Res. 2010;40(1):109–18.
Tang L. Engineering expression of the ClinConc model for prediction of free and total chloride ingress in submerged marine concrete. Cem Concr Res. 2008;38(8–9):1092–7.
Barneyback RS, Diamond S. Expression and analysis of pore fluids from hardened cement paste and mortars. Cem Concr Res. 1981;11(2):279–85.
Designation, A. S. T. M. "C876–15," Standard Test Method for Corrosion Potentials of Uncoated Reinforcing Steel in Concrete," ASTM International, West Conshohocken, PA, 2015."
Jindal BB. Investigations on the properties of geopolymer mortar and concrete with mineral admixtures: a review. Constr Build Mater. 2019;227(10): 116644.
Lee T, Lee J, Kim Y. Effects of admixtures and accelerators on the development of concrete strength for horizontal form removal upon curing at 10 °C. Constr Build Mater. 2020;237: 117652.
Heikal M. Effect of calcium formate as an accelerator on the physicochemical and mechanical properties of pozzolanic cement pastes. Cem Concr Res. 2004;34:1051–6.
Reddy PN, Naqash JA. Properties of concrete modified with ultra-fine slag. Karbala Int J Mod Sci. 2019. https://doi.org/10.33640/2405-609X.1141.
Kumar MP, Rangarajan M, Mini KM. Enhancement of mechanical properties and corrosion behaviour of concrete due to addition of ultrafine GGBS, in: RILEM International Conference on Advances in Construction Materials and Systems – ICACMS (2017).
Amba JC, Balayssac JP, Détriché CH. Characterisation of differential shrinkage of bonded mortar overlays subjected to drying. Mater Struct. 2010;43(1–2):297.
Reddy PN, Naqash JA. Experimental study on TGA, XRD and SEM analysis of concrete with ultra-fine slag. Int J Eng. 2019;32(5):679–84.
Roychand S, Silva SD, Setunge S. Micro and nano engineered high volume ultrafine fly ash cement composite with and without additives. Int J Concrete Struct Mater. 2016;10(1):113–24.
Reddy PN, Naqash JA. Effectiveness of polycarboxylate ether on early strength development of alccofine concrete. Pollack Periodica. 2020;15(1):79–90.
Narasimha Reddy P, Ahmed Naqash J. Effect of alccofine on mechanical and durability index properties of green concrete. Int J Eng. 2019;32(6):813–9.
Acknowledgements
The authors express their gratitude to SVCET(A), Chittoor, for providing laboratory testing facilities.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
Initial idea and literature review done by A. Uday Kumar and Kunamineni Vijay. The experimental work done by Panga Narasimha reddy, Bode Venkata Kavyatheja, G. Gautham Kishore Reddy, and Avuthu Narender Reddy. Manuscript written by Panga Narasimha Reddy and A. Uday Kumar.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Reddy, P.N., Vijay, K., Kavyatheja, B. et al. Impacts of corrosion inhibiting admixture and supplementary cementitious material on early strength concrete. Discov Appl Sci 6, 378 (2024). https://doi.org/10.1007/s42452-024-06032-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-024-06032-8