Abstract
Ultrasonic techniques are frequently used in organic molecule conformation analysis. Mixtures of ternary liquid complexes are extensively used for comprehending the strength as well as the characteristics of the interactions between molecules. Experimentally, the thermo physical properties of ternary liquid mixtures such as viscosity (η), density (ρ) and velocity (u) of K-contin, (2-Aminoacetamido)acetic acid and non-aqueous medium were determined over temperature ranges of 298.15, 308.15 and 318.15 K. The experimental data were used to ascertain the thermoacoustic parameters such as free volume, internal pressure, adiabatic compressibility, solvation number, specific acoustic impedance and intermolecular free length. These parameters are more useful for predicting and validating molecular interactions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
It is crucial to assess the materials’ physical and chemical features regardless of how they are produced. Exposure to ultrasonic sound waves can give insight into the properties of solid or liquid materials. Liquid-Liquid mixtures and the combinations of liquids have many applications in the healthcare, pharmaceutical, chemical, solvent and allied sectors 1.
Ultrasonic studies have increasingly focused on understanding molecular interactions that occur in both pure liquids and mixtures of liquids [2,3,4]. Assessment of the velocity of ultrasonic waves is frequently used for investigating the physical and chemical behaviour of liquid mixtures [5, 6]. The potassium ion is the predominant intracellular cation in most bodily tissues. It plays cardinal roles innerve impulse transmission, contraction of smooth muscles, skeletal muscles and cardiac muscles. Potassium ions play an important role in several physiological processes, including maintaining proper renal function [7]. Low blood potassium levels can be treated and prevented with potassium chloride. The potassium ion is used in the treatment of hypokalemia, fertilizers and explosives. (2-Aminoacetamido)acetic acid is used in the synthesis of more complex peptides.
Moreover, the potassium ion aids in the solubilization of recombinant proteins in Escherichia coli. Specifically, it cleaves Staphylococcus aureus peptidoglycans, which are generally derived from staphylococci. Measurements of ultrasonic velocity and density were conducted for both pure components and ternary mixtures of quinoline, methanol, and toluene across the entire composition range at 30, 35, 40 and 45 °C is investigated by Kavitha et al. [8].The molecular interactions of the antibiotic doxycycline hyclate were investigated using ultrasonic and viscometric methods [9]. At varied temperatures, the interactions between molecules within the ternary liquid mixtures have been investigated through thermodynamic studies [10].
This current study involves measuring the density, viscosity, ultrasonic velocity and additional thermoacoustic measurements for the ternary system of K-contin and (2-Aminoacetamido)acetic acid in non-aqueous formamide at 298.15, 308.15 and 318.15 K. As ternary mixtures contain molecular interactions and the results are explained and discussed.
2 Materials and methods
The polar protic solvent used in the present study is formamide, (2-Aminoacetamido)acetic acid, which forms the solute and K-contin which functions as the co˗solute. Varying molalities (0.001, 0.005, 0.01, 0.025 and 0.05 m) of solute, (2-Aminoacetamido) acetic acid) and a set molality (0.01 m) of the co-solute, K-contin were used to prepare the solutions. The salts were weighed using a Kern electronic balance, which has an accuracy of ± 10−4 g, to prepare the non-aqueous solutions.
2.1 Ultrasonic velocity measurement
Ultrasonic velocity was measured using digital ultrasonic interferometer of fixed frequency 2 MHz with on accuracy of ± 0.2 m s-1 (Model F-81 Mittal enterprises, New Delhi). The measuring cell of the interferometer is a specially designed double walled vessel with provision for maintaining temperature constant. A digital constant temperature bath operating in the temperature range 0 to 90 °C with an accuracy of ± 0.1 °C has been used to circulate water through the outer jacket of double walled measuring cell containing the experimental liquid/solution.
2.2 Density measurement
An Anton Paar DMA 4100 Digital density meter was used to calculate the density of non-aqueous peptides dissolved in electrolyte solutions with an accuracy of 0.0001 g/cc.
2.3 Viscosity measurement
The viscosities of the solutions are measured using a specially designed Cannon Fenske viscometer (± 0.1% error) with the experimental solution is immersed in a temperature controlled water bath. The time of flow was measured using a stop watch with an accuracy of 0.1 s.
3 Computation analysis
There are several different concentrations of ternary mixtures of density (ρ), viscosity (η) and ultrasonic velocity (u) at various different temperatures as shown in Table 1 and Figs. 1, 2 and 3.
The following equations were used to define ‘u’, ‘ρ’ and ‘\(\eta\)’.
where, ‘f’= frequency of ultrasonic waves, ‘λ’= wavelength of ultrasonic waves.
where, ρ1 and W1 are the density and weight of the solution respectively; ρ2, W2 indicate the density and weight of the solvent respectively.
where η1 and η2 are viscosity of solutions and solvent respectively; ρ1, and ρ2 are the density of solutions and solvent respectively; t1 = time of flow of solutions, t2 = time of flow of solvent.
The standard relations have been used to calculate the following parameters based on the measured data.
The value of ‘b’ represents cubic packing and is equal to 2. The constant k is dimensionless and is not dependent on the temperature or characteristics of fluids, with a specific value of 4.281 × 109. T = Absolute temperature in Kelvin, R = Universal Gas Constant, Meff= effective molecular weight, \(\eta\) = Viscosity of solution (N s m−2), ρ = Density of the solution (kg m−3) and u = ultrasonic velocity of the solution (m s−1).
where, Meff = ΣmiXi. Here, mi= molecular weight of each constituent, Xi= mole fraction of that constituent in the mixture.
where u and ρ are as defined for Eq. (4)
The parameter K, known as Jacobson’s constant, is directly proportional to the square root of the absolute temperature and can be expressed as K α \(\sqrt{\text{T}}\), where α denotes the proportionality.
where u and ρ are as defined for Eq. (4).
where Sn is the primary solvation number, ni moles of ions are solvated with niSn moles of incompressible solvent, ns moles of solvent corresponding to a total volume V of solution, β = the compressibility of solution and β0 = the compressibility of the solvent.
4 Results and discussion
In Table 1, the density, viscosity and ultrasonic velocity of K-contin and (2-Aminoacetamido) acetic acid ternary mixtures in non-aqueous formamide calculations are performed across the entire mole fraction range at various temperatures 298.15 K, 308.15 and 318.15 K.
Based on all experimental temperatures, Tables 2 and 3 report the values for the derived thermo-acoustic parameters.
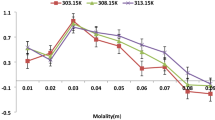
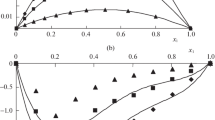
Figures 1, 2, 3, 4, 5, 6, 7, 8 and 9 show how the ultrasonic velocity, density and viscosity varied as a function of molality across a temperature range as well as how the thermodynamic parameters such as internal pressure and free volume which can be evaluated across a range of temperatures. They also show the variation of intermolecular free length as well as specific acoustic impedance and adiabatic compressibility as well as a function of internal pressure
From Table 1, the variations in ultrasonic velocity provide the details regarding the structural variations within samples. As the mole fraction increases, the viscosity of solution increases, indicating a close association between solute, co-solute and solvent molecules.
With respect to concentration, ultrasonic velocity increases due to molecular interactions in these solutions, which may be ascribed to the cohesion created in the samples used for the study. With increasing temperature, the cohesion and frictional forces between molecules decrease due to the increased thermal motion of the molecules.
The density of the solutions increases as the concentration increases, which may be attributed to the more compact packing of molecules within the solution. It was observed that the density increases with temperature. Due to the presence of solvent molecules, the density decreases with the mole fraction due to the shrinkage in volume.
Viscosity is a critical parameter that helps delineate the structures and interactions between molecules occurring in solutions. Increase in ultrasonic velocity occurs as the mole fraction increases, demonstrating a moderately strong electrolytic relationship between the solute and the solvent. Thus, these mixtures exhibit increased velocity due to molecular interactions. The decrease in viscosity as temperature increases may be attributed to the enhanced movement of molecules and ions within the solution [11]. Ternary systems can be studied with the help of the variation in internal pressure [12] to reveal the strength and nature of intermolecular forces [13]. From Table 2, it is found that internal pressure (πi) increases as the concentration increases.
The free volume (Vf) of a sample decreases with an increase in concentration and increases with a temperature rise (Table 2). In ternary mixtures, there is a strong association between the molecules, causing a decrease in free volume [14, 15]. Free volume changes in ternary solutions are opposite to internal pressure, changes can occur as a result of variations in both concentration and temperature. The attraction between molecules potentially leads to a reduction in the compressibility value due to structural re-arrangements of the constituents [16]. Decrease in adiabatic compressibility (\({\beta }_{ad}\)) as the mole fraction of K-contin and (2-Aminoacetamido)acetic acid increases in non-aqueous formamide, indicates a close-set packing and increased ionic repulsion.
The reduction in intermolecular free length with an increase in the concentration of the solution suggests the presence of significant interactions between the peptide and drug. This results in a decrease in the distance between adjacent molecules within the solution. This decrease in intermolecular distance could potentially be as a result of the formation of dipolar associations and hydrogen bonds between the molecules in solution [17].
According to the current study, a reduction in intermolecular free length increases ultrasonic velocity and decreases adiabatic compressibility. As the sound energy propagates through a medium, its specific acoustic impedance (Z) offers opposition [18]. The acoustic impedance increases with increasing mole fraction and decreases with increase in temperature of K-contin and (2-Aminoacetamido)acetic acid in non-aqueous formamide, indicates that significant interactions between the molecules component.
Ion-solvent interactions are often studied using the solvation method. Pertaining to K-contin and (2-Aminoacetamido)acetic acid in non-aqueous formamide at below room temperature, negative solvation numbers are observed for almost all concentrations. Above room temperature, positive solvation numbers are observed for all concentrations. A solvation number that is positive implies that the solution’s compressibility at all concentrations could be lower than that of the solvent. Conversely, a negative solvation number implies that the solvent is less compressible than the solution. When the interaction between the ion and the solvent is the same as the intermolecular interaction energy, the solvation number is zero. The solvation number’s decline with increasing molality could be attributed to either a limited number of solvent molecules available for all the ions or preferential ion-pairing [19, 20]
5 Conclusion
Ultrasonic is a technique that is non-destructive and versatile and is valuable for investigating the different physicochemical features of solutions, such as adiabatic compressibility, internal pressure, free volume and intermolecular free length. In this study, ultrasonic studies were carried out on solutions of K-contin and (2-Aminoacetamido)acetic acid in non-aqueous formamide at different temperatures. K-continis commonly used as an electrolyte supplement in medications. Based on experimental readouts of density, viscosity, ultrasonic velocity, among other derived thermoacoustic parameters, it was found that there exists molecular association and solute, co-solute and solvent interactions between components in the ternary mixtures of K-contin and (2-Aminoacetamido)acetic acid in non-aqueous formamide medium. This study uses variations in temperature to clearly show that the strength of the intermolecular interactions decreases as temperature increases and increases with increasing the concentration.
Data availability
Not applicable.
Code availability
Not applicable.
References
Sridevi G (2016) Study of Excess Thermodynamic Parameters of Binary Liquid Mixtures of Methyl Benzoate with 1-Nonanol at different temperatures. J Chem Bio Phy Sci 6:486–497
Santosh MS, Krishna Bhat D, Aarti Bhat S (2010) Volumetric and Ultrasonic investigation of Glycylglycine in Aqueous FeCl3 Solutions. J Solution Chem 39:1763–1773
Chithra Devi R, Mathammal R, Kesavasamy R, Rohini V (2018) Ultrasonic studies of Molecular interaction in the Ternary Liquid Mixtures of Acrylonitrile + Benzene + N Methyl Aniline at Different Temperatures. Mater Today Proc 5:16855–16862
Singh RD, Gupta M (2017) Molecular interaction study in binary mixture of DMSO with formamide and N, N-dimethyl formamide. Indian J Pure Appl Phys 55:729–736
Mary Girija A, Armstrong Arasu MM, Devi D (2015) Ultrasonic and Viscometric Studies of Molecular Interaction in binary liquid mixtures of propylene glycol and dimethyl sulphoxide at various temperatures. Int J Sci Res Sci Technol 3:89–96
Ali A, Nain A, Chand D, Lal B (2007) Ultrasonic, volumetric and viscometric studies of molecular interactions in binary mixtures of dimethylsulphoxide with polar substituted cyclohenes at 30°C. Phys Chem Liq 45:79–91
Zacchia M, Abategiovanni ML, Stratigis S, Capasso (2016) Potassium: from physiology to clinical implications G. Kidney Dis (Basel) 2:72–79. https://doi.org/10.1159/000446268
Kavitha C, Ratnakar A, Narendra K (2016) Study of molecular interactions in ternary liquid mixtures. Int Lett Chem Phy Astron 63:77–82
Kumar R, Mohamed Kamil MG, Shri Prasad S, Gayathri GS, Shabeer TK (2013) Ultrasonic and viscometric study of molecular interactions of antibiotic doxycycline hycalte. Indian J Pure Appl Phys 51:701–707
Asghar J, Liakath F, Khan A, Subramani K (2013) Thermodynamic studies of molecular interactions in ternary liquid mixtures at various temperatures. Rasayana J Chem 3:697–704
Jennifer Dunlap MD, Rita M, Brij Bihari M (2011) Association of JAK2 Mutation Status and Cytogenetic Abnormalities in Myeloproliferative Neoplasms and Myelodysplastic/Myeloproliferative Neoplasms. Am J Clin Pathol 135:709–719. https://doi.org/10.1309/AJCPS6C8EVYCQNRM
Padmavathy R, Dhanalakshmi K, Radha N, Jasmine Vasantha Rani E (2017) Densitometric, viscometric and conductometric study of non-aqueous solutions of peptide and Electrolyte. Int J Innov Eng 2:8–13
Kalyanasundaram S, Manuel Stephen A, Gopalan A (1997) Ultrasonic studies on Poly(Methylmethacrylate) in dioxane. Acta Acust United Acust 83:74–77
Aruna P, Natarajan S, Suryanarayana CV (1991) The internal-pressure at the miscibility point in some ternary‐systems. Ind J Chem 29:537–540
Baluja S, Oza S (2003) Ultrasonic studies of some derivatives of 6-ethylbenzene-1,3-diol in 1,4-dioxane. Fluid Phase Equilib 208:83–89
Palani R, Balakrishnan S, Sudhamani KA (2010) Molecular interaction studies of glycylglycine in aqueous sodium halide solutions at 303, 308 and 313K. ARPNJ Eng Appl Sci 5:58–64
Syal VK, Chauhan S (2005) Ultrasonic velocity of binary mixtures of acetone and dioxane with dimethylsulphoxide as one component. Indian J Pure Appl Phys 43:844–848
Aruldhas G (2007) Molecular Structure and Spectroscopy, 2nd edn. PHI Learning Private Limited, Delhi
Ubagaramary D, Enoch VMV, Mullainathan S (2018) Correlation between Surface Tension on Aqueous Solution of 5-Fluorouracil with heterocyclic compound at various temperatures. Anal Chem Ind J 18(1):128–140
Mistry AA, Bhandakkar VD, Chimankar OP (2012) Acoustical studies on ternary mixture of toluene in cyclohexane & nitrobenzene at 308K using ultrasonic technique. J Chem Pharm Res 4:170–174
Acknowledgements
The authors are thankful to management of Seethalakshmi Ramaswami College, Tiruchirappalli, for the support and motivation.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
1. RG—Conceptualization, Data Curation, Formal Analysis, Writing—Original Draft Preparation. 2. RP—Investigation, Resources, Supervision, Validation. 3. RRM—Supervision, Validation.
Corresponding author
Ethics declarations
Conflict of interest
I am submitting herewith my original research article entitled “Molecular interactions in ternary system of K-contin and (2-Aminoacetamido)acetic acid at various temperatures—Ultrasonic and viscometric analysis” I assure you that the work described has not been applied for any other journal that is not under consideration for any journal.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Geetha, R., Padmavathy, R. & Muhamed, R.R. Molecular interactions in ternary system of K-contin and (2-Aminoacetamido)acetic acid at various temperatures–ultrasonic and viscometric analysis. SN Appl. Sci. 5, 383 (2023). https://doi.org/10.1007/s42452-023-05597-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-023-05597-0